Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

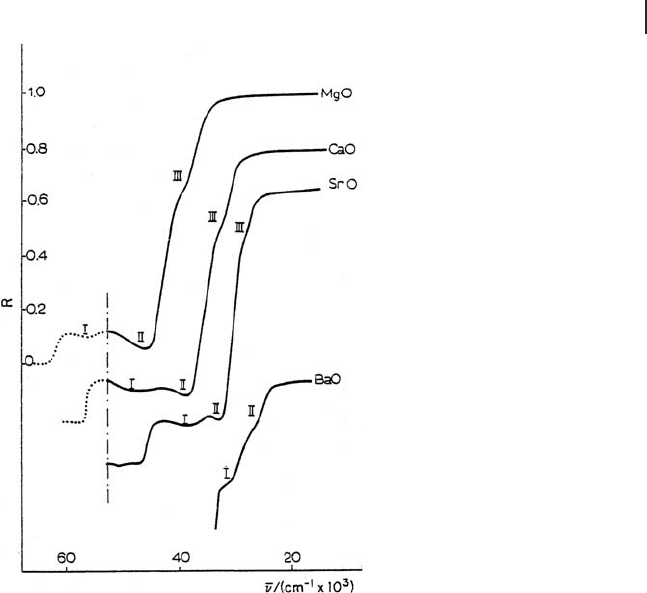
and a weak shoulder (III), and a similar pattern is exhibited by the spectrum of
CaO (edge beyond the instrumental limit). In the case of MgO, where the edge is
known to lie at even higher energies, only one band (II) and a weak shoulder (III)
are present below 52 000 cm
− 1
, although a spectral infl ection near the limit suggests
the presence of another band (I) just above that position. The intensity of absorp-
tions labeled as II and III is a function of the specifi c surface area of the powders
used, decreasing in the order MgO (210 m
2
g
− 1
) > CaO (110 m
2
g
− 1
) > SrO (6 m
2
g
− 1
).
For such a reason, in the spectrum of BaO ( < 1 m
2
g
− 1
) bands due to surface states
appear only as shoulders.
Absorptions related to surface states must be affected by interaction with adsor-
bates, and a typical example of the evolution in such a process of near UV bands
of AEO is reported in Figure 2.6 , where the spectral patterns obtained by contact-
ing MgO with water vapor are shown. The solid line is the spectrum of the sample
2.3 UV-Vis-NIR Absorption Spectroscopy 63
Figure 2.5 Diffuse refl ectance spectra of
polycrystalline AEO outgassed at 1073 K.
Spectra of MgO, CaO and SrO were
recorded in the presence of ∼ 10
2
Pa of non -
reactive gas (e.g. O
2
) to quench possible
fl uorescence emission. The R scale is
displaced vertically to avoid overlapping of
spectra. The dotted portions of the MgO and
CaO spectra at ν = 52 000 cm
− 1
(the vacuum
UV) are extrapolations. Reprinted from ref.
[46] with permission from Francis & Taylor
Ltd.
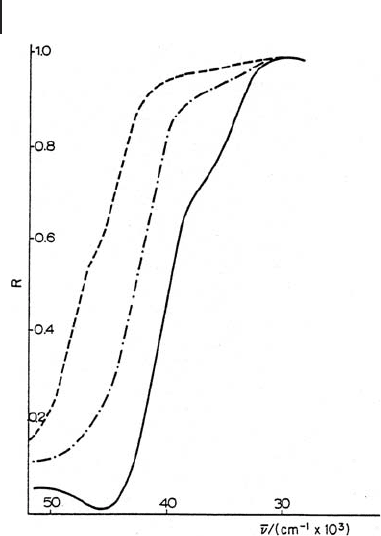
64 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
outgassed at 1073 K, while the other two curves are spectra recorded after contact
with increasing small doses of H
2
O vapor (with coverages lower than monolayer).
The main feature to be noticed is that the low - frequency shoulder is eroded pref-
erentially. In addition, as the chemical attack progresses, the band at 46 000 cm
− 1
is depleted and the low - energy tail of another band can be now observed, support-
ing the hypothesis of the presence of an absorption just above the instrumental
limit.
The increase of the sensitivity towards surface reactions along the band series
III > I I > I (when observed) accounts well for the involvement in such absorptions
of surface ions with coordination number decreasing in the same order III < I I <
I. As indicated above, AEOs have the rock salt structure, with both cations and
anions octahedrally six - coordinated in the bulk. Their surface morphology results
from the intersection of nano {001} planes, which leads to the exposure of fi ve -
(5C), four - (4C) and three - (3C) coordinated ions of facelets, edges and corners
respectively. A general consensus has been reached that such sites are responsible
for the optical transitions resulting in bands I, II and III, respectively. On this
basis, the trend in the intensities of such bands for each AEO (Figure 2.5 ) can also
Figure 2.6 The effect of H
2
O vapor adsorption at 293 K on the
diffuse refl ectance spectrum of polycrystalline MgO initially
outgassed at 1073 K: (a) original spectrum, (b) after
adsorption of a fi rst sub - monolayer dose of H
2
O, (c) after
adsorption of a second dose. Reprinted from ref. [46] with
permission from Francis & Taylor Ltd.

be rationalized, with sites in corner positions being defi nitely less abundant than
those on edges, which are in turn much less numerous that those on faces. In
addition, this assignment accounts well for the changes in relative intensity of the
three bands in dependence on the specifi c surface area of the material also, the
sensitivity of the relative amount of surface sites to this parameter following
the scale 3C >> 4 C >> 5C. On such a basis, the relative intensity of near - UV bands
in the spectra of a series of powder samples of an AEO differing in surface mor-
phology can be coupled with reactivity data to elucidate the possible structure
sensitivity of a catalytic process occurring at its surface [47] .
Besides being a probe of the presence of sites in different coordination states
and of their different reactivity, near - UV excitonic bands of insulating oxides can
be further analyzed to obtain insights into the electronic features of surface sites
responsible for such transitions, and the reasons for the peculiar reactivity related
to a type of surface site/structure. To achieve this, it must be recalled that the main
model for the quantitative prediction of the energies of surface states of highly
ionic solids has been developed by Levine and Mark [48] , where the exciton gap
for surface ions, E
s
, is expressed as:
EAIV
ss
=−+2
(2.6)
where A (the electron affi nity of the anion) and I (the ionization energy of the
cation) are assumed to be the same as for ions in the bulk, and V
s
is the Madelung
potential for surface ions, which (neglecting the sign) is given by
Vzea
ss
=α
2
(2.7)
where z is the charge of the ions, α
s
the surface Madelung constant and a the
anion – cation distance.
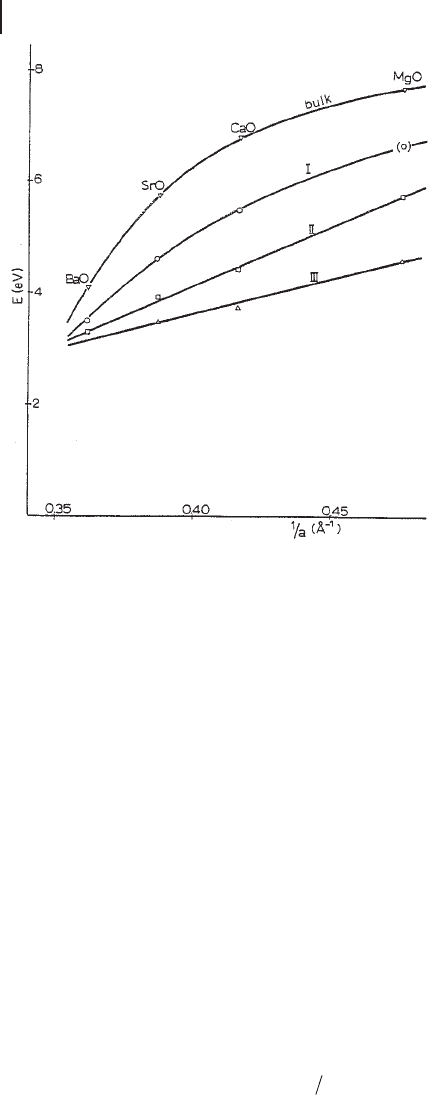
Equivalent relations can be derived for excitonic bulk transitions, by considering
the bulk Madelung potential. Independently of the type of material, which deter-
mines the values of A, I and a , the dependence of E
s
on α
s
should be similar, so a
plot of E
s
against 1/ a for isostructural ionic materials should be a family of curves
with no tendency to intersect. However, this is not the case when the values of the
excitonic absorptions of AEO are considered, including those due to bulk excitons
(Figure 2.7 ). The rationalization of such behavior has been provided by assuming
that A and I depend not only on the material but also on the co - ordinative state of
the ions. In chemical terms, this means that the ionicity of the surface has to vary
according to different coordination situations; in particular, ionicity decreases as
the ion coordination decreases. Departure from ideal ionicity is then least for sites
responsible for excitonic band I, for which a 5C surface confi guration is proposed,
and greatest for those responsible for excitonic band III, that contains 3C ions.
Ions exposed at the surface in different co - ordinative states are then character-
ized by different electronic features, which, in the case of oxygen anions of an
AEO, result in an increase of their nucleophilic character so relevant to the appear-
ance of the strong basic reactivity typical of these oxides.
2.3 UV-Vis-NIR Absorption Spectroscopy 65
66 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
2.3.4
UV Absorption Bands of Semiconductor Oxides
Many oxides employed as catalysts/photocatalysts, supports for catalysts or, vice
versa, supported catalysts, such as SnO, ZnO, Fe
2
O
3
, TiO
2
, ZrO
2
, CeO
2
, V
2
O
5
,
Nb
2
O
5
and WO
3
, are semiconductors, and their absorption edge due to the inter-
band electron transition fall in the near UV - Vis range. As reported in Section 2.3 ,
in semiconductors excitons undergo ionization, so the optical behavior of these
solids is dominated by the fundamental adsorption edge, which, by using the
appropriate equation, can be used to determine the E
g
of the material.
Among the various possibilities, it is worth noting that in semiconductor oxides
where the fundamental absorption edge is due to allowed transitions between
indirect valleys (see Figure 2.4 B), such as TiO
2
[49, 50] , WO
3
[51] and MoO
3
[52] ,
the absorption of photons can occur by coupling with the absorption or emission
of a phonon.
Hence, two adsorption coeffi cients must be considered [53] , one ( α
a
) for the
process with phonon absorption:
αν ν
agpp
hAhEE EkT
()
=−+
()()
−
[]
−21
1exp
(2.8)
and a second one ( α
e
) for the process with phonon emission:
Figure 2.7 Energies and surface transition versus the
reciprocal of the anion - cation distance. Reprinted from ref.
[46] with permission from Francis & Taylor Ltd.
αν ν
egpp
hAhEE EkT
()
=−−
()
−−
()
[]
−21
1exp
(2.9)
where E
g
and E
p
are the energy and phonon energy, respectively, and the terms in
square brackets contain the dependence on the number of phonons of energy
E
p
.
Since both phonon and emission absorption are possible when h ν > E
g
+ E
p
, the
absorption coeffi cient is then
αν α ν α νhhh
()
=
()
+
()
ae
(2.10)
which results in the type of dependence of α
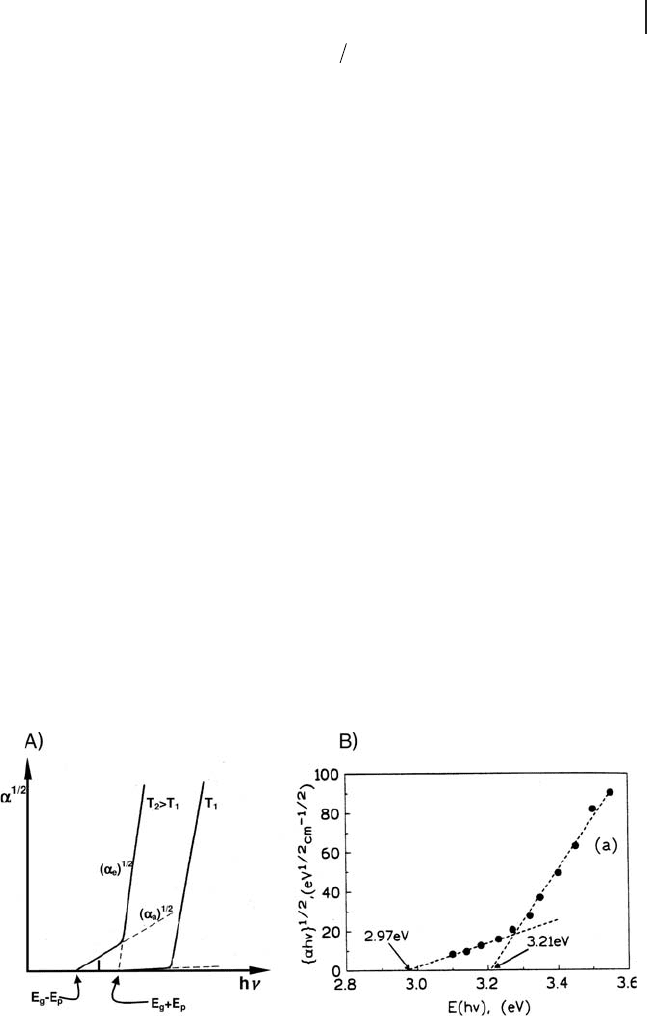
1/2
on h ν displayed in Figure 2.8 A,
from which the value of E
g
can be easily obtained. It is worth drawing attention
to the fact that that α
a
( h ν ) and α
e
( h ν ) exhibit a different dependence on the tem-
perature also, as when T is very low (as T
1
in Figure 2.8 A), the phonon density is
very small (large denominator in Equation 2.11 ), and therefore α
a
( h ν ) is very small.
Turning to experimental data, a similar trend in the dependence of α on h ν has
been obtained by Serpone and coworkers [54] in analyzing the spectra of colloidal
TiO
2
particles (Figure 2.8 B).
For each type/family of semiconductor oxide the value of E
g
is related to signifi -
cant dimensional, structural and/or functional properties, and so the features of
the optical absorption edge is a source of relevant insight into such materials.
As for the functional properties directly dependent on the optical absorption
edge, a special place is held by the photocatalytic behavior of titania [49, 50] . In
this respect, the determination of E
g
of TiO
2
materials by electronic spectroscopy
data is of great interest. This allows assessment of the effectiveness of treatment/
preparation methods intended to decrease the wide E
g
(3.2 eV for the anatase form,
usually the most active photocatalytically). This is of interest in the quest for visible
2.3 UV-Vis-NIR Absorption Spectroscopy 67
Figure 2.8 Plot of α
− 1
against E ( h ν ): panel (A) as from
equations and panel (B) for TiO
2
colloidal particles (three
samples: 2.1, 13.3 or 26.7 nm) suspended in an aqueous
medium (15 g L
− 1
). Panel (A): adapted from ref [26] ; panel (B):
reprinted with permission from ref. [54] . Copyright 1995
American Chemical Society.
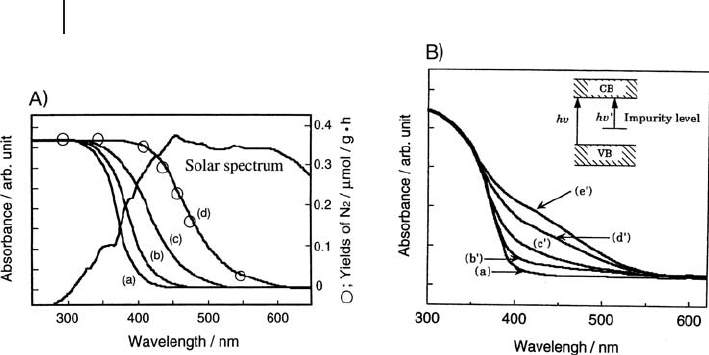
68 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
light - driven photocatalysts, since the UV light required for the parent oxides is
only ca. 5% of ground - level solar radiation (the most desirable irradiation source).
Signifi cant results have been obtained in the development of the so - called “ second
generation ” TiO
2
photocatalysts [55] obtained by implantation of TMIs, resulting
in an actual red - shift of the transition edge (Figure 2.9 A), instead of the appearance
of new spectral components assignable to transitions involving localized states
(Figure 2.9 B). Band gap narrowing has been also obtained by using another physi-
cal method, radio - frequency magnetron sputtering, to prepare TiO
2
in the form of
thin fi lms [55] . Another important possibility for narrowing the TiO
2
band gap is
offered by the preparation of TiO
2
materials doped with non - metal impurities, also
introduced by using more conventional preparation methods (e.g. sol – gel). In
recent years this area has been the object of intense research activity (e.g. N and
C doping, see ref. [56] ).
Turning to the dependence of E
g
on dimensional properties, particle size
( < 100 nm) can have an effect on the spectral properties of semiconductors when
it becomes comparable with the size of the exciton. This gives rise to the so - called
Q - size effect (explained in chemical valence terminology in ref. [57] ) which, with
constant bonding geometry, results in a blue - shift of the absorption edge [58] .
Equations relating the band gap shift ∆ E
g
(measured spectroscopically) to dimen-
sional parameters have been established [57] , and a relationship between the
particle size estimated in this way and that obtained by other methods, typically
TEM, was found (see, for example, ref. [30] ). This relationship notwithstanding,
in some cases other effects can be responsible for the adsorption edge blue - shift.
For instance, the spectroscopic behavior exhibited by TiO
2
particles with decreas-
Figure 2.9 DR UV - Vis spectra of TiO
2
doped
with Cr ions with different methods. Panel A:
(a) unimplanted pure TiO
2
, (b) – (d) TiO
2
implanted with increasing amounts of Cr
ions: 2.2, 6.6 and 13 × 1 0
− 7
mol Cr/g TiO
2
respectively. Open circles are the action
spectrum of the material (d) for the
photocatalytic decomposition reaction of NO.
Panel B: (a) undoped TiO
2
, (b ′ ) – (d ′ ) TiO
2
chemically doped with increasing amounts of
Cr ions: 1.6, 20, 100, 200 × 1 0
− 6
mol Cr/g
TiO
2
, respectively. Reprinted from ref. [11]
with permission from Elsevier.

ing particle size upon anaerobic illumination has been alternatively interpreted on
the basis of the Burstein – Moss effect [59] , which is a consequence of the fact that
the Fermi level of electrons in particles is a function of the irradiation intensity.
However, at spectrophotometric light intensities normally used to record absorp-
tion spectra, the Burstein – Moss effect should be of little consequence.
In the fi eld of oxide catalysts, besides the case of the optical behavior of nano-
metric powders of TiO
2
(see ref. [54] and references therein), a typical manifesta-
tion of the Q - size effect is the blue - shift of the absorption edge exhibited by very
small three - dimensional particles formed by increasing the loading when semi-
conductor oxides (e.g. V
2
O
5
, MoO
3
, WO
3
, Fe
2
O
3
) dispersed on a support (SiO
2
,
TiO
2
, ZrO
2
, Al
2
O
3
) are prepared.
Higher levels of dispersion (usually obtained at lower loadings) result in the
formation of bidimensional patches and monodimensional ribbons of MO
x
(M =
metal) species, which can be recognized on the basis of a third feature of E
g
, that
is, its sensitivity to bonding geometry/structure of semiconductor species. A sys-
tematic experimental and theoretical study on polyoxometallates [60, 61] demon-
strates that the fundamental optical absorption strongly relates to the number of
nearest MO
x
polyhedral neighbors and the number of bonds between each of those
neighbors (corner - or edge - shared polyhedra). For instance, a larger fraction of
edge - sharing polyhedra relative to corner - sharing ones should result in a greater
molecular orbital overlap between such units, and thus a smaller E
g
because of
the more extensive sharing of electron density between polyhedra. Conversely, the
local symmetry around M
n +
centers in polymeric structures and the metal – oxygen
bond lengths were found to have less infl uence. On this basis, and by considering
appropriate reference materials (another key point in the elucidation of structure/
optical feature relationships [60] ), the determination of E
g
values from the optical
absorption edges can trace the evolution of the structure of supported species as
a function of loading. Furthermore, the trend obtained can be combined with that
exhibited by some functional aspect of interest, to elucidate structure/function
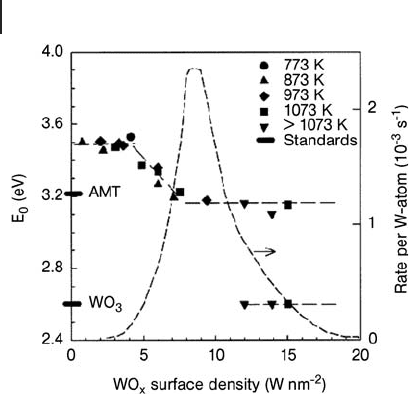
relationships, as performed in the case of WO
x
/ZrO
2
(Figure 2.10 ) [62, 63] and
VO
x
/ox (ox = SiO
2
, ZrO
2
, Al
2
O
3
) catalysts [64] .
The validity of the approach based on the evaluation of E
g
from spectroscopic
data disappears as the size of such MO
x
decreases towards the limit of isolated
species, as bands of energy are turned into discrete energy levels. Hence, the
optical behavior can be described in terms of localized charge - transfer transitions
involving molecular orbitals. This will be the subject of the next section.
2.3.5
Highly Dispersed Supported Oxo - Species and TMI
2.3.5.1 LMCT Transition Bands as Source of Structural Insight
Highly dispersed surface species, with the limiting form of single - site active
centers, play a primary role in a number of catalytic materials because of their
peculiar features in terms of activity and selectivity. Both oxo - species and transition
metal ions supported on oxides (or in zeotype materials) belonging to these types
2.3 UV-Vis-NIR Absorption Spectroscopy 69
70 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
Figure 2.10 Indirect absorption edge energies
of WO
x
- ZrO
2
samples at several oxidation
temperatures and W loadings. Two crystalline
W oxide materials (monoclinic WO
3
and
ammonium metatungstate) are shown for
reference. Dashed curve is a summary of o -
xylene isomerization rates per W atom (523 K,
0.66 kPa o - xylene, 100 kPa H
2
). Reprinted with
permission from ref. [62] ; Copyright 1999
American Chemical Society.
of species can be effectively investigated through their ligand to metal charge -
transfer absorptions falling in the UV - Vis range. The main features related to the
nature of such transitions have been reported in Section 2.2 , while this part will
be devoted to examples showing how structural insights into supported species
can be provided by the analysis of the features of LMCT bands. Some information
arises from the sensitivity of LMCT to the number of ligands surrounding the
metal ions, resulting in a decrease of the transition energy as the coordination
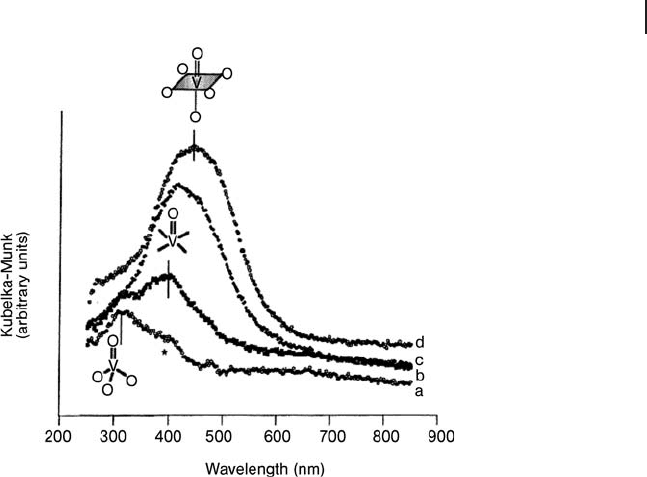
number increases. A typical example is depicted in Figure 2.11 , dealing with the
evolution of the local structure of isolated V
5+
oxo - complexes grafted on the inner
walls of a MCM - 48 silica support as a function of the hydration level [65] .
For each type of coordination number, the position of LMCT bands are sensitive
to the presence of M
n +
–
O
–
M
n +
linkages. This is found, for example, for species
ranging from supported chromates to dichromates and polychromates [66, 67] ,
and for isolated or polymeric VO
4
tetrahedra present in Na
3
VO
4
and NH
4
VO
3
,
respectively, which are considered as reference compounds for the interpretation
of the spectra of supported V
5+
species [68] .
As a further aspect, it must be considered that in the context of a type of struc-
ture, differences in shape and positions of LMCT bands can monitor the occur-
rence of peculiar local geometries or distortions. Such spectral features can usually
be analyzed in more detail, with a consequent higher information output, in the
case of catalysts with active centers that are quite homogeneous in structure and
with simpler spectra, as more commonly occurs in the case of highly isolated
species. These are the conditions for the observation of spectral behavior that can
be rationalized in terms of differences in the bond angles connecting the metal
active sites to a crystalline or amorphous host framework, as in the case of Ti(IV)
dispersed in silicalite or at the surface of amorphous silica [69] . Furthermore, it
must be noted that changes in the local structure of supported species can occur
owing to the adsorption of probe molecules, as in the interaction of NH
3
with Fe
3+
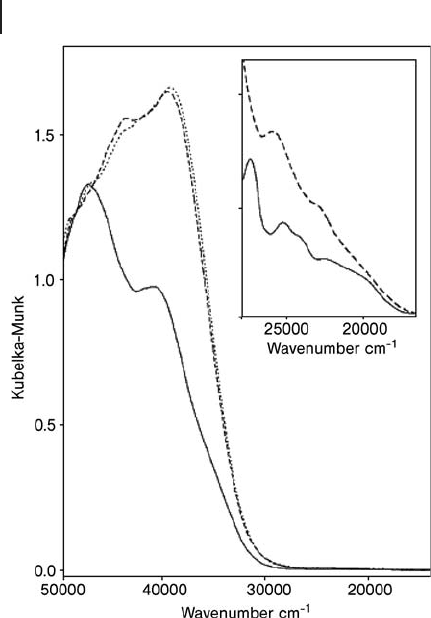
sites, again in silicalite [70] . As shown in Figure 2.12 , in the presence of NH
3
, dis-
tinct CT absorptions were present (solid line), corresponding to framework Fe
3+
species in an almost perfect tetrahedral symmetry, as the charge balancing of
the negative charge on the zeolite framework can be made by NH
4
+
, while after
vacuum treatment the CT bands appeared more complex and broader (broken and
dotted lines) indicating the formation of Fe
3+
species with reduced symmetry.
Finally, according to the empirical optical electronegativity theory, CT absorp-
tions are sensitive to factors affecting the electronegativity of the fi rst coordination
shell surrounding the metal center, such as the composition of the second shell.
Such dependence can be useful in the elucidation of the types of links saturating
the coordinative demand of a metal center exposed on a surface, in terms of the
possible presence of OH groups, instead of only surface lattice oxygen atoms.
This has been the case of tripodal [(OH)Ti(OSi)
3
] versus tetrapodal [Ti(OSi)
4
]
Ti(IV) sites anchored to the inner walls of mesoporous MCM silicas [71] . On the
basis of ab initio calculations, the presence of OH groups in the coordination
sphere was expected to produce a CT sub - band at wavelengths longer than 220 nm,
in agreement with the experimental evidence provided by the comparison of the
2.3 UV-Vis-NIR Absorption Spectroscopy 71
Figure 2.11 Evolution of DR UV - Vis spectra as a function of
hydration time (under ambient conditions) for a VO
x
/MCM - 48
catalyst (V/Si = 0.05): (a) time 0, (b) 10 min, (c) 30 min,
(d) 2 h. Reprinted with permission from ref. [65] ; Copyright
1996 American Chemical Society.
72 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
experimental DR UV - Vis spectra of original Ti
–
MCMs with those of the materials
after silylation, which converted surface OH groups Ti
–
OH and Si
–
OH into
Ti
–
OSi(CH
3
)
3
and Si
–
OSi(CH
3
)
3.
The latter actually appears to be characterized
by a decrease of adsorption intensity in the 220 – 240 nm region (Figure 2.13 ).
2.3.5.2 d – d Transition Bands as a Source of Structural Insight
When oxide catalysts contain TMI in d
1
≤ d
n
≤ d
9
confi guration, additional detailed
information on the structure of metal ion sites can be provided by the analysis of
the features of their d – d bands. As indicated in Section 2.1 , for such an analysis
a huge background of literature is available. Selected examples of the different
spectral features that can be considered for the derivation of structural and chemi-
cal insight for TMI sites in catalysts, or on the evolution of the TMI sites during
catalyst preparation, will be presented.
Figure 2.12 Effect of vacuum treatment and NH
3
adsorption
on the DR UV - Vis spectra of a Fe - silicalite catalyst (Fe =
1.71 wt%; calcined in air at 773 K): broken line, outgassed at
673 K; full line, after contact with 60 Torr NH
3
; dotted line:
outgassed at 673 K. Inset: spectral behavior related to d – d
transitions; related comments in the original paper. Reprinted
from ref. [70] , with permission from Elsevier.
