Ishihara T. (Ed.) Perovskite Oxide for Solid Oxide Fuel Cells (Fuel Cells and Hydrogen Energy)
Подождите немного. Документ загружается.


occur. Sunde et al. [7] have recently pointed out some of these effects when
using the potential step method. By far the most direct method of measure-
ment is the use of isotope exchange followed by secondary ion mass specto-
metry (SIMS) depth profiling, as descri bed in our earlier publications [8,9],
often known as the isotope exchange depth pro filing tech nique (IE DP).
This technique yields the tracer diffusion coefficient, D,andthemajority
of the data included in this review were obtained using the IEDP/SIMS
method.
5.1.3 The Surface Exchange Coefficient
The oxygen surface exchange coefficient, k, is another important kinetic
parameter associated with the measurement of oxygen transport rates in
these oxide materials. It is a measure of the neutral oxygen exchange flux
crossing the surface of the oxide, at equilibrium, as described by the following
reaction [10]:
1
2
O
2
þ V
o
þ e
0
$ O
x
o
(5:9)
This flux will be dependent upon the surface vacancy concentration, the
surface electron concentration, and the dissociation rate of the dioxygen mole-
cule; however, at present, the rate-limiting step has yet to be identified. Kilner
et al. [11] have derived a simple relationship for the surface exchange coefficient
in terms of the bulk and surface vacancy concentrations, in an attempt to
explain the apparent correlation found between the activation enthalpy for
the surface exchange coefficient and the diffusion coefficient, in a number of
La-based perovskites. Adler et al. [12] have also arrived at a similar relationship
for k, by consideration of the AC electrode behavior of symmetrical cells with a
‘‘double’’ cathode structure. As already mentioned, the exact mechanisms of
oxygen surface exchange remain elusive; however, the vacancy concentration is
clearly a very important parameter.
On a more practical level, the influence of the surface exchange on
cathode performance has been recently emphasized by both Adler et al.
[12] and St eele [13 ]. They have both stressed the importance of a character-
istic length L
c
defined as the ratio of the two parameters D/k. This length is
the c hangeover point at which the permeation flux of oxygen through a
thin mixed conducting membrane (such as a cathode) changes fr om being
limited by diffu sion in the membrane to being limited by the surface
exchange process. For a more detailed discussio n of the interpretation of
the surface exchange c oefficient, the reader is referred to the work of Maier
[14] and De Souza [15].
98 J.A. Kilner et al.
5.1.4 Defect Chemistry and Oxygen Transport
A large number of different materials adopt the perovskite structure, however
in terms of the oxygen transport the most studied are materials for applications
in solid oxide fuel cells (SOFCs) and oxygen separation membranes. The
materials are usually 3,3-perovskites (with the general formu la A
3þ
B
3þ
O
3
)
with lanthanum on the A site, a transition metal on the B site, and acceptor
doped on the A site, usually with strontium. One of the main feat ures of these
mixed conducting perovskites is that they show a large range of stoichiometry,
which can vary from hypostoichiometric to hyperstoichiometric; this will alter
both the electrical conductivity and the oxygen exchange and transport proper-
ties. To understand the oxygen transport properties, we first look at the theo-
retical defect structure of these mixed con ducting perovskites and then compare
the predicted behavior with experimental results.
5.1.5 Defect Equilibria
The defect structure of these materials is far from simple, with a number of
coincident defect equilibria contributing to the observed behavior. First, we
must consider the intrinsic defect processes for a hypothetical mixed conductor
La
1–x
Sr
x
BO
3d
, which undergoes Schottky disorder together with intrinsic
electronic disorder. Following an analysis given in [16]:
‘‘0’’ ! V
000
La
þ V
000
B
þ 3V
O
(5:10)
‘‘0’’ ! e
0
þ h
(5:11)
Equation (5.11) can also be viewed as the dissociation of the effectively
neutral B cation (+3) into two charge states (+2 and +4):
2B
x
B
! B
0
B
þ B
B
(5:12)
For perovskites with variable valent B cations, the redox processes that occur
in the lattice must also be taken into account. Oxidation of the lattice can lead to
oxygen excess material in which cation vacancies form [17].
3
=
2
O
2
þ 6B
x
B
! 3O
x
O
þ V
000
La
þ V
000
B
þ 6 B
B
(5:13)
In this oxygen excess region, the formation of cation vacancies will have the
effect of strongly decreasing the oxygen vacancy concentration through the
Schottky equilibrium.
5 Diffusivity of the Oxide Ion in Perovskite Oxides 99
Compensation of the acceptor can occur either by elect ronic or by a vacancy
mechanism, or perhaps more importantly for these materials, by a combination
of the two.
2SrO þ
1
=
2
O
2
þ 2B
x
B
! 2Sr
0
La
þ 3O
x
O
þ 2B
B
(5:14)
2SrO ! 2 Sr
0
La
þ 2O
x
O
þ V
O
(5:15)
The resulting neutrality condition then contains all the possible defect species
3 V
000
La
þ 3 V
000
B
þ Sr
0
La
þ B
0
B
¼ 2 V
O
þ B
B
(5:16)
To construct a Brouwer diagram to depict the relative concentration of these
defects with P
O2
, it is necessary to define a series of approximations to the full
neutrality condition. The first of these corresponds to a region (R I) where d > 0
and where the material is hyperstoichiometric.
3 V
000
La
þ 3 V
000
B
¼ B
B
(5:17)
This region is followed by a stoichiometric region (R II) where the material
acts a s a controlled valence semiconductor and d ¼0.
Sr
0
La
¼ B
B
(5:18)
One of the most important features of these materials is that the compensa-
tion of the acceptor does not change abruptly from electronic to vacancy
compensation, but there is an extensive region in which the compensation is
mixed. Here the material is hypostoichiometric; d now becomes negative and
lies between 0 and –x/2, where x is the concentration of the acceptor (R III).
Sr
0
La
¼ 2 V
O
þ B
B
(5:19)
Next there is a region (R IV) where the material is vacancy compensated and
the value of d is fixed at d ¼x/2.
Sr
0
La
¼ 2 V
O
(5:20)
And finally, the material becomes reduced (R V), and we get the production
of oxygen vacancies and electrons:
B
0
B
¼ 2 V
O
(5:21)
100 J.A. Kilner et al.
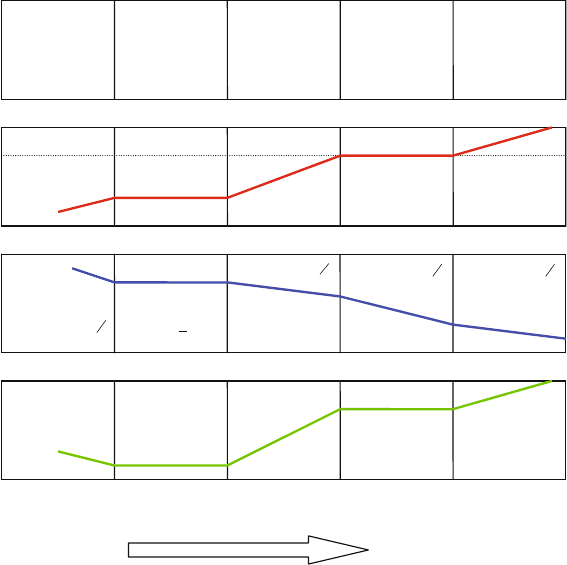
The five regions are shown in Fig. 5.1 for a general 3,3 acceptor (A
2þ
)-doped
perovskite with the rare earth ion (RE) on the A site, RE
1x
A
x
BO
3
.
To compare with experimental data, we now need to e xamine a number of
materials. Nonstoichiometry data for three 3,3 acceptor-doped perovskites
with the B cations Mn, Fe, and Co are shown in Fig. 5 .2 as a function of P
O2
at 10008C [18,19]. It is clear that the manganite is hyperstoichiometric (R I, d > 0)
at high P
O2
s(P
O2
1), even though it is acceptor doped. As the P
O2
is lowered,
the manganite shows a plateau corresponding to R II behavior, i.e., d ¼ 0. This
behavior implies that the oxygen vacancy concentration will be very low indeed,
and this remains true even for quite heavily acceptor doped material. The
manganite material shown in Fig. 5.2 does not become hypostoichiometric
(R III, d < 0) until oxygen partial pressures approaching 10
10
atm. are reached.
Thus, under normal SOFC cathode operating conditions, the manganite
materials are expected to have low vacancy concentrations and consequently
IIIIIIIVV
][2
••
=
O
Vn
][
][2
/
RE
O
A
V =
••
][2
][
/
••
−
=
O
RE
V
Ap
][
/
RE
Ap =
][6
///
B
Vp =
+δ
3
–δ
]Log[V
O
••
Log
n-type p-type
p-type
p-type
6
1
2
][
−
••
∝
O
O
PV
][][
/
2
1
REO
AV =
••
4
1
2
][
−
••
∝
OO
PV
2
1
2
][
−
••
∝
OO
PV
8
1
2
][
−
••
∝
OO
PV
Neutrality
condition
2
O
LogP
Fig. 5.1 Brouwer diagram for an acceptor-doped RE
1x
A
x
BO
3
oxide showing the oxygen
content, d, oxygen vacancy concentration, V
O
, and electrical conductivity, s, as a function
of oxygen partial pressure [59]
5 Diffusivity of the Oxide Ion in Perovskite Oxides 101
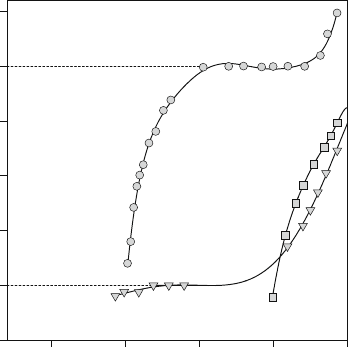
low oxygen self-diffusivities. A more extensive set of nonstoichiometery
isotherms for the manganite compositions has been published by Tagawa
et al. [20].
Incomparisontothemanganite,thecobaltiteandferriteareseentobein
the mixed compensation region (R III) at high P
O2
s,wherethevalueofd lies
between 0 and x/2. Thus, fairly high oxygen vacancy concentrations, and
consequently oxygen diffusivities, are to be expected under normal cathodic
conditions. For the ferrite, a plateau is seen at the lower P
O2
that corresponds
to R IV behavior, indicating that the acceptor is vacancy compensated and the
material would become a predominantly ionic conductor. From these data,
and the preceding analysis, it is obvious that there are significant changes in
the defect populations in these acceptor-doped materials that depend on the
temperature, oxygen partial pressure, and perhaps most importantly, the
identity of the transition m etal in the B-cation site. We now look at the way
in which composition changes affect the oxygen transport properties of t hese
oxides.
5.2 Diffusion in Mixed Electronic-Ionic Conducting
Oxides (MEICs)
MEICs display some very interesting electrochemical properties and because
of this are used as cathodes for the SOFC, both at high and intermedi ate
temperatures, and in permeation membranes for the separation of oxygen.
log
10
[
P
O
2
/(atm)
]
–20 –15 –10 –5 0
3 - δ
2.75
2.80
2.85
2.90
2.95
3.00
3.05
MnO
3–δ
La
0.6
Sr
0.4
FeO
3–δ
La
0.7
Sr
0.3
La
0.8
Sr
0.2
CoO
3–δ
Fig. 5.2 Nonstoichiometry
data for the acceptor-doped
perovskites La
1x
Sr
x
BO
3d
(B ¼ Mn, Fe, and Co) as a
function of P
O2
at 10008C
[18, 19]
102 J.A. Kilner et al.
The materials chosen for the development of commer cial application are based
on perovskite MEICs; however, most of them have very complex compositions
involving both A- and B-site substitution. This situation can lead to problems in
any discussion of the literature on these materials because families of materials
are often referred to by the use of acronyms. For example, LSM and LSCF are
often used to denote cathode materials, but these acronyms cover a range of
materials of various levels of Sr substitution, B-site substitution, and often A-
site deficiency, leading to sets of materials with very different properties. The
exact composition must thus be used to compare the oxygen transport proper-
ties within and between each group of materials. As a further note of caution, it
is also rare to see detailed analysis of the purity of the materials, which might
affect the transport of oxygen, particularly in ceramic samples where the grain
boundaries can affect the overall transport rates.
5.2.1 Effect of A-Site Cation on Oxygen Diffusivity
Two types of substitution into the A site in the perovskite structure are possible.
Aliovalent doping occurs when the oxidation stat e of the substituting ion is
different from the host ions, thus introducing effective charges for the substitute
ion. To mainta in electrical neutralit y, these charges have to be compensated by
the formation of oppositely charged defects. This state can be achieved either
by changing the oxidation state of the B cations (electronic compensation) or by
the formation of oppositely charged vacancies (ionic compensation), as was
discussed earlier for acceptor-doped materials. Isovalent doping occurs when
the oxidation state of the substituting and host ions is identical. As a result, no
charges are introduced into the A-site sub-lattice and no charge compensation
is required; however, there will be elastic strain effects because of the size
mismatch of the host and the substituting cation.
Several combinations of elements have been used as A-site host and substitu-
tional ions. Historically, lanthanum has been a host ion of a choice for the past
several decades due to its large ionic radius and relative availability. Alkaline
earth elements have been used as substitutional ions due to their close size match
to the lanthanides and thermodynamic stability at the operational conditions of
the SOFC. The effect of alkaline earth doping on the oxygen tracer diffusion
coefficient in several families of perovskite compounds is shown in Fig. 5.3a
(for Sm
1x
Sr
x
CoO
3
at 7938C[21]andLa
1x
Sr
x
CoO
3
at 8008C[22–24])and
Fig. 5.3b (for La
1x
Ca
x
CrO
3
at 9008C [25], La
1x
Sr
x
MnO
3
at 9008C [23, 26],
and La
1x
Sr
x
FeO
3
at 10008C [27]). All these data were obtained from experi-
ments carried out at high oxygen partial pressures ( 1 bar), to simulate opera-
tion in an SOFC cathode environment. Note from observation of Fig. 5.3a that
although all the isothermal diffusivities increase with an increase in the acceptor
doping, the changes seen for the cobalt-based materials (by six orders of
5 Diffusivity of the Oxide Ion in Perovskite Oxides 103
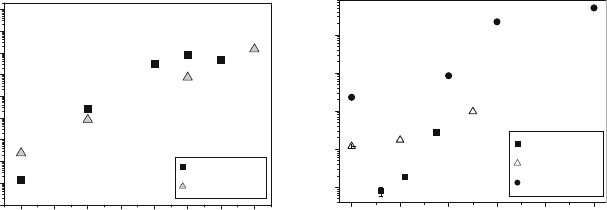
magnitude) are much larger that the relatively modest increases seen for the Cr
and Mn analogues.
It is generally accepted that oxygen diffusion in these perovskite compounds
occurs via oxygen vacancy mechanism. Consequently, the increase in the diffu-
sion coefficient with doping is most likely caused by the increased concentration
of oxygen vacancies. However, as we have seen, the identity of the B cation will
determine which of the neutrality approximations is valid for each group of
materials (i.e., RI, II, III, etc.). Thus, the spectacular increase of the oxygen
tracer diffusion coefficient in cobaltites with doping is related to the large values
of oxygen hypostoichiometry observed in those compounds (R III) [28]. How-
ever, some care is needed in this interpretation at this stage as it must be
remembered that the diffusivity arises as a result of the product of the vacancy
concentration and the vacancy diffusion coefficient (Eq. 5.8), which we examine
in more detail below.
5.2.2 The Effect of B-Site Cation on Oxygen Diffusivity
Only one systematic study has been carried out to investigate the effect of B-site
cation on oxygen diffusivity [23]. The effect of doping La
0.8
Sr
0.2
MnO
3d
with
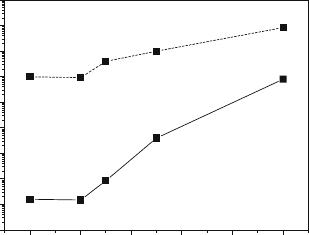
cobalt is shown in Fig. 5.4. The substitution enhances the diffusivity by five
orders of magnitude and the surface exchange coefficient by two orders of
magnitude at 10008C. This is quite an interesting finding, because the level of
strontium substitution on the A site remains constant. Clearly, the nature of the
B cation is again determining the nature of the neutrality approximation, and it
would be apparent from the earlier data given in Fig. 5.2 that we are moving
from La
0.8
Sr
0.2
MnO
3+d
(R I) to La
0.8
Sr
0.2
CoO
3d
(R III).
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7
10
–5
10
–6
10
–7
10
–8
10
–9
10
–10
10
–11
10
–12
10
–13
10
–4
Sm
1-x
Sr
x
CoO
3
La
1-x
Sr
x
CoO
3
D
*
(cm
2
s
–1
)
Site fraction Sr (x)
a)
10
–10
10
–11
10
–12
10
–13
10
–14
0.0 0.2 0.4 0.6 0.8 1.0
La
1-x
Ca
x
CrO
3
La
1-x
Sr
x
MnO
3
La
1-x
Sr
x
FeO
3
D
*
(cm
2
s
–1
)
Site fraction Sr and Ca (x)
b)
Fig. 5.3 Effect of aliovalent doping on the tracer diffusion coefficient in (a)RE
1x
Sr
x
CoO
3
(RE ¼ La, Sm at 8008C [21–24]) and (b)La
1x
A
x
TmO
3
(A ¼ Sr, Ca; Tm ¼ Cr, Mn at 9008C
[22, 25, 26], and Tm ¼ Fe at 10008C [ 27]). Nominal pressure during the experiments was 1 atm
except for [22, 27] where a pressure of 34 and 40 torr was used, respectively
104 J.A. Kilner et al.
5.2.3 The Effect of A-Site Cation Vacancies
on Oxygen Diffusivity
The effect of A-site vacancies on oxygen diffusion in perovskite materials has
been studied in lanthanum-deficient La
1y
MnO
3
(y ¼ 0, 0.1) [26]. It was found
that introduction of 10% La vacancies has no effect on the values of oxygen
tracer diffusion coefficient. The activation energy for oxygen diffusion
increased from the value of 2.49 eV in a stoichiometric sample to 3.05 eV in
La
0.9
MnO
3
. It was assumed that oxygen vacancies formed by the introduction
of La deficiency, are bound in to the defect complexes (e.g., V
000
La
V
O
0
).
Similarly, no effect of A-site deficiency was observed on the parameters of
chemical diffusion of oxygen in (La
0.85
Sr
0.15
)
s
CoO
3d
(s ¼ 0.98, 1 [29]).
5.2.4 Temperature Dependence of the Oxygen
Diffusion Coefficient
In addition to following the isothermal effects, it is also quite important to
understand the changes that take place in the observed activation energy for
diffusion as a function of the composition. The ease of oxygen ion diffusion in
perovskite structure is usually attributed to the value of the apparent activation
energy estimated from the Arrhenius plots of diffusion coefficient. This appar-
ent activation energy, E
a
, however, consists of several terms, as indicated in the
following equation:
E
a
¼ H
m
þ H
f
þ H
a
(5:22)
Here H
m
is the enthalpy of vacancy migration, H
f
is the enthalpy of vacancy
formation, and H
a
is the enthalpy of vacancy–dopant association, e.g.:
Sr
0
La
þ V
o
, Sr
0
La
V
o
(5:23)
0.0 0.2 0.4 0.6 0.8 1.0
10
–4
10
–5
10
–6
10
–7
10
–8
10
–9
10
–10
10
–11
10
–12
10
–13
D
*
(cm
2
s
–1
) and k(cm s
–1
)
Site fraction Co (x)
D
*
k
Fig. 5.4 The oxygen self-
diffusion coefficient, D, and
surface exchange coefficient,
k, for La
0.8
Sr
0.2
Mn
1x
Co
x
O
3d
at 10008C as a function
of Co site y [23]
5 Diffusivity of the Oxide Ion in Perovskite Oxides 105
H
f
affects the stoichiometric vacancy concentration wher eas H
a
affects
the mobile vacancy concentration and H
m
only enters into the vacancy diffu-
sion coefficient. Thus, if we can determine the stoichiometric vacancy concen-
tration (or, more accurately, the mobile vacancy concentration), then we can
extract the value of D
v
, leading to a value for H
m
.
First, let us look at the overall effect of alkaline earth doping on the apparent
activation energy of tracer diffusion in La
1x
A
x
TmO
3
(A ¼ Sr, Ca Tm ¼ Mn,
Fe, Cr), and RE
1x
Sr
x
CoO
3
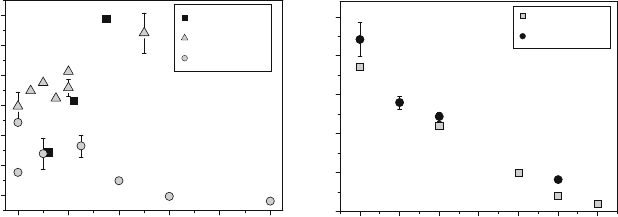
(RE ¼ La, Sm), shown in Fig. 5.5(a) and 5.5(b),
respectively. Although a large scatter of data is present, presumably due to
differences in oxygen partial pressure during diff usional anneals, it is evident
that the activation energy increases with doping for Mn- and Cr-b ased perovs-
kites and decreases with doping for Fe- and Co-based perovskites.
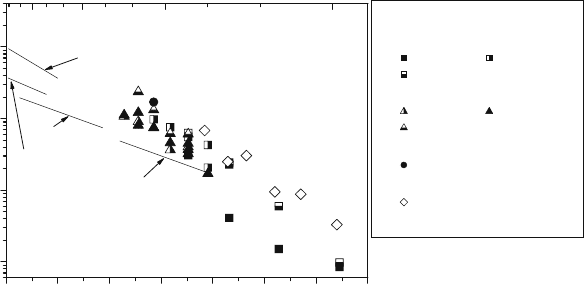
To clarify some of this differing behavior, the vacancy diffusion coefficients
have been estimated from oxygen tracer diffusion experiments and using ther-
mogravimetric studies to provide the stoichiometric vacancy concentration. A
correlation factor, f, of 0.69 was used in the calculation. The calculated values of
the vacancy diffusion coefficient in several families of perovskite materials are
shown in Fig. 5.6. Remarkably, and as mentioned earlier, the values calculated
for perovskite oxides, with significantly different oxygen diffusion coefficients
and temperature dependencies, appear to have very similar values of vacancy
diffusion coefficient. The activation energy of vacancy diffusion in the perovs-
kite structure is 0.94 0.07 eV irrespective of the nature of the constituent
ions. This value is close to the migration enthalpy for oxygen vacancies calcu-
lated by atomistic simulation techniques for 0.67 eV (LaMnO
3d
) [30] and in the
range 0.6–0.8 eV reported for several perovskites by Islam [31].
0.0 0.2 0.4 0.6 0.8 1.0
1.0
1.5
2.0
2.5
3.0
3.5
4.0
E
act.
(eV)
Site fraction Sr and Ca (x)
La
1-x
Ca
x
CrO
3
La
1-x
Sr
x
MnO
3
La
1-x
Sr
x
FeO
3
a)
E
act.
(eV)
0.0 0.1 0.2 0.3 0.4 0.5 0.6
1.0
1.5
2.0
2.5
3.0
3.5
Site fraction Sr (x)
Sm
1-x
Sr
x
CoO
3
La
1-x
Sr
x
CoO
3
b)
Fig. 5.5 The effect of aliovalent doping on the apparent activation energy of tracer diffusion
in La
1x
Sr
x
MnO
3
(a) at 1 atm [23,26] and 0.13 atm [60], La
1x
Ca
x
CrO
3
(a) at 0.13 atm [25],
La
1x
Sr
x
FeO
3
(a) at 0.06 atm [27, 36], La
1x
Sr
x
CoO
3
(b) at 1 atm [23,61], and 34 tor [22],
Sm
1x
Sr
x
CoO
3
(b) at 1 atm [21]
106 J.A. Kilner et al.
There are several very interesting implications from this finding. It would
suggest that the differences seen between the activation energies measured for
the different pe rovskite materials are the result of changes in the values of either
the association or vacancy formation energies. It is difficult to discriminate
between the two components; however, some observations are helpful.
1. The majority of the materials under discussion here are composed of perovskites
made nonstoichiometric by the substitutionofLabySr.Theextentofvacancy
trapping, of the form shown in Eq. (5.23), can be estimated from atomistic
calculations of the association enthalpy, H
a
. This value has been calculated by
Islam [32] for the related Sr-doped lanthanum gallate material to be essentially
zero, implying that trapping would not occur and that the vacancies are essen-
tially free to participate in the migration process. The major part of this trapping
energy has been shown to be the elastic contribution due to the size mismatch
between the host and the substitutional cation [2], and hence the close size match
of the La
3þ
(1.36 A
˚
) and Sr
2þ
(1.44 A
˚
) ions leads to a minimization of this term.
2. The close match of D
v
from very different materials was obtained using the
stoichiometric vacancy concentrations determined by Thermogravimetric
Analysis (TGA); this implies that all these vacancies are mobile and hence
that trapping is negligible.
If we can discount the effects of vacancy trapping, then the major differ-
ences seen in the activation enthalpy are ascribable to differences in the
vacancy formation energy. Tagawa et al. [33] have noted that the partial
0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2
1E-7
1E-6
1E-5
1E-4
1500 1200 900 600
La
1-x
Sr
x
CoO
3
x = 0.1 [34],
x=0.2 [23],
x = 0.5 [23]
La
1-x
Sr
x
FeO
3
x = 0 [36],
x = 0.1 [34],
x = 0.25 [34]
La
0.9
Ca
0.1
CrO
3
[62]
La
0.6
Sr
0.4
Co
0.2
Fe
0.8
O
3−δ
[37]
D
V
(cm
2
s
–1
)
T
–1
x 10
3
(K
–1
)
Zr
0.85
Ca
0.15
O
1.85
UO
1.99
Zr
0.88
Mg
0.12
O
1.88
CeO
1.92
Temperature (°C)
Fig. 5.6 Temperature dependence of the oxygen vacancy diffusion coefficient in perovskites:
La
1x
Sr
x
CoO
3d
(x ¼ 0.1 [34], x ¼ 0.2 [23], x ¼ 0.5 [23]), La
1x
Sr
x
FeO
3d
(x ¼ 0 [36], x ¼ 0.1
[34], x ¼ 0.25 [34]), La
0.9
Ca
0.1
CrO
3d
[62], La
0.6
Sr
0.4
Co
0.2
Fe
0.8
O
3d
[37]. Data for fluorite-
based oxides (Zr
0.85
Ca
0.15
O
1.85
,Zr
0.88
Mg
0.12
O
1.88
,UO
1.99
, and CeO
1.92
) have been taken from
reference [34]
5 Diffusivity of the Oxide Ion in Perovskite Oxides 107
