Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

In the case of semiconductor systems initially containing TMI in a d
0
state and
subsequently reduced, the presence of band - like absorptions assignable to d – d
transitions, instead of edge - like features, arises from the formation of localized
reduced centers and not the injection of electrons into the conduction band of the
support. This has been discussed in the case of the V
2
O
5
/WO
3
/TiO
2
EUROCAT
SCR catalyst [72] .
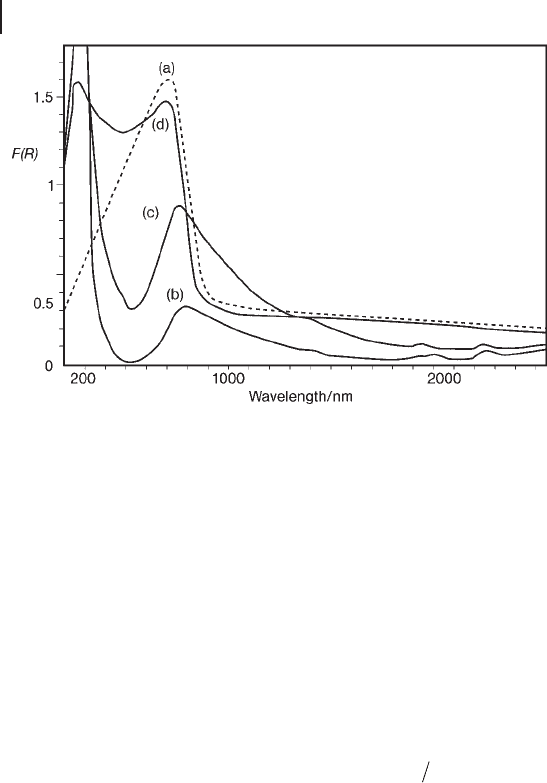
Focusing on the shape of d – d bands, a profi le asymmetry due to the distortion
of local structures by the Jahn – Teller effect can be useful in monitoring the isola-
tion of TMI sites with respect to aggregated structures exhibiting a sharp band - gap
transition in a similar position. An example is provided by the dependence of the
spectral features of CuO/Al
2
O
3
catalysts on the copper content (Figure 2.14 ). It can
be observed that Cu
2+
octahedra are strongly distorted by the Jahn – Teller effect,
changing from O
h
to D
4h
symmetry.
Besides asymmetry, band width may be a source of information also. The width
of the d – d bands of TMI complexes adsorbed on an oxidic support can be compa-
rable to or even narrower than those observed for the aqueous precursor com-
plexes. This can be related to a high homogeneity of the molecular environment
of TMI on the surface, suggesting a possible molecular recognition character of
the adsorption process [73] .
2.3 UV-Vis-NIR Absorption Spectroscopy 73
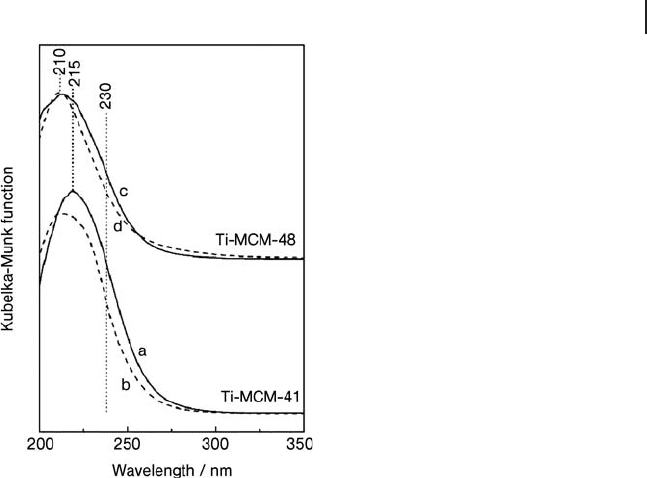
Figure 2.13 DR UV - Vis spectra of (a) calcined Ti - MCM - 41, (b)
silylated Ti - MCM - 41, (c) calcined Ti - MCM - 48, silylated Ti -
MCM - 48. In all cases the Ti loading was of ca 2 wt%; the
samples were outgassed at 523 K for 2 h before spectroscopic
measurements. Reprinted with permission from ref. [71] .
Copyright 2007 American Chemical Society.
74 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
Of course, a relevant source of information on the features of a TMI center at
the surface of a catalyst, or on its evolution during the catalyst preparation, is the
position of d – d bands. These have a dependence upon the composition of the
ligand sphere, including oxygen atoms/hydroxy groups at the surface of an oxidic
support. If the system can be treated with the model of the cubic ligand fi eld
(i.e. octahedral and tetrahedral stereochemistry) and, once known the nature of
the ligands, it is possible to analyze the band positions by applying the law of
average environment [15] . This states, taking a [MA
n
B
6 - n
] complex as an example,
that the ligand fi eld will be
∆∆ ∆
00 0
66
TOT A B
=+−
()
[]
nn (provided that the partial
substitution of B with A ligands results in negligible splitting of the octahedral
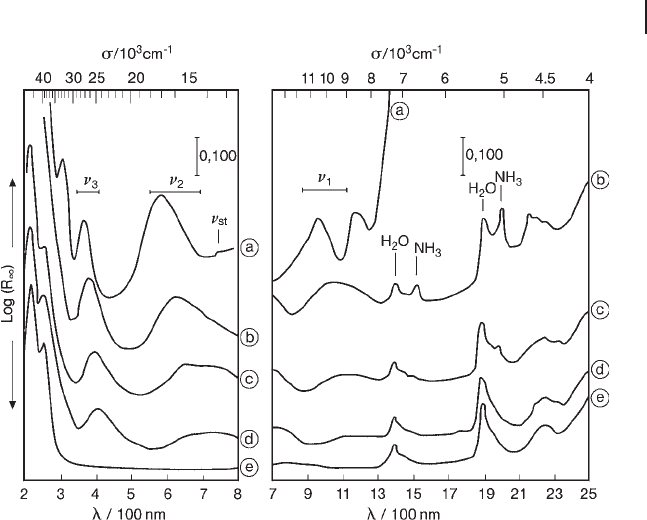
terms). For instance, using this approach, analysis of the progressive shift of d – d
bands along a series of preparation steps of Ni/SiO
2
catalysts (Figure 2.15 ), has
allowed evaluation of the ∆
0
related to surface
≡
SiO
−
present in the ligand sphere
of supported Ni
2+
ions [74] . Such an approach extended to a larger series of oxidic
supports gave rise to a “ spectrochemical series of supports ” (alumina, zeolite Y
and silica) [75] :
∆∆∆
000
AlO ZO SiO
()
<
()
<
()
consistent with what was found for square - planar Pd
2+
complexes [76] .
Furthermore, the comparison of the values of the Racah parameter, B , obtained
from the d – d spectra of a TMI dispersed on a series of supports can give informa-
tion on the difference in the covalence level of the TMI – support interaction among
Figure 2.14 DR UV - Vis spectra of copper oxide supported or
not on Al
2
O
3
in the fresh state: (a) bulk copper oxide diluted
into Al
2
O
3
; (b) 2.1 wt% CuO/Al
2
O
3
; (c) 4.8 wt% CuO/Al
2
O
3
;
(d) 9.2 wt% CuO/Al
2
O
3
. Reprinted from ref. [116] , with
permission from the Royal Society of Chemistry.
the various cases. Again in the case of Ni
2+
ions, the following “ nephelauxetic series
of supports ” was proposed [75] :
βββAlO ZO SiO
()
<
()
<
()
indicating that the weaker - fi eld surface ligands have more covalent bonding to the
supported ions.
Finally, in deriving structural information from the features of d – d spectra of
TMIs, it must be considered that, because of the Laporte selection rule, ion sites
with octahedral symmetry can contribute to the spectra only to a very limited
extent, and so can escape spectroscopic detection. However, this behavior can be
turned into a tool to monitor the distribution of TMIs in sites with different struc-
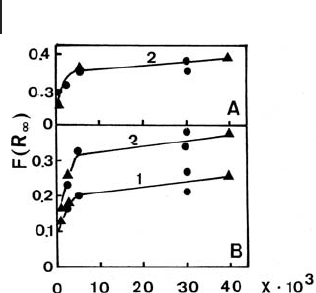
ture as a function of loading, as in the case of CoAPO zeotype materials. In this
case, the attainment of a plateau level of the intensity of the d – d bands due to Co
2+
ions with tetrahedral symmetry that became inserted in the framework indicated
the formation of extra - framework species, containing “ d – d silent ” octahedral Co
2+
sites with increasing loading (Figure 2.16 ) [77] .
2.3 UV-Vis-NIR Absorption Spectroscopy 75
Figure 2.15 DR UV - Vis - NIR spectra of a 1.7 wt% Ni
(II)
/SiO
2
catalyst after different preparation steps: (a) wet after
centrifugation; (b) fi ltered and dried at 293 K in air for 15 h; (c)
fi ltered and dried at 353 K in an oven for 15 h; (d) calcined at
773 K in O
2
and rehydrated for 1 year; (e) unexchanged
ammoniated SiO
2
dried in an oven at 353 K for 15 h.
Reprinted from ref. [74] with permission from Elsevier.
76 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
2.4
UV - V is - NIR Photoluminescence Spectroscopy
Photoluminescence spectroscopy studies the emission of electromagnetic radia-
tions by a system that returns to its ground electronic state from an excited one
previously populated by light absorption. This kind of electronic spectroscopy
is a powerful tool in determining the structure, coordination and reactivity of
metal centers, especially for metal loadings below 1 – 2 wt%. In fact, at higher metal
loading, a concentration quenching may occur and the photoluminescence
becomes less informative.
Photoluminescence ( PL ) is widely applied to investigate surfaces and surface
chemical phenomena with a high degree of sensitivity. The technique provides
extremely rich information when applied to the study of photoluminescence sites
on bulk oxides with a large surface to volume ratio; on sites located on the surface
of a support, for example oxide - supported catalysts; on sites that can be modifi ed
by thermal treatments (calcination, reduction, etc.); and when the local environ-
ment of the emitting sites is altered by the adsorption of molecular probes. By way
of introduction, basic photophysical aspects essential for the rationalization of PL
data will be summarized.
2.4.1
Franck – Condon Principle
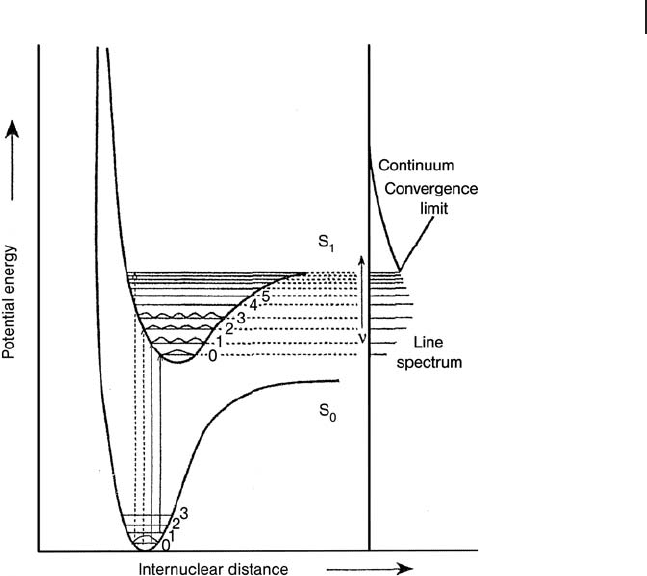
The Frank – Condon principle is based on the fact that the time of an electronic
transition (of the order of 10
− 16
s) is shorter than that of a vibration (of the order
of 10
− 14
s). This means that during an electronic transition the nuclei do not change
their positions. This phenomenon can be illustrated using the Morse potential
energy curves for diatomic molecules (Figure 2.17 ). The series of horizontal lines
Figure 2.16 Plot of the intensity of the KM
function [ F ( R
∞
)] against the Co - content in
CoAPO - 5 catalysts: (A) as synthesized;
(B) after activation in O
2
at 373 K. Curve 1:
NIR band; curves 2: Vis bands. Co content
is expressed as the value “ x ” in the formula
(Co
x
Al
y
P
z
)O
2
· n (N/T) · m H
2
O [ x + y = 0.5; z =
0.5; n = 0.38 (triethylamine, T), 0.25 (N,N -
diethylethanolamine, N); m = 8.5 (with T),
10.3 (with N). Reprinted from ref. [77] , with
permission from Elsevier.
represents the vibrational states of the anharmonic oscillator either in the ground
singlet state (S
0
) in which all electrons are paired or are in the electronic excited
singlet state (S
1
) in which the unpaired electrons remain with opposite spin. Con-
sequently the electronic transitions, in both absorption and emission processes,
occur at constant internuclear distance and should be drawn vertically, as shown
in Figure 2.17 .
Upon absorption of UV - Vis light, the system moves to an electronic excited state.
There are many ways by which the system can return to the ground state involving
radiative or non - radiative decays. In the case of radiative decay, the system loses
the excitation energy as a photon and two different mechanisms can be involved:
fl uorescence and phosphorescence. Fluorescence is an emission process between
states with the same spin multiplicity (i.e. singlet – singlet transitions), while phos-
phorescence involves electronic states with different spin multiplicity (i.e. triplet –
singlet transitions). Fluorescence lifetimes are normally very short (10
− 9
s for
organic molecules). Phosphorescence lifetimes are longer (ranging from 10
− 3
s
to minutes) which is because transitions between states of different multiplicity
are forbidden by the selection rule ∆ S = 0. They thus have very low probability.
Frequently, non - radiative decay may also compete.
Figure 2.17 Potential energy curves for ground (S
0
) and
excited (S
1
) states. Reprinted from ref. [11] , with permission
from Elsevier.
2.4 UV-Vis-NIR Photoluminescence Spectroscopy 77
78 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
In the case of solids, the excited ion pair is subject to the infl uence of its lattice
environment, which produces a vibrational relaxation process. The lattice environ-
ment, however, is not able to accept the larger energy difference needed to lower
the ion pair to the ground electronic state and, therefore, the ion pair may survive
long enough to undergo spontaneous emission, releasing the remaining excess of
energy as radiation. This electronic transition, denoted as fl uorescence, is repre-
sented in accordance with the Frank – Condon principle by a vertical line. The fl uo-
rescence occurs at a frequency lower than that of the absorption process and the
difference between the frequencies is called the Stokes shift.
The light emission normally occurs after some vibrational energy has been dis-
sipated into the surroundings; consequently the fl uorescence intensity depends
on the ability of the lattice environment (or the surrounding gas - phase molecules)
to accept the electronic and vibrational quanta. Thus, it has been observed that
molecules with widely spaced vibrational levels, such as molecular oxygen, are able
to accept the large quantum of electronic energy and quench the fl uorescence. On
the other hand, fl uorescence emission may be increased by decreasing the tem-
perature, for instance working at liquid nitrogen temperature because, at low
temperatures, lattice vibrations are less favored.
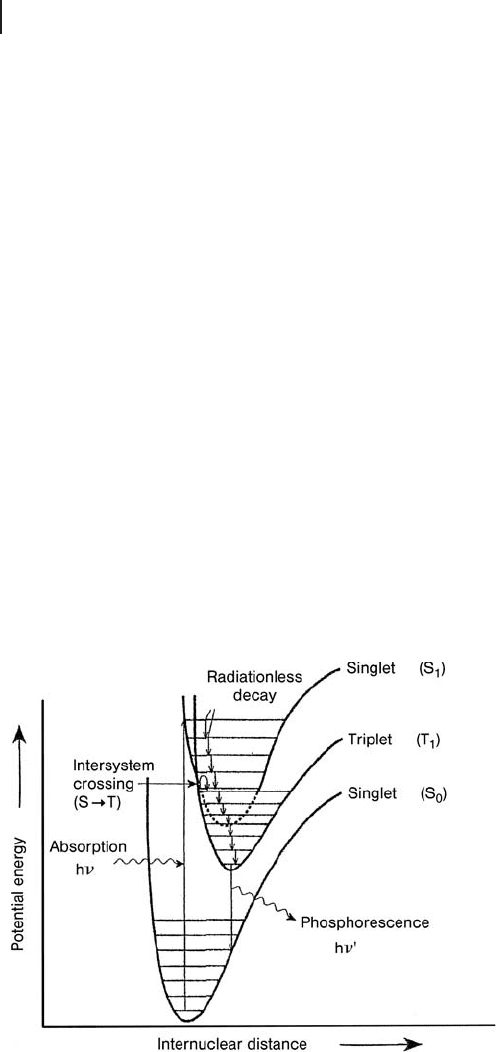
When a spin fl ip occurs for an electron in the S
1
state, an excited triplet state
(T
1
) is fi lled. The T
1
state has a lower potential energy than the S
1
state since
electron – electron repulsions are lower (Figure 2.18 ).
Phosphorescence can be observed only by populating the T
1
electronic state,
which occurs when the S
1
potential energy curve that has been populated by
Figure 2.18 Potential energy curves for ground (S
0
) and
excited singlet (S
1
) and triplet (T
1
) states. Reprinted from
ref. [11] , with permission from Elsevier.

absorption phenomena, intersects the T
1
curve sharing a common geometry. The
mechanism for changing the spin state of the S
1
is called Intersystem Crossing
( ISC ). Singlet – triplet transitions may occur by spin – orbit coupling and for this
reason the ISC mechanism is expected to take place more effi ciently in a molecule
with heavy atoms or in an ion pair in a oxidic system since the corresponding
spin – orbit coupling constant is large. Upon ISC, the ion pair moves from a vibra-
tional level of S
1
to an iso - energetic vibrational level of the T
1
state, where it con-
tinues to release energy into the surroundings, moving down the vibrational levels
until it reaches the lowest vibrational state. From this vibrational level, the ion pair
may return to the ground state (S
0
) by emitting radiation; this phenomenon is
called phosphorescence. The T
1
– S
0
transition is forbidden by the ∆ S = 0 selection
rule and consequently the intensity of phosphorescence is normally lower than
that of fl uorescence. Generally, phosphorescence is enhanced when the material,
such as a catalyst, has highly dispersed metal ions or highly localized emitting
sites. In these cases, the energy transfer becomes less effi cient and there is time
for ISC mechanism to take place, as the singlet excited state steps slowly past the
intersection point (Figure 2.18 ).
Finally, we note that radiative decay transitions have the same nature as absorp-
tion ones and consequently they obey the same selection rules (see Sections 2.1
and 2.2 ).
2.4.2
Quantum Effi ciency and Lifetime
The quantum effi ciency or yield ( Φ e) and lifetime ( τ ) are important parameters
for identifi cation of the emitting species.
The quantum effi ciency, or yield, of photoluminescence ( Φ e) is defi ned as the
ratio of the numbers of photons emitted from an excited species to the number
of photons absorbed. It can be expressed as
ΦΦ
eeeIC e
*=+
()
=kkk kτ
(2.11)
where k
e
is the radiative rate constant and k
IC
is the sum of the unimolecular rate
constants of non - radiative processes from the excited states. Φ * and τ are the
concentration of species and the experimental lifetime of the excited state respec-
tively. To achieve a high quantum effi ciency it is necessary to minimize k
IC
, for
instance by cooling the sample to low temperature (77 K or 4 K) in order to reduce
the contribution of non - radiative processes.
For a single exponential decay, the radiative lifetime ( τ
0
), obtained from the
decay curves of the intensity measured as a function of time, is defi ned by the
mean time the species spends in the excited state prior to return to the ground
state. Generally, lifetimes are longer for the upper states reached through a weakly
absorbing transition and also as the energy separation between the ground and
excited states is larger.
2.4 UV-Vis-NIR Photoluminescence Spectroscopy 79
80 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
The lifetime is expressed as
τ
0
1=+
()
kk
eIC
(2.12)
In the presence of a quenching molecule, the lifetime ( τ ) is given by
τ= + +
[]
()
1 kk k
eICq
Q
(2.13)
where k
q
is the rate constant of the quenching process and [Q] the concentration
of the quencher.
Under conditions of steady and constant illumination, the concentration of the
excited state is given by
Φ
00
=+
()
Ikk
eIC
(2.14)
but if a quencher molecule is present the concentration becomes
Φ= + +
[]
()
Ik k k
eICq
Q
(2.15)
When the intensity of the excitation light and the concentration of the emitting
species are kept constant, the photoluminescence intensity is proportional to the
concentration of the excited species.
The presence of a quencher molecule decreases the intensity of photolumines-
cence since the fl uorophore is often returned to the ground state during a diffusive
encounter with the quencher. In this process the fl uorophore is not chemically
modifi ed. For collisional quenching the decrease in intensity is described by the
Stern – Volmer equation:
ΦΦ
00
11
()()
=+
[]
+
()
=+
[]
kkk k
qeIC q
QQτ
(2.16)
A wide variety of molecules can act as quenchers, including oxygen, halogens,
amines and electron - defi cient molecules.
2.4.3
General Remarks on Methodologies Applied for PL Measurements
A PL spectrum reports the emission intensity as a function of the wavelength of
the emitted light when the excitation wavelength and the intensity of the exciting
light are fi xed at constant values. In some cases, it is possible to observe the vibra-
tional fi ne structure in the photoluminescence band, especially if the spectra are
collected at 77 K. As well as the emission spectrum, the excitation spectrum can
also be collected. In this case, the intensity of the emission at a fi xed wavelength
is plotted as a function of the wavelength of the excitation light, which varies with
the extinction coeffi cient of the absorbing species. Therefore, the excitation spec-
trum is similar to the absorption one; the advantage of collecting excitation spectra
compared to absorption measurements is their greater sensitivity, even for low
concentrations of photoluminescent species.

Details on the instruments available for collecting PL spectra are reported in
refs. [11, 78] . One of the main points to be stressed is that, because of the high
sensitivity of the PL technique, the sample cell has to be made from high - quality
fused silica with no impurities, such as Suprasil.
PL is typically collected at 90 ° to the transmitted light for transparent liquid
samples, whilst in the case of pellets or powered samples it is necessary to collect
the spectra in front - face geometry, meaning that a swing - away mirror is positioned
to allow the collection of the PL of the sample at an angle 45 ° > θ > 22 ° (depending
on the instrument) with respect to the incident radiation. When oxide catalysts
have to be studied it is important to collect the spectra in vacuo to remove any
gases that might act as a quencher molecules, such as oxygen.
2.4.4
Characterization of Oxide Catalysts by PL
2.4.4.1 Insulating Oxides: the Case of AEO
The possibility of using PL to study insulating oxide catalysts is limited by the
position of the absorption bands that can be used for excitation. For this reason,
most PL investigations on such types of material were focused on alkaline earth
oxides that, as reported above, exhibit excitonic absorptions in the near - UV. In this
respect, the background knowledge in this area is based on the series of investiga-
tions carried out in the late 1970s and early 1980s on MgO, CaO, SrO and BaO
[3, 79 – 83] . These studies demonstrated that the emission and excitation spectra of
AEOs are extremely rich in information, owing to the presence of several bands
associated with ions with different local coordination on the surface.
The PL spectra were actually very sensitive to the overall surface structure
and this allowed the study of the behavior of each type of luminescence center
upon thermal treatment or adsorption of probe molecules. These studies have also
shown that, by the way of an energy - transfer process, emission can arise from
surface sites that are not necessarily those that absorbed light in the fi rst step of
the PL phenomenon. For instance, at 300 K, the energy absorbed by 5 - and 4 -
coordinated sites is transferred to the 3 - coordinated ones, whilst at 77 K, the
energy - transfer process is largely suppressed and the original emission profi les of
4 - and 5 - coordinated centers can be observed [41] .
From these early studies, the PL technique, which was initially aimed at confi rm-
ing the observation of surface sites, was gradually extended to explore other related
aspects, such as surface structure, decay of the excited states and surface reactivity.
In addition, the adsorption of quencher molecules, such as O
2
and H
2
, on AEOs
has allowed the clarifi cation of the nature of the luminescence sites present at the
surface [81] . In particular, in the case of H
2
adsorption on SrO, a change in the
excitation band shape was observed. H
2
may react at different rates with species
absorbing in different parts of the excitation band, producing a change in the band
shape. No corresponding change in band shape was observed in the emission
spectrum. By contrast, O
2
adsorption did not change the shape of either the excita-
tion or the emission bands; only a decrease in intensity was observed. This
2.4 UV-Vis-NIR Photoluminescence Spectroscopy 81

82 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
spectroscopic evidence suggested (i) that O
2
and H
2
are adsorbed on different sites,
(ii) that the absorption and emission processes occur at different, but closely
related, sites on the surface and (iii) that energy can be transferred along the
surface. In general, O
2
adsorption is associated with regions of the surface that are
oxygen - defi cient or have a local excess of cations, whilst hydrogen is expected to
react with areas that are cation - defi cient or have a local excess of oxygen ions. In
the case of SrO, most of the intensity loss of the emission was not reversible and
H
2
must be strongly held on the surface, probably as hydroxyls. Using analysis of
such spectroscopic behavior, it was proposed that the sites on the surface respon-
sible for the light emission are oxygen - defi cient or have a local excess of cations,
whereas the sites responsible for the light absorption are cation - defi cient or have
a local excess of oxygen ions [81] .
The luminescence band of the AEO, outgassed at 1200 K, was shifted to lower
energy as the cation size increased, as reported in Table 2.4 and in Figure 2.19 .
The excitation spectra were at lower energy than the band gap of the corresponding
bulk materials and were similar to the absorption spectra [44, 45] . The excitation
processes were, therefore, associated with anions and cations in low coordination
located at the surface and led to the formation of excitons (electron - hole pairs):
MO MO
LC LC LC LC
22+− +−
+→hν
(2.17)
where LC denotes low coordination.
Surface excitons require less energy in their formation than bulk excitons, owing
to the reduced Madelung constant of the coordinatively unsaturated ions at the
surface [81] .
Furthermore, analysis of the excitation spectra of AEOs has revealed that the
excitation bands are characterized by several components, which is evidence that
centers with different coordination are present on the surface. The lower the
energy, the lower the coordination number. It was established that the three main
components identifi ed in the excitation spectra were associated with three surface
states with different coordination of the ion involved: O
C5
2−
on extended (001) faces,
O
C4
2−
on the edges and O
C3
2−
on the corners. In the bulk lattice of an AEO the ions
are octahedrally coordinated.
Table 2.4 Peak positions of the absorption, excitation and
emission spectra of the alkaline earth oxides.
Oxide Absorption (eV) Excitation (eV) Emission (eV)
MgO 5.70; 4.58
> 5.40; 4.52
3.18
CaO 5.52; 4.40
> 5.40; 4.40
3.06
SrO 4.64; 3.96 4.43; 3.94 2.64
BaO 3.60; 3.22 3.70 2.67
