Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

2.2 Types of Electronic Transitions Producing UV-Vis-NIR Bands 53
In the following, the main features related to the nature of each type of electronic
transition and their dependence on the physical - chemical properties of the absorb-
ing/emitting systems are presented.
2.2.1
Metal - Centered Transitions
d → d transitions occur among the electron energy levels in incompletely fi lled d
n
subshells (1 ≤ n ≤ 9), and so are typical for transition metal ions ( TMI ). The
number and relative position of such levels in TMI complexes (in solution, solid –
liquid interfaces [14] or in solid frameworks) results from the splitting of the free
ion terms by the ligand fi eld, electron interactions (orbital, spin, spin – orbital
angular momentum coupling), confi guration interactions and the Jahn – Teller
effect [15, 16] . These features in turn are controlled by the oxidation state of the
metal ions, the number and kind of ligands and the geometry of their arrangement
around the ions. Because all these dependences exist, the correct interpretation of
a d – d spectrum can yield relevant information about the nature of metal ions and
the composition and structure of the sites where they are hosted. In this respect,
effective, quantitative support is provided by the use of Orgel diagrams (from the
weak fi eld method) and Tanabe – Sugano diagrams (from the strong fi eld method)
[15 – 18] . Both of these allow the calculation of the crystal fi eld parameter ∆
0
, which,
for given oxidation states and symmetry of the environment, depends on the
nature of the ligands, related to the spectrochemical series [15, 16] . In addition,
from Tanabe – Sugano diagrams, the value of the Racah parameter, B , for com-
plexed metal ions can be obtained. The ratio between B and the corresponding B
0
for free ions (available in the literature) results in the parameter β = B / B
0
, which
refl ects the covalent character of the metal ion/ligand interaction, which has been
arranged in the nephelauxetic series. It may be of interest to note that once the
oxidation state and the symmetry are defi ned, ∆
0
and B values can also be derived
by combining spectral data and qualitative d term energy levels reported in the
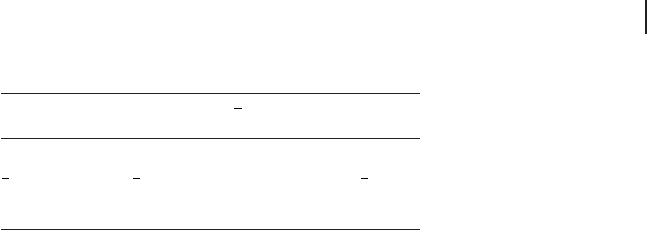
literature, such as those depicted in Figure 2.2 .
For instance, in the case of d
8
metal ions in a weak ligand environment
with an octahedral (or quasi - octahedral, such as C
2v
) symmetry, ∆
0
and B
can be easily calculated on the basis of the position (e.g. in cm
− 1
) of ν
1
(
3
A
2g
→
3
T
2g
), ν
2
[
3
A
2g
→
3
T
1g
(F)] and ν
3
[
3
A
2g
→
3
T
1g
(P)] absorption bands,
Table 2.1 Conversion factors among wavelength λ (nm),
wavenumber ν (cm
− 1
), energy E (eV).
λ (nm)
n
(cm
− 1
) E (eV)
λ (nm)
1
(1/ λ ) ∤ 1 0
− 7
1240/ λ
ν
(cm
− 1
) (1/
ν
) ∤ 10
7
1
ν
/8065.5
E (eV) 1240/E E ∤ 8065.5 1
54 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
because the energy level scheme (right side) allows the determination of ∆
0
(= ν
1
) and B (= ( ν
2
+ ν
3
− 3 ν
1
)/15).
Normally, d – d transition bands are of weak intensity because they are forbidden
by the Laporte (orbital) selection rule (allowed transitions: ∆ l = ± 1; for systems
with inversion center: g → u or u → g). However, transitions that are forbidden
in octahedral symmetry may be partially allowed in the tetrahedral one, because
of d – p mixing in the t
a
2
orbital. Moreover, d – d transitions are subject to the spin -
selection rule which requires that spin multiplicity of the levels involved be the
same ( ∆ S = 0). However, neither the orbital nor the spin selection rules hold very
strictly, because of some relaxation afforded by vibronic coupling, spin – orbit cou-
pling or exchange interaction (in the case of polymetallic systems). Connected with
the relaxation of the Laporte rule by vibronic coupling is the phenomenon of
“ intensity stealing ” which can occur when a forbidden and a fully allowed transi-
tion involve excited terms close enough in energy to allow a mixing of the elec-
tronic wavefunctions, via a vibrational mode of proper symmetry. This is the
reason why certain d – d bands which lie close to charge - transfer (see below) bands
exhibit abnormally high intensity. Representative values of the intensity of d – d
bands in TMI complexes are listed in Table 2.2 .
Finally, for the sake of completeness, it must be considered that metal ions of
catalytic interest can exhibit absorption bands due to metal centered transitions of
other types, as summarized in Table 2.3 .
Figure 2.2 Weak fi eld splitting scheme for maximum
multiplicity terms of d
8
ions in tetrahedral (left side) and
octahedral (right side) complexes. Adapted from ref. [17] .
2.2 Types of Electronic Transitions Producing UV-Vis-NIR Bands 55
2.2.2
Charge - Transfer ( CT ) Transitions
The intense color shown by several inorganic complexes, such as d
0
systems (e.g.
MnO
4
−
) in which no d – d transitions can occur, is due to transition between elec-
tronic states where the electron moves from one group of atoms to another [19] .
These charge - transfer ( CT ) transitions, which are Laporte allowed, produce very
intense bands, when the ∆ S = 0 selection rule is obeyed, and are sensitive to the
nature of both donor and acceptor atoms, to the local and general symmetry of
the absorbing centers and to their bonding geometry.
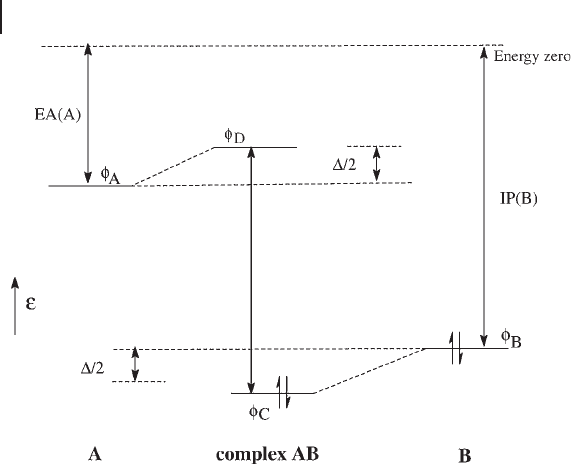
The main features of CT transitions are illustrated in Figure 2.3 . If the interac-
tion between the orbitals φ
A
and φ
B
(orbitals located on a single atom or groups of
atoms) is small, the new orbital φ
C
is composed mainly of φ
B
, and φ
D
is composed
mainly of φ
A
. In the case of MnO
4
−
,
the lower energy orbital φ
B
is located on the
oxygen ligands and φ
A
is a metal d orbital. The transition φ
C
→ φ
D
, called a ligand
to metal charge - transfer transition ( LMCT ), produces a decrease by 1 in the metal
oxidation state. In other systems, for instance metal carbonyls, where there are
high - lying empty ligand orbitals, φ
B
may be a metal d orbital and φ
A
a ligand orbital.
In this case, a metal to ligand charge - transfer transition ( MLCT ) occurs and pro-
duces an increase by 1 in the metal oxidation state. In fact, the charge transfer is
Table 2.2 Representative values for the intensities of d – d transitions in TMI complexes.
Type of d – d transition
Approximate e
Spin - forbidden, Laporte forbidden 0.1
Spin - allowed, Laporte forbidden 10
Spin - allowed, Laporte forbidden, with d – p mixing (tetrahedral symmetry) 100
Spin - allowed, Laporte forbidden, but with “ intensity stealing ” 1000
Table 2.3 Types of metal - centered transitions other than d – d.
Type of transition Comments Type of ions of
catalytic interest
f – f Laporte forbidden; slightly sensitive to the
surroundings; produce weak narrow bands
rare earth ions L
3+
(e.g. Ce
3+
)
( n − 1)d → n s
Laporte forbidden; often too high in energy to
produce bands below 200 nm
Cu
+
, Ag
+
(bands
in the UV - Vis)
( n − 1)f → n d
Laporte allowed Ce
3+
, U
4+
n s → n p
Laporte allowed Sn
2+
, Sb
3+
, Bi
3+
56 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
never complete and all electronic transitions involve a certain amount of electron
redistribution in the excited state compared with the ground state.
LMCT and MLCT transitions depend on the symmetry, on the oxidation state
of the metal and on the nature of the ligand or of the other metal atoms. In par-
ticular, the energy of CT transitions increases with the optical electronegativity
difference between the metal and the ligand [20 – 22] .
Optical electronegativity values ( χ
opt
) are assigned to elements in a given oxida-
tion state and Pauling electronegativity values for halide ions are used as refer-
ences. The wavenumber of the fi rst CT absorption band is expressed as [23, 24]
χχχcm ligand metal
opt opt
−
()
=
()
−
()
[]
1
30000
(2.1)
CT transitions can involve metal ions in all electronic confi gurations, and then,
as in the case of TMIs, are also responsible for optical bands for systems contain-
ing d
0
or d
10
TMIs, that, conversely, do not produce d – d absorption.
Intra - valence, or metal to metal, CT transitions can also occur within systems
containing ions of different oxidation state, as Fe
2+
→ F e
3+
in Fe
3
O
4
or Fe
2+
→ T i
4+
in sapphire [4] .
2.2.3
Transitions between Electron Energy Bands in Solids
The assembly of atoms into an array to form a solid leads to the formation of bands
of allowed states separated by an energy gap. This results from the fact that when
Figure 2.3 Schematic representation of CT transitions.
EA(A) = electron affi nity of species A; IP(B) = ionization
potential of species B; ∆ = stabilization energy of AB.
Adapted from ref. [19] .

2.2 Types of Electronic Transitions Producing UV-Vis-NIR Bands 57
similar “ building blocks ” (atoms, unit cells) approach each other the wavefunc-
tions of their electrons start to overlap and, as a consequence of Pauli ’ s exclusion
principle, the energy states of all spin - paired electrons shared to form bonds are
slightly shifted from the original values when the building blocks are isolated. For
instance, by packing N building blocks the 2 N electrons originally occupying the
same orbital must be spread over 2 N different states, forming a band instead of
discrete levels. The distribution of the states so formed depends on the actual dis-
tance of the building blocks in the solids, leading to the appearance of a gap in
the energy band ( E
g
). The lower band, which can contain as many states as elec-
trons, and hence be completely fi lled, is the valence band. The upper band, which
may contain no electrons at all or fewer electrons than states, is the conduction
band. The extent of the energy gap and the relative electron population determines
whether a solid is a metal, a semiconductor or an insulator. This last kind of mate-
rial has an E
g
larger than 3 eV and a negligible concentration of electrons in the
upper band (and practically no holes in the lower band). Semiconductors, however,
usually exhibit an E
g
lower than 3 eV and a density of electrons in the upper band
(or holes in the lower band) lower than 10
20
c m
− 3
. Promotion of electrons from the
valence to the conduction band (or to states localized in the gap) can occur by
absorption of energy from electromagnetic radiation, thus generating optical tran-
sitions. Finally, in metals, the conduction band is populated by electrons, with a
concentration of the order of 10
23
c m
− 3
.
A second fundamental aspect to be considered in relation to optical transitions
in solids, is that electron states, other than defi nite energy assignments, are also
characterized by a distribution in the momentum space, related to the movement
(i.e. to the kinetic energy) of electrons in the solid. For the sake of pictorial sim-
plicity, bidimensional models of crystals, conceived as a square well potential, are
usually employed in this respect, portraying the “ parabolic valley ” dependence (in
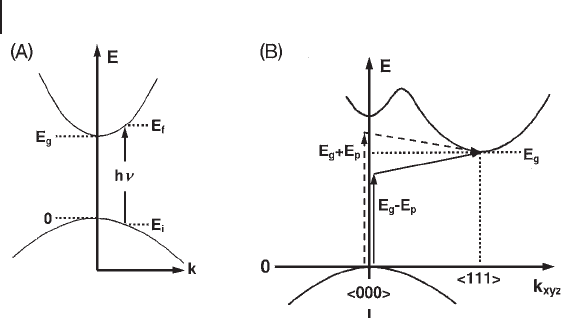
one direction) of energy from the “ momentum vector ” k , as schematized in Figure
2.4 A. The signifi cance of the downward curvature of the valence band is that if
electrons could have a net motion in such a band (i.e. if it were not completely
fi lled), they would be accelerated in the opposite direction with respect to those in
the conduction band.
It is important to note that in solids distances between nearest atoms can vary
in different directions, and hence the minimum of the valley may not occur at
k
x
= k
y
= k
z
= 0, but at some point defi ning a specifi c direction, as shown in Figure
2.4 B for a crystalline solid. In an optical transition, both energy and momentum
must be conserved. Because the momentum of a photon, h / λ ( λ is the wavelength
of light which is typically thousands of å ngstr ö ms), is very small compared to the
crystal momentum h / a ( a is the lattice constant, typically a few Å ngstr ö ms), the
photon - absorption process should conserve the electron momentum.
Thus, the absorption coeffi cient α ( h ν ) for a given photon energy h ν is propor-
tional to the probability, P
if
, for the transition from the initial state to the fi nal state
(governed also by the conservation of momentum), the density of electrons in the
initial state, n
i
, and also the density of electrons in the fi nal states, n
f
. This process
must be summed for all possible transitions between states separated by an energy
difference equal to h ν :
58 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
ανhAPnn
if i f
()
=
∑
(2.2)
Depending upon the relationship between the momentum in the initial and
fi nal states (which, in turn, depend on the profi le of the “ parabolic energy valley ” )
direct or indirect transitions can occur, as shown in Figure 2.4 , and this affects all
the three terms P
if
, n
i
and n
f
. It must be noted that in transitions between indirect
valleys (Figure 2.4 B) momentum is conserved via interaction with a phonon (i.e.
a quantum lattice vibration), which can be either emitted or adsorbed. Some addi-
tional detail on such transitions will be given in the section dealing with semicon-
ductor oxides.
By using the parabolic valley model, an expression of α ( h ν ) for each type of
transition (allowed or forbidden in dependence on selection rules) has been
obtained [25 – 27] . As a common feature, for crystalline solids, α ( h ν ) appeared
expressed in all cases in the form:
αν νhBhE
n
()
=−
()
g
(2.3)
where B contains the dependence on P
if
, n
i
and n
f
specifi c for each type of transi-
tion (that essentially rules the transition edge width), E
g
is the interband energy
gap and n can take values 1/2, 3/2, 2, 3 for direct allowed, direct forbidden, indirect
allowed and indirect forbidden transitions, respectively. The value n = 2 holds for
amorphous materials also, irrespective of the type of transition found in corre-
sponding crystalline materials. Actually, the momentum vector is not conserved
in amorphous phases, and to obtain α ( h ν ) an integration over the density of states
must be made, as in the case of indirect transitions in crystalline systems.
A plot of [ α ( h ν )]
− n
against E ( h ν ) (values of the energy range the absorption edge
is spread over) should then result in a linear relationship, the intercept with the
Figure 2.4 Energy versus momentum and possible interband
transitions in: (A) a direct - gap two - band system and
(B) a solid with conduction band valleys at k = < 000 > and
k = < 111 > . Adapted from ref. [26] .

E ( h ν ) axis being E
g
. Examples of the use of this relation will be provided in the
section devoted to semiconductor oxides.
As a third point, it must be considered that when an electron is raised to the con-
duction band, it leaves behind an unoccupied state (a positive hole) in the valence
band. Thus the excited electron moves in the fi eld of this positively charged hole,
and an e
−
/h
+
pair forms which is called an exciton. A detailed discussion of such
kinds of states has been the subject of the work of Knox [28] . The binding energy
of such a couple slightly decreases the amount of energy required to cause an exci-
tonic transition with respect to a true valence to conduction one, where the excited
electron and the corresponding hole are free to move independently in the solids.
Hence, excitons generate electron states within the interband energy gap of a solid.
The binding energy of excitons depends on the effective e
−
and h
+
masses and the
dielectric constant of the solid. In semiconductors, such a dependence results in
very small binding energy, of the order of 0.01 eV. In solids of such type, excitons
exist in principle but will be completely ionized, the corresponding optical transi-
tions vanishing in the edge - shaped E
g
absorption. In a typical insulator, on the other
hand, effective masses are larger and the dielectric constant is signifi cantly smaller
than in semiconductors. Ionized excitons cannot exist at room temperature (or at
higher temperatures), generating discrete bands at energy below the E
g
edge
absorption. Examples will be reported in the section devoted to insulating oxides.
2.3
UV - V is - NIR Absorption Spectroscopy
2.3.1
Theory of Diffuse Refl ectance ( DR ) Spectroscopy
In the case of powdered catalysts, incident light is almost completely diffused and
it is necessary to use Diffuse Refl ectance ( DR ) instead of transmission spectros-
copy. Mie theory can be applied to describe a single scattering of light which occurs
when light interacts with isotropic and non - interacting small particles. This theory
is not suitable for describing the optical properties of powders in which multiple
scattering occurs. In this case, it is necessary to use a phenomenological theory
that considers the absorbance and scattering coeffi cients. The Schuster – Kubelka –
Munk ( SKM ) model is widely accepted to describe matt surfaces [29] . The refl ec-
tance of a solid includes specular refl ectance and diffuse refl ectance. This latter,
characterized by an angular distribution independent of the incident angle, pre-
vails in the case of powdered solids. Accordingly to the SKM approximation, the
diffuse refl ectance of a layer of infi nite thickness R
∞
is linked to the absorption K
and the scattering S coeffi cients by the expression:
KS FR R R=
()
=−
()()
∞∞∞
12
2
(2.4)
F ( R
∞
) is the remission, or SKM, function [29] .
2.3 UV-Vis-NIR Absorption Spectroscopy 59

60 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
The refl ectance depends upon the K / S ratio and not on the absolute values of
K and S . The scattering S coeffi cient is independent from the wavelength over
wide spectral ranges and therefore the SKM function refl ects the trend of the
absorption K coeffi cient. Moreover, because SKM is a function of α ( h ν ), a plot of
( F ( R
∞
) h ν )
1/ n
against h ν can be used to determine E
g
in powdered semiconductor
oxides.
The SKM theory has some limitations and can be applied only when the follow-
ing experimental conditions are fulfi lled:
i) The incident radiation is monochromatic and completely diffused. The latter
condition can be obtained using samples with a high diffusion and low
absorption.
ii) The layer is of infi nite thickness (generally obtained with a layer depth of
1 – 2 mm), to avoid loss of radiation by transmission.
iii) The absorption intensity is quite weak [ F ( R
∞
) ≤ 1].
iv) Photoluminescence is absent.
Moreover, diffuse refl ectance depends on the particle sizes ( d ). When the particle
size decreases, the S coeffi cient increases since S ∝ 1 / d (for d ≥ 1 µ m) and this
results in an increase of refl ectance. It is worth noting that in the case of thin fi lms
(layers), the UV absorption spectra are complicated by the superposition of inter-
ference effects. The interference oscillations facilitate the semiquantitative deter-
mination of the layer thickness ( δ ), although they prevent the accurate determination
of the fi lm absorption spectrum. In addition, a further uncertainty arises from
specular and diffuse refl ectance by the fi lm. It is possible to solve both problems
by measuring the transmittance ( T ) and refl ectance ( R ) of the light in all directions
using the integrating sphere. The fraction of the truly absorbed light is (1 − R −
T ) and the plot of − ln( R + T ) against λ gives a spectrum in the scale of optical
densities. Since the refl ected and transmitted beams are phase shifted by n δ ( n is
the refractive index), this type of plot is, moreover, smoothed by compensation of
the interference effects [30] .
Finally, it must be noted that an interesting consequence of the Kubelka – Munk
( KM ) equation is that DR spectroscopy has excellent properties for the analysis of
trace quantities of absorbing centers dispersed in non - absorbing matrices. As
shown in the following equation:
12 10
2
−
()()
==
()
∞∞
R R K S ac Sln
(2.5)
where c is the concentration of the absorbing species and a is the (base 10) absorp-
tivity, the signal in a DR measurement is given by the difference between the
refl ectance of the reference and the refl ectance of the sample, that is, (1 − R
∞
). For
trace amounts of the analyte, the denominator in the KM function (2 R
∞
) is approxi-
mately equal to 2 and does not vary greatly when the concentration of the analyte
is changed, provided that ac / S is small. As (1 − R
∞
)
2
is proportional to c, the signal
(1 − R
∞
) is proportional to c
1/2
. The noise across a refl ectance spectrum is approxi-
mately constant. Thus, provided that R
∞
is less than about 0.9, the signal - to - noise

ratio ( S/N ) is proportional to c
1/2
. A reduction in concentration of the absorbing
species by a factor of 100, therefore, only leads to a reduction in S/N by a factor
of 10. The downside of this effect is that small impurities present in the catalyst
(e.g. in the support) can often lead to quite intense bands in the refl ectance
spectrum.
2.3.2
General Remarks on Methodologies for DR UV - V is - NIR Measurements
As indicated in the Introduction, considerations related to the instruments, attach-
ments, cells and experimental setup have been extensively reported, so only a few
fundamental points will be considered here. In the DR UV - Vis experiments, the
light scattered by the solid sample and by the reference is recorded by an integrat-
ing sphere coated with a white standard showing a high diffuse refl ectance, such
as MgO, BaSO
4
and more recently polytetrafl uoroethylene ( PTFE ). Experimental
details of this attachment and cells that can be used for the measurements have
been reported in several articles [4, 9, 13, 31] . By using an appropriate system, in
situ measurements on catalysts treated following specifi c protocols and kept in
controlled atmosphere are possible; and it may be of interest to report that kinetic
aspects of the catalyst activation or of catalyzed reactions can be evaluated (see, for
example, refs. [32, 33] , respectively). Recently, great effort has been applied to
develop operando techniques which refer to the spectroscopy of a working catalyst
in combination with on - line activity measurements [34 – 35] .
2.3.3
UV Absorption Bands of Insulating Oxides: Excitonic Surface States
Insulators such as alumina, silica and alkaline earth oxide s ( AEO ) employed as
catalyst supports or as catalysts have been studied by UV - Vis spectroscopy since
the beginning of the application of optical methods to the investigation of fi nely
divided materials. The basic feature to be considered is that the band gap of
these oxides is so wide that electron transitions from the valence to the conduc-
tion band can be promoted only by electromagnetic radiation in the far/vacuum
UV range ( λ < 192 nm; ν > 52 000 cm
− 1
), requiring special equipment. Neverthe-
less, optical absorptions involving energy states within the main gap produce
optical absorption in the near - UV, and these can be observed using conventional
spectrophotometers (equipped with an integrating sphere, see above). Among
such states are those related the surface, which can be due to the saturation of
surface valence states with
–
OH groups, the presence of supported species (e.g.
TMIs) or coordinative unsaturation (typically appearing after dehydration). As for
surface hydroxyls, their electronic excitation by laser irradiation at 514.5 nm was
found to be responsible for the so called “ fl uorescence ” background of η - Al
2
O
3
and MgO, which strongly affects the ability to measure laser Raman spectra of
species adsorbed on these oxides [36] . Surface states related to the presence of
supported species produce optical absorptions that can be interpreted indepen-
2.3 UV-Vis-NIR Absorption Spectroscopy 61

62 2 The Application of UV-Visible-NIR Spectroscopy to Oxides
dently of the band state model of the support, and these will be considered in
the following sections. Conversely, near - UV - Vis spectroscopy can be considered
a useful tool to obtain specifi c, and in some cases unique, insights on electronic
surface states related to unsaturated sites exposed by insulating oxide - based
materials. UV bands at 240 and 320 nm were observed for a series of zeolites
(faujasite, mordenite, ZSM - 5, erionite, offretite). The fi rst band was related to
framework Al - O units easily removable by dealumination and dehydroxylation,
while the second one was attributed to transitions occurring in an oxoaluminum
structure inside the zeolite matrix [37] . Optical absorption of silica materials has
been thoroughly investigated by physicists [38] , and their results were useful for
the assignment of near - UV bands exhibited by silicas (not structured sol – gel
derived and mesoporous FMS - 16 and MCM - 41) outgassed at T > 673 K to surface
≡
SiO ˙ and
≡
Si ˙ defects sites, produced by dehydroxylation, responsible for the
activity in photometathesis of propene [39] . However, the overwhelming part of
the investigation carried out on the electronic surface states of insulating oxides
has been focused on AEOs, which is still an area of direct interest in base cataly-
sis [40] . In addition, AEOs are regarded as useful model systems for a number
of reasons:
i) Most of them can be obtained in the form of highly dispersed nanocrystalline
powders, with specifi c surface area ranging from ca. 200 to 10 m
2
g
− 1
.
ii) They have a simple crystalline structure (rock salt, based upon interpenetrating
face centered cubic sub - lattices).
iii) {001} cube faces are predominant in the nanocrystals, as evidenced by trans-
mission electron microscopy, allowing even highly rough AEO surfaces to be
described as resulting from the intersection of {001} microfaces [41, 42] .
Nelson and Hale [43] published the fi rst DR spectra of AEO powders. They
showed that highly dispersed systems have UV optical absorption bands that are
not present in the spectra of pure single crystals, which are conversely character-
ized by an extremely low specifi c surface area, and hence an almost negligible
contribution from surface states. Additional evidence was obtained in the follow-
ing years [44, 45] , and a comprehensive analysis of such bands has been reported
by Garrone et al. [46] . The results of this work are an effective example of the wide
set of fundamental information which can be derived from the spectroscopic data
on the nature of optical transitions, structure and reactivity (and their inter - rela-
tionships) of surface sites.
Figure 2.5 summarizes the DR UV spectra of well outgassed samples of MgO,
CaO, SrO and BaO powders considered by those authors. Owing to bulk excitonic
transitions, the edges are clearly visible in the case of SrO and BaO, while for MgO
and CaO they lie beyond the near - UV range (in the dotted part of the spectra). In
the spectrum of each oxide some absorptions at frequencies below that edge are
present, which are assigned to excitonic transitions involving surface sites (and
expected to produce discrete absorptions). Considering SrO fi rst, this latter part
of the spectrum is constituted by two absorptions of comparable intensity (I, II)
