Ishihara T. (Ed.) Perovskite Oxide for Solid Oxide Fuel Cells (Fuel Cells and Hydrogen Energy)
Подождите немного. Документ загружается.

where A and B are larger and smaller cations. The crystal structure of A
2
BO
4
-
based oxides consists of one perovskite-type block and one rock salt-type AO
block. Thus, it is interesting to study the structural disorders and diffusion path
of oxide ions in the A
2
BO
4
-based oxides and compare the results with those
of perovskite-type materials. Here we also review the structural disorder
and diffusion paths of oxide ions in the K
2
NiF
4
-type (Pr
0.9
La
0.1
)
2
(Ni
0.74
Cu
0.21
Ga
0.05
)O
4+d
[15].
6.2 High-Temperature Neutron Powder Diffractometry
Some ABO
3–d
perovskite-structured materials, where A and B represent larger
and smaller cations, are ionic conductors, while some other ABO
3–d
perovskite-
type compounds are mixed conductors. Heavy elements such as La and Ba
occupy the A site, but because the mobile O anion is a light element, conventional
X-ray powder diffractometry is not sensitive to positional and occupational
disordering of oxide ions. To investigate the diffusion path of mobile oxide
ions, and structural disorder and crystal structure in perovskite-structured ionic
and mixed conductors [5, 6, 8, 10–14], we applied a high-temperature neutron
powder diffraction method. Our reasons for choosing this method were as
follows [24]:
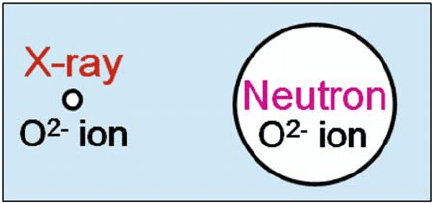
1. The coherent scattering length of the O atom is relatively large compared
with its X-ray scattering factor. Figure 6.1 illustrates the relative scattering
abilities of the oxygen atom in both methods.
2. At high temperatures, the sample surface is often altered by processes such as
sintering, grain growth, cracking, evaporation, and thermal expansion; this
can lead to shifts in diffraction peak intensity and position. Thus, it is often
difficult to perform structural refinement and electron density analysis using
conventional high-temperature X-ray diffraction data measured with
Fig. 6.1 Circles representing the relevant sizes of the square of the X-ray scattering factor (left)
and the neutron scattering length (right) of oxygen atoms in (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
where the size of the square of the X-ray scattering factor of the cation is assumed to be the same
as that of the neutron scattering length of the cation
118 M. Yashima
Bragg–Brentano geometry. In contrast, structural analysis based on high-
temperature neutron powder diffraction data is less subject to the influence
of sintering, grain growth, cracking, evaporation, and thermal expansion.
3. The lack of electronic interference allows for a simple density map. An
electron-density map from X-ray diffraction data includes not only the struc-
tural disorders but also the electron clouds. In contrast, the nuclear density
map from neutron diffraction data does not include the electron clouds.
4. The neutron form factor is independent of the scattering angle, which allows
for high precision in the elucidation of atomic displacement parameters and
structural disorder.
5. The low absorption of neutron by the furnace itself is less damaging to the
data quality.
We devised and fabricated a new furnace for high-temperature neutron
diffraction measurements (Fig. 6.2) [24], using molybdenum silicide heaters to
heat the sample. The merits of the molybdenum silicide heater are as follows:
1. It can be used in air for long periods at temperatures of up to 1900 K without
degradation.
2. A furnace based on this heater is superior to a mirror furnace in terms of
temperature homogeneity.
3. Low-temperature degradation, which is often seen in LaCrO
3
heaters, does
not occur.
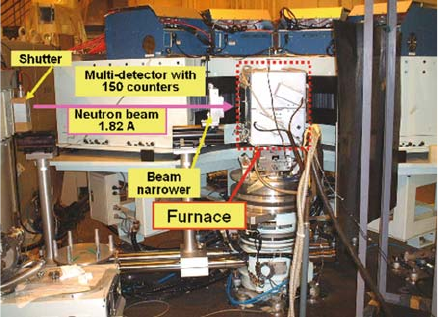
Using the furnace, neutron powder diffraction measurements were con-
ducted in air from room temperature to 1850 K using a 150-detector system,
HERMES [25], i nstalled at the JRR-3 M reactor at the Japan Atomic Energy
Agency, Tokai, Japan (Fig. 6.2) [24, 26]. The furnace was placed on a sample
table, and neutrons with wavelength 1.82 A
˚
were obtained using the (331)
reflection of a Ge monochromator. Although diffraction data where d
spacingislessthan0.93A
˚
cannot be measured using HERMES, the
Fig. 6.2 Photograph of the
furnace [24] placed on the
sample table of the neutron
diffractometer HERMES [25]
6 Perovskite-Type Oxides and Related Materials 119
diffractometer has sufficient intensity and power to collect data with good
counting statistics for nuclear density analysis. Diff raction data were col-
lected in the range 2y ¼38–1578 at step interval s of 0.18. The sample tempera-
ture was kept constant during data collection, and was monitored using a Pt/
Pt-13 wt% Rh thermocouple in contact with the sample.
6.3 Data Processing for Elucidation of the Diffusion Paths of
Mobile Oxide Ions in Ionic Conductors: Rietveld Analysis,
Maximum Entropy Method (MEM), and MEM-Based Pattern
Fitting (MPF)
The experimental diffraction data were analyzed by a combined technique
involving Rietveld analysis, the maximum entropy method (MEM), and
MEM-based pattern fitting (MPF) [10–15]. Rietveld analysis, which is used to
refine the crystal structure from the powder diffraction data by a least squares
method, was carried out using the RIETAN-2000 program [27], which yields
structure factors and their errors after structural refinement. It is known that
MEM can be used to obtain a nuclear density distribution map based on
neutron structure factors and their errors [5, 6, 8, 10–15, 26–29]; any type of
complicated nuclear density distribution is allowed so long as it satisfies the
symmetry requirements. MEM calculati ons were carried out using the PRIMA
program [29]. To reduce the bias impos ed by the simple structural model in the
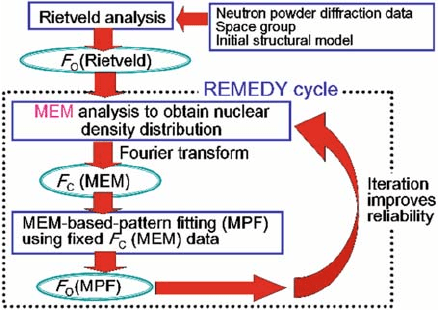
Rietveld refinement, an iterative procedure known as the REMEDY cycle [29]
was applied after MEM analys is (Fig. 6.3). In this procedure, structure factors
Fig. 6.3 Flow chart of the combined technique involving Rietveld analysis, MEM and MPF.
The REMEDY cycle, in which MEM and MPF are performed alternately and repeatedly,
improves the reliability of the nuclear density. F
O
(Rietveld) is the observed structure factor
obtained from the Rietveld analysis. F
C
(MEM) is the structure factor calculated from the
MEM nuclear density. F
O
(MPF) is the observed structure factor, which is obtained from the
MPF analysis
120 M. Yashima
F
C
(MEM) were calculated by Fourier transform of the nuclear densities
obtained by MEM analysis. In the subsequent MEM-based pattern fitting
(MPF), the structure factors F
C
(MEM) obtained in the previous MEM analysis
were fixed, and parameters irrelevant to the structure—e.g., scale factor, pro-
file, unit cell, and background parameters—were refined using RIETAN-2000
[27]. The obs erved structure factors evaluated after the MPF, F
O
(MPF), were
then analyzed again by MEM. MPF and MEM analyses were alternated until
the reliability indices no longer decreased (REMEDY cycle). The REMEDY
cycle allowed us to obtain a reliable nuclear density distribution (Fig. 6.3).
When the MEM is successful in obtaining a nuclear density, the reliability factors
based on the structure factors (R
F
) and on the Bragg intensities (R
I
or R
B
)in
the MPF analysis are lower than those in the Rietveld analysis.
6.4 Diffusion Path of Oxide Ions in the Fast Oxide Ion Conductor
(La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
[10]
6.4.1 Introduction
Lanthanum gallate-based materials with an ABO
3–d
perovskite-type structure
have higher oxide ion conductivity than conventional yttria-stabilized zirconias
[9, 30]. The crystal structure of these materials ha s been the subject of a number
of investigations [31–39], and the diffusion path of oxide ions in lanthanum
gallates has been studied by computational methods [40, 41] and by diffracto-
metry [36]. Here, we describe the temperature dependence of the diffusion paths
and structural disorder of oxide ions in (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
at
1665, 1471, and 1069 K [10]. For comparison, we also describe the nuclear
density distribution of LaGaO
3
at 1663 K [42]. Comparison of structural
disorder in (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
and LaGaO
3
is interesting
because the oxide ion conductivity of (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
is
about 10
3
times higher than that of LaGaO
3
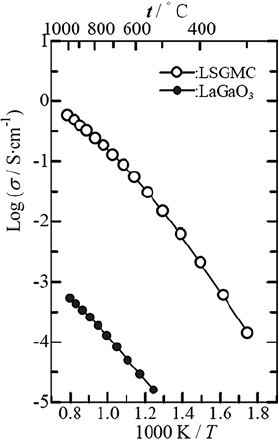
(Fig. 6.4 [42]).
6.4.2 Experiments and Data Processing
In this work, we used a material with the chemical composition (La
0.8
Sr
0.2
)
(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
because doping of Co, Sr, and Mg into lanthanum
gallate effectively enhances oxide ion conductivity (Fig. 6.4 [42]) [43]. A high-
purity sample was synthesized via solid-state reactions [10]. Chemical analysis of
the final product showed a composition of (La
0.80(3)
Sr
0.20(3)
)(Ga
0.80(6)
Mg
0.15(6)
Co
0.050(7)
)O
2.8(3)
, where the number in parentheses is the error in the last
digit. Neutron powder diffraction experiments were carried out at 1069.2
1.6, 1470.7 1.3, and 1664.6 1.4 K in air using a furnace with MoSi
2
heaters
[24], as described above, and the HERMES diffractometer [25]. Neutron
6 Perovskite-Type Oxides and Related Materials 121
diffraction data for LaGaO
3
were obtained in air at 1663 K. The wavelength of
the incident neutrons was 1.8207 A
˚
. Powder patterns were obtained in the range
2y ¼58–1558. The experimental diffraction data were analyzed using a combina-
tion of the Rietveld method and MPF, with the RIETAN-2000 program [27], and
MEM, using the PRIMA program [29].
6.4.3 Results and Discussion
The crystal structure of (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
was successfully
refined, assuming an ideal perovskite-type structure with the space group
Pm
3m at 1665 K (Fig. 6.5) and at 1471 K. At 1069 K, the material was analyzed
assuming R
3c symmetry, because the R
3c reflections forbidden for the Pm
3m
phase exist at this temperature [10]. The refined crystallographic parameters are
shown in Table 6.1. The unit-cell volume of the pseudo-fluorite lattice increases
with temperature due to thermal expansion. The atomic displacement para-
meters of the oxygen atom are large and anisotropic (Fig. 6.5(a) and Table 6.1).
The isotropic atomic displacement parameters of all cations, and the equivalent
isotropic atomic displacement parameters of the oxide ions, increase with
increasing temperature (Table 6.1), corresponding to higher oxide ion conduc-
tivity at higher temperatures (Fig. 6.4 [42]) [43]. The equivalent isotropic atomic
displacement parameters of the oxide ions are higher than those of the cations,
suggesting higher diffusivity for the oxide ions.
Fig. 6.4 Arrhenius plot of
oxide ion conductivity
of LaGaO
3
(closed circles)
and (La
0.8
Sr
0.2
)
(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
(LSGMC, open circles) [42].
The structural origin of the
difference in ionic
conductivity between the
two materials can be seen in
Fig. 6.6(a,d)
122 M. Yashima
MEM analysis was carried out using the structure factors obtained by
Rietveld analysis; 17, 16, and 56 structure factors were obtained for data
measured at 1665, 1471, and 1069 K, respectively. We measur ed the peak
intensity of cubic 100 reflection at the lowest 2y position, because the intensity
of the 100 reflection contributes the most information to MEM analysis. MEM
calculations were performed using 64 64 64 and 96 96 235 pixels for the
cubic and trigonal structures, respectively. The R factor based on the Bragg
intensities, R
I
, was considerably improved by the REMEDY cycle (Table 6.1),
indicating the validity of these nuclear density distributions for (La
0.8
Sr
0.2
)-
(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
. The isosurface of nuclear density obtained from the
REMEDY cycle provided much information on the complexity of structural
disorder and diffusion paths of oxide ion in the crystal (Figs. 6.5(b) and 6.6).
Simple models consisting of atom sph eres were no longer appropriate to
describe the positional distribution of the oxide ions.
To visualize the structural disorder and diffusion paths, the MEM nuclear
density distribution map in the (100) plane is shown in Fig. 6.6. The ox ide ions
in the cubic Pm
3m phase exhibit a large anisotropic distribution, corresponding
to large anisotropy in the atomic displacement parameters (Table 6.1). The
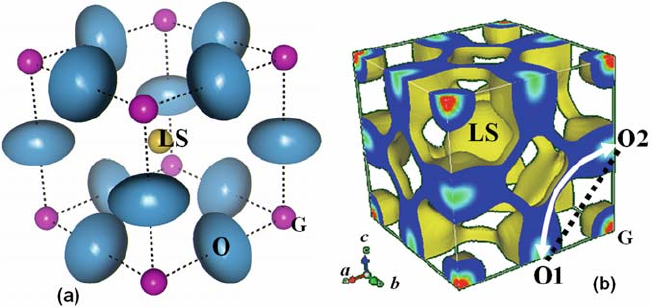
most striking feature is the diffusion path of the oxide ions. Roughly speaking,
the diffusion paths are along the [110], [011], and [101] directions, forming a
three-dimensional network of pathways. The diffusion path does not follow the
edge of the BO
6
[= (Ga
0.8
Mg
0.15
Co
0.05
)O
5.6
] octahedron along the <110>
direction (shown as straight dotted line between the ideal O1 and O2 positions
in Fig. 6.5(b)), but displays an arc shape (curved solid line with arrows),
maintaining a constant distance from the B-site cation (G in Fig. 6.5(b)). This
curved feature is consistent with the results obtained by computational methods
Fig. 6.5 (a) Refined crystal structure and (b) isosurface of nuclear density at 0.05 fm A
˚
3
of
cubic Pm
3m (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
at 1665 K [10]. LS, (La
0.8
Sr
0.2
) cation; G,
(Ga
0.8
Mg
0.15
Co
0.05
) cation
6 Perovskite-Type Oxides and Related Materials 123

Table 6.1 Crystallographic parameters and reliability factors for (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
[10]
Temperature, space group
Unit-cell parameters
Unit-cell volume
1069.2 1.6 K, R
3c
a=b=5.5587(9) A
˚
; c=13.629(3) A
˚
V=364.7(1) A
˚
3
(V
p
=60.78(2) A
˚
3
)***
1470.7 1.3 K, Pm
3m
a=3.9618(2) A
˚
V=62.184(4) A
˚
3
1664.6 1.4 K, Pm
3m
a=3.9744(2) A
˚
V=62.779(4) A
˚
3
La
0.8
Sr
0.2
Wyckoff
Position / g
6a / 1.0 1b / 1.0 1b / 1.0
x, y, z 0, 0, 1/4 1/2, 1/2, 1/2 1/2, 1/2, 1/2
Atomic displacement parameters U
11
=U
22
=2U
12
=0.025(4) A
˚
2
;
U
33
=0.033(9) A
˚
2
;
U
13
=U
23
=0 A
˚
2
; U
eq
=
0.028 A
˚
2
U=0.0391(9) A
˚
2
U=0.0454(9) A
˚
2
Ga
0.8
Mg
0.15
Co
0.05
Wyckoff
Position / g
6b / 1.0 1a / 1.0 1a / 1.0
x, y, z 0, 0, 0 0, 0, 0 0, 0, 0
Atomic displacement parameters U
11
=U
22
=2U
12
=0.016(4) A
˚
2
;
U
33
=0.018(7) A
˚
2
;
U
13
=U
23
=0 A
˚
2
U
eq
=0.017 A
˚
2
U=0.0274(9) A
˚
2
U=0.0343(9) A
˚
2
O Wyckoff
Position / g
18e / 0.9333 3d / 0.9333 3d / 0.9333
x, y, z 0.529(4), 0, 1/4 1/2, 0, 0 1/2, 0, 0
Atomic displacement parameters U
eq
=0.028 A
˚
2
; U
11
=0.039(10) A
˚
2
;
U
22
=2U
12
=0.018(8) A
˚
2
;
U
33
=0.087(11) A
˚
2
;
U
13
=0.5U
23
=0.012(3) A
˚
2
U
eq
=0.0712 A
˚
2
;
U
11
=0.0268(12) A
˚
2
;
U
22
=U
33
=0.0935(13) A
˚
2
;
U
12
=U
13
=U
23
=0 A
˚
2
U
eq
=0.0817 A
˚
2
;
U
11
=0.0292(13) A
˚
2
;
U
22
=U
33
=0.0935(13) A
˚
2
;
U
12
¼U
13
¼U
23
¼0A
˚
2
Reliability factors in the Rietveld
refinement*
R
wp
=6.53%, R
p
=4.97%,
Goodness of fit ¼1.492,
R
I
=3.16%, R
F
=1.90%
R
wp
=6.90%, R
p
=5.20%,
Goodness of fit ¼1.610,
R
I
=3.63%, R
F
=2.23%
R
wp
=6.35%, R
p
=4.94%,
Goodness of fit ¼1.485,
R
I
=2.68%, R
F
=2.39%
Reliability factors in the final
MEM-based whole-pattern fitting*
R
I
=1.92%, R
F
=1.52% R
I
=2.03%, R
F
=1.24% R
I
=1.91%, R
F
=1.34%
Reliability factors in the final MEM
analysis**
R
F
(MEM) ¼1.74%
wR
F
(MEM) ¼2.04%
R
F
(MEM) ¼1.18%
wR
F
(MEM) ¼1.35%
R
F
(MEM) ¼1.48%
wR
F
(MEM) ¼1.46%
Note: g, occupancy; x, y, z, fractional coordinate.
*Standard Rietveld indices; **reliability factors: MEM analysis; ***V
p
, unit-cell volume of the pseudo-perovskite cell.
124 M. Yashima
[40, 41] and with the potential map obtained using a probability density func-
tion technique [36]. Here, for the first time, we have obtained a diffusion path
from the nuclear density distribution and demonstrated its temperature depen-
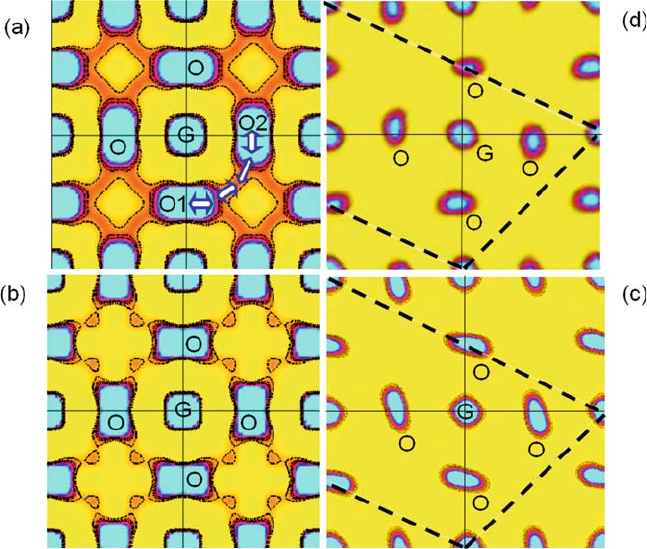
dence [10]. The nuclear density in the area of the diffusion path is greater at
1665 K (Fig. 6.6(a)) than at 1471 K (Fig. 6.6(b)), which is consistent with
an increase in oxide ion conductivity with increasing temperatur e (Fig. 6.4
[42]) [43]. Notably, the oxide ions in the low-temperature tri gonal phase are
localized near the equilibrium positions (Fig. 6.6(c)), although they are spread
over a wide area between the ideal positions in the high-temperature cubic phase
(Fig. 6.6(a, b)). This interesting distribution indicates that the more symmetrical
Fig. 6.6 Nuclear density distributions on the (100) plane of cubicPm
3m (La
0.8
Sr
0.2
)-
(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
at (a) 1665 K and (b) 1471 K, and (c) on the (012) plane of trigonal
R
3c (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
at 1069 K, with contours in the range from 0.3 to
4.0 fm A
˚
3
(0.3 fm A
˚
3
step) [10]. (d) Nuclear density distribution on the (012) plane of
trigonal R
3c LaGaO
3
at 1663 K [42]. G and O denote the B-site cation (Ga
0.8
Mg
0.15
Co
0.05
)
and the oxide ion, respectively. The diffusion path of the oxide ion is not along the straight line
between the ideal positions, but along the curved solid line avoiding the G ion [white arrows in
(a)]. The thin black straight line and thick black dashed line in Fig. 6(c, d) show the Pm
3m and
R
3c unit cells, respectively. In the low-temperature trigonal structure, the oxide ions are
localized near the equilibrium position, while in the high-temperature cubic phase the oxide
ions are spread over a wide area between the ideal sites
6 Perovskite-Type Oxides and Related Materials 125
Pm
3m phase has a lower activation energy for the migration of oxide ions. As
shown in Fig. 6.6(a,d), the oxide ions in cubic (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
-
Co
0.05
)O
2.8
have a greater distribution than in trigonal LaGaO
3
. This finding
is consistent with the difference in oxide ion conductivity between the two
compounds (Fig. 6.4 [42]).
6.5 Diffusion Path of Oxide Ions in an Oxide Ion Conductor,
La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
, with a Double Perovskite-Type
Structure [11]
6.5.1 Introduction
As mentioned earlier, some perovskite-related ABO
3–d
phases possess high
oxide ion conductivity. In Section 6.4, we described the diffusion path of mobile
oxide ions in a solid solution of cubic perovskite-type doped lanthanum gallate
(La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
[10]. However, although there is a wide
variety of perovskite-related structures (e.g., A-site-deficient doubl e perovs-
kite-type structure), there were no reports concerning diffusion paths in such
materials. The lanthanum titanate solid solution La
(2x)/3
(Ti
1–x
M
x
)O
3–d
(M ¼Al
or Nb, 0.05 x 0.20), where d denotes the concentration of oxygen defects,
has an A-site-deficient layered perovskite-type structure [19–21, 44–53], and
exhibits high oxide ion conductivity at high temperatures [21, 44]. Yoshioka [21]
studied the electrical properties of La
(2–x)/3
(Ti
1–x
Nb
x
)O
3–d
(x = 0.05–0.15) and
reported that a sample with x = 0.10 displayed the highest ionic conductivity
(10
–2
Scm
1
at 973 K). In this section, we describe the crystal structure and
pathway of oxide ion diffusion in La
0.64
(Ti
0.92
Nb
0.08
)O
3–d
(x = 0.08) [11].
6.5.2 Experiments and Data Processing
The La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
specimen was prepared via solid-state reactions
[11, 20]. ICP-OES chemical analysis indicated that the chemical formula of the
final product was La
0.636(1)
(Ti
0.921
Nb
0.079(1)
)O
2.993(1)
. The value for oxygen
(2.993) was calculated based on electrical neutrality and suggests that the
amount of oxygen defects is small. Neutron powder diffraction data for
La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
were collected at 769 K, 1281 K, and 1631 K using
the furnace [24] and HERMES diffractometer [25] as described above. Incident
neutrons with a fixed wavelength of 1.8143 A
˚
were used. Powder diffraction
data were measured over the range 2y =38–152.628. The sample temperature
was maintained to within 1.5 K during each measurement. The diffraction
data were analyzed by the Rietveld method, followed by application of MEM
and M PF, using the RIETAN-2000 [27] and PRIMA [29] programs.
126 M. Yashima

6.5.3 Results and Discussion
Rietveld analysis of the neutron powder diffraction patterns of
La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
at 769 K, 1281 K, and 1631 K was performed assuming
a tetragonal P4/mmm structure (a ¼b a
p
, c 2a
p;
subscript p denotes
pseudo-cubic perovskite-type structure) . The refined unit-cell and structural
parameters and R factors are summ arized in Table 6.2 [11]. The unit-cell
parameters increase with temperature, as does the unit-cell volume due to
thermal expansion. Figure 6.7(a) shows the crystal structure of the material
Table 6.2 Refined crystallographic parameters and reliability factors in Rietveld and MPF
analyses for La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
[11]
Temperature 769 K 1.5 K 1281 K 1.5 K 1631 K 1.5 K
Atom/Site Parameter
a (A
˚
) 3.8827(2) 3.9019(2) 3.9172(2)
c (A
˚
) 7.8684(4) 7.9118(4) 7.9249(5)
La1 U ( 10
2
A
˚
2
) 1.33(7) 2.83(9) 3.99(12)
La2 U ( 10
2
A
˚
2
) 0.8(2) 1.9(3) 1.3(3)
Ti,Nb z
U ( 10
2
A
˚
2
)
0.2625(6)
0.54(10)
0.2640(7)
1.95(12)
0.2621(9)
2.51(14)
O1 U
11
( 10
2
A
˚
2
)
U
33
( 10
2
A
˚
2
)
U
eq
( 10
2
A
˚
2
)
2.5(2)
1.3(3)
2.12
4.5(2)
2.3(3)
3.76
5.1(3)
2.8(4)
4.32
O2 U
11
( 10
2
A
˚
2
)
U
33
( 10
2
A
˚
2
)
U
eq
( 10
2
A
˚
2
)
3.7(2)
0.2(2)
2.58
5.5(2)
1.1(3)
4.05
6.4(3)
1.9(4)
4.91
O3 z
U
11
( 10
2
A
˚
2
)
U
22
( 10
2
A
˚
2
)
U
33
( 10
2
A
˚
2
)
U
eq
( 10
2
A
˚
2
)
0.2340(3)
2.6(2)
0.11(10)
3.16(12)
1.97
0.2344(4)
4.4(2)
1.04(11)
4.81(14)
3.41
0.2373(5)
5.6(3)
1.53(14)
5.9(2)
4.36
Reliability factors* R
wp
=5.76%,
R
p
=4.28%
Goodness of fit:
3.16
R
I
=5.38%,
R
F
=3.59%
R
wp
=5.21%,
R
p
=3.83%
Goodness of fit:
2.87
R
I
=4.19%,
R
F
=4.36%
R
wp
=5.00%,
R
p
=3.73%
Goodness of fit:
2.82
R
I
=4.33%,
R
F
=5.06%
Reliability factors** R
I
=6.03%,
R
F
=3.27%
R
I
=4.17%,
R
F
=3.13%
R
I
= 4.05%,
R
F
=3.33%
Note: * Reliability factors in Rietveld analysis. ** Reliability factors in MEM-based
pattern fitting. Tetragonal space group P4/mmm (No. 123) Z =2. U, Atomic displacement
parameter; z, fractional coordinate. Occupancies for La1, La2, O1, O2, and O3 sites are
assumed to be 1.0, 0.271, 1.0, 0.9972, and 0.9972, respectively. Occupancies of Ti and Nb
atoms at the Ti,Nb site are assumed to be 0.9209 and 0.0791, respectively. In analyses, atom
positions were: La1 1a (0, 0, 0); La2 1b (0, 0, 1/2); Ti,Nb 2h (1/2, 1/2, z); O1 1c (1/2, 1/2, 0); O2
1d (1/2, 1/2, 1/2); O3 4 i (1/2, 0, z). Site symmetries give constraints: U
12
= U
13
= U
23
=0 at
O1, O2, and O3 sites; U
11
= U
22
at O1 and O2 sites. Only independent atomic displacement
parameters are given.
6 Perovskite-Type Oxides and Related Materials 127
