Ishihara T. (Ed.) Perovskite Oxide for Solid Oxide Fuel Cells (Fuel Cells and Hydrogen Energy)
Подождите немного. Документ загружается.

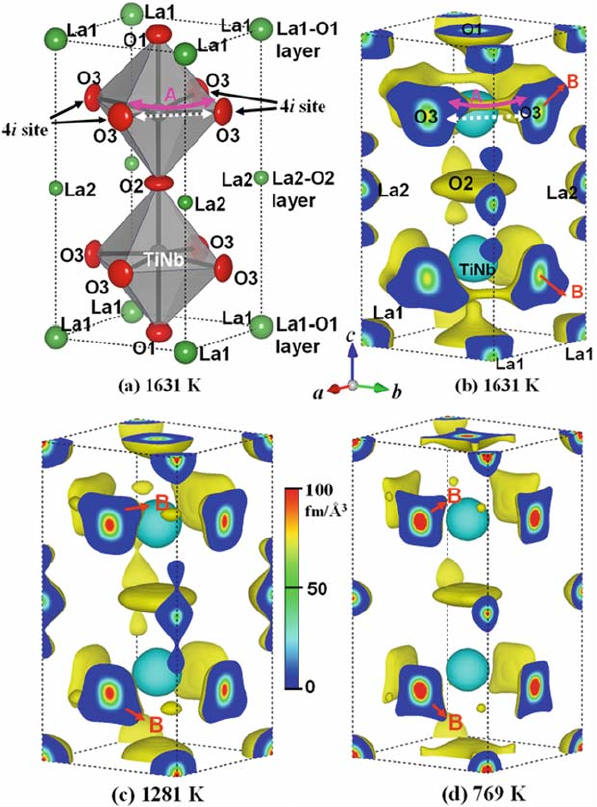
Fig. 6.7 (a) Refined crystal structure of double perovskite-type La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
at 1631 K,
and (b, c, d) isosurfaces of nuclear density distribution at –0.1 fm A
˚
3
(light blue)and+0.1fmA
˚
3
(yellow) and nuclear density on the (100) and (001) planes in La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
at 1631 K (b),
1281 K (c), and 769 K (d) [11]. Since the Ti atom has negative scattering length, the Ti,Nb site is
drawn with light blue equi-density surface at –0.1 fm A
˚
3
. Oxygen atoms at the O3 site have a large
spatial distribution to the <101> directions shown by the line with an arrow (B). The O3 atoms do
not move along the straight line shown by the dotted line with arrows but along the curved solid line
with arrows (A in (a)and(b))
128 M. Yashima

drawn with the refined crystallographic parameters [11]. This is the high-
temperature form of La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
, which has an A-site-deficient
perovskite-type structure with double perovskite ABO
3–d
units along the
c axis (number of chemical formula in a unit cell: Z = 2), where A=La
0.64
and B = (Ti
0.92
Nb
0.08
). The occupancy factors for La at the La1 and La2 sites
are g(La1) = 1.00 and g (La2) = 0.271 [11]. The dissimilarity of these values
reflects the chemical ordering of La-occupied La1-O1 and La-defective La2-O2
layers [Fig. 6.7(a)]. All the refined atomic displacement parameters increase
with temperature (Table 6.2). The equivalent isotropic atomic displacement
parameters of the oxygen atoms are larger than those of the cations, suggesting
a larger diffusion coefficient for the oxide ions (Table 6.2). The oxygen atoms
also display large anisotropy in terms of atomic displacement parameters,
suggesting directionality in the movement of oxide ions around the stable
positions. Similar large and anisotropic thermal motions of oxide ions were
observed for the cubic perovskite-type oxide ion conductor (La
0.8
Sr
0.2
)-
(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
(Fig. 6.5) [10].
MEM analysis was conducted using diffraction data in the range 2y ¼4.08–1408,
corresponding to d > 1.0 A
˚
(d, spacing of lattice planes), with the structure
factors obtained by Rietveld analysis. A total of 59 structure factors were
obtained for all data measured at three different temperatures. The 001 reflec-
tion appearing at the lowest 2y position (138) was included, as this peak
provides information on the disordered arrangement of the oxide ions. MEM
calculations were performed with the unit cell divided into 64 64 128 pixels.
Use of the REMEDY cycle resulted in significant improvement in the R factors
based on the Bragg intensities (R
I
) and structure factors (R
F
) (Table 6.2). Figure
6.7(b, c, d) shows the isosurface of nuclear density and the nuclear density
distributions on the (100) and (001) planes obtained afte r the REMEDY cycle.
Figure 6.8 shows the temperature dependence of the nuclear density contour
map at z = 0.2 on the ab plane. Figures 6.7(b, c, d) and 6.8 provide much
information on the positional disorder and diffusion paths of mobile oxide
ions compared to the simple atomistic model (Fig. 6.7(a)).
At 769 K, the O3 atoms are localized near the stable 4i site (1/2, 0, 0.234). The
O3 atoms display small bulges in the <101> direction (B in Fig. 6.7(d)), which
become larger at 1281 and 1631 K (Fig. 6.7(c,b)). The probability density of
each O3 atom is connected with that of its nearest neighbor O3 atoms, indicat-
ing diffusion paths (A in Fig. 6.7(a, b)). The diffusion path is along the [100] or
[010] direction near the stable O3 positions, and along the [110] or ½1
10
direction around the center of the paths. The O3 atoms migrate to the nearest
neighbor 4i site through a triangle formed by adjacent La1, La2, and (Ti,Nb)
atoms. The spatial distribution of the O3 atoms becomes larger with increasing
temperature (Figs. 6.7 and 6.8). Such an increase in the density of oxide ions
with increasing temperature is consistent with the higher conductivity observed
at higher temperatures [21]. The O3 atom migr ates following a curved route to
maintain a relatively constant distance from the (Ti,Nb) atoms (solid curves
A in Figs. 6.7(a, b) and 6.8(a)), rather than a direct linear path along the <110>
6 Perovskite-Type Oxides and Related Materials 129
direction (straight dotted lines with arrows between regular positions in
Figs. 6.7(a, b) and 6.8(a)). Similar curved migration pathways were found in
the nuclear density distribution of an ideal cubic perovskite-type compound,
(La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
[10]. Computer simulations [40, 54] for
perovskite-structured LaBO
3
(B = Co, Mn, Ga, Cr, and Fe) comp ounds also
revealed deviations of the migration pathway from the direct path.
The oxide ion conductor (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
, which has an
ideal perovskite-type structure, exhibits diff usion paths along the [110], ½1
10,
[011], ½01
1, [101] and ½101 directions to form a three-dimensional network
of equivalent diffusion pathways (Fig. 6.5(b)) [10]. In contrast, in the present
double pe rovskite-type material La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
, a two-dimensional
diffusion pathway, by which O3 atoms migrate along the [110] and ½ 1
10
directions (Fig. 6.7(b)), is present. This two-dimensional feat ure is attributable
to the layered structure of the material, which consists of La-occupied La1-O1,
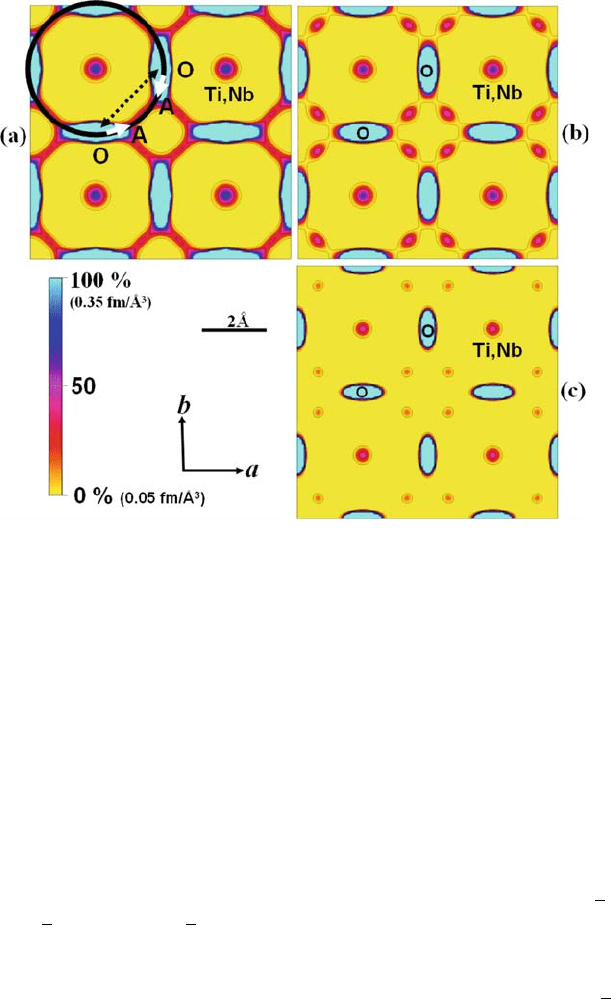
Fig. 6.8 Nuclear density distribution in the ab plane at z = 0.2 (0 < x, y < 2 ) of double
perovskite-type P4/mmm La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
at (a) 1631 K, (b) 1281 K, and (c) 769 K
[11]. Contours are in the range 0.05–0.35 fm A
˚
3
with steps of 0.05 fm A
˚
3
. The solid line in (a)
denotes the curved diffusion path of the oxide ions, and the dotted line denotes the direct path
between ideal positions. At low temperature (769 K), oxide ions are localized near the
equilibrium position (see (c)); at high temperature (1631 K), the oxide ions are dispersed
over a wide area between the regular positions (see (a))
130 M. Yashima

(Ti,Nb)-O, and La-deficient La2-O2 layers (Fig. 6.7(a)). Two-dimensional
lithium catio n conduction has also been reported in the orthorhombic layered
perovskite-type compound La
0.62
Li
0.16
TiO
3
[55], in which the Li cation exists
and migrates only near the La-deficient La2-O2 layer. This work has thus
revealed that oxide ion diffusion in an ionic conductor with a double perovskite
structure is two dimens ional.
6.6 Crystal Structure and Structural Disorder of Oxide Ions in
Cathode Materials, La
0.6
Sr
0.4
CoO
3–d
and
La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
, with a Cubic Perovskite-Type
Structure [12, 13]
6.6.1 Introduction
The lanthanum strontium cobaltites La
1–x
Sr
x
CoO
3–d
and La
1–x
Sr
x
Co
1–y
Fe
y
O
3–d
,
which ha ve a perovskite-type structure, are promising cathode materials for
use in conjunction with doped lanthanum gallate electrolyte in SOFCs [56–58].
The crystal structure of trigonal R
3c La
1–x
Sr
x
CoO
3–d
has been the subject of
a number of investigations [59–65]. However, far less attention has been
paid to the high-temperature cubic phase of La
1–x
Sr
x
CoO
3–d
and La
1–x
Sr
x
Co
1–y
Fe
y
O
3–d
, which is important for application in SOFCs. In this section,
we describe the crystal structure and structural disorder of cubic Pm
3m
perovskite-type oxides La
1–x
Sr
x
CoO
3–d
at 1531 K [12] and La
0.6
Sr
0.4
-
Co
0.8
Fe
0.2
O
3–d
at 1533 K [13].
6.6.2 Experiments and Data Processing
La
0.6
Sr
0.4
CoO
3–d
and La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
specimens were prepared by a
solid-state reaction method. Neutron powder diffraction data of
La
0.6
Sr
0.4
CoO
3–d
were collected in air using the HERMES diffractometer [25]
at room temperatur e and at 1531 K. Neutr on diffraction data of
La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
were measured in air using the HERMES at 667 K
and at 1533 K. The powder patterns were measured in the range 2y ¼58–1558.
The wavelength of the incident neutrons was 1.8207 A
˚
. The sample temperature
was kept constant during each data collection, using the furnace with MoSi
2
heaters [24]. The diffraction data of La
0.6
Sr
0.4
CoO
3–d
at 1531 K and of
La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
at 667 K and 1533 K were analyzed by the Rietveld
method and MPF analysis with the RIETAN-2000 program [27] and the MEM
analysis with the PRIMA [29].
6 Perovskite-Type Oxides and Related Materials 131
6.6.3 Results and Discussion
6.6.3.1 Crystal Structure and Disorder of La
0.6
Sr
0.4
CoO
3–d
The neutron diffraction data of La
0.6
Sr
0.4
CoO
3–d
at room temperature indi-
cated that the specimen consisted of a single phase of trigonal R
3c
La
0.6
Sr
0.4
CoO
3–d
. All the peaks in the neutron diffraction pattern of
La
0.6
Sr
0.4
CoO
3–d
at 1531 K were indexed by a cubic perovskite-type structure
with Pm
3m symmetry (Fig. 6.9(a)), indicating phase transformation from a
low-temperature trigonal to a high-temperature cubic phase. Rietveld analysis
was performed using the diffraction data in the range 2y ¼208–1538, based on a
cubic perovskite-type structure (Fig. 6.9( a)). The La and Sr atoms were placed
at the special positions 1b 1/2, 1/2, 1/2 in the Pm
3m symmetry. The Co and O
atoms were placed at the 1a 0, 0, 0 and 3d 1/2, 0, 0 sites, respectively. Isotropic
and anisotropic atomic displacement parameters were used for cations and
anions, respectively (Table 6.3). The refined crystallo graphic parameters and
reliability fact ors are shown in Table 6.3. The atomic displacement parameters
of the O atom exhibited a large anisotropy (Fig. 6.9(a) and Table 6.3). The
occupancy factor of O atoms at the 3d site was estimated to be 0.886(6),
indicating an oxygen deficiency of d ¼0.34(2) in the La
0.6
Sr
0.4
CoO
3–d
at
1531 K. The averaged valence of the Co cations was estimated to be 2.72 at
1531 K, which is consistent with the calculated bond valence sum of 2.8. Here,
the average value of the bond valence parameters, 1.698, was used for the
calculation [66].
MPF analysis was conducted using diffraction data in range 2y ¼208–1538,
corresponding to d > 1.07 A
˚
, based on the structure factors obtaine d by
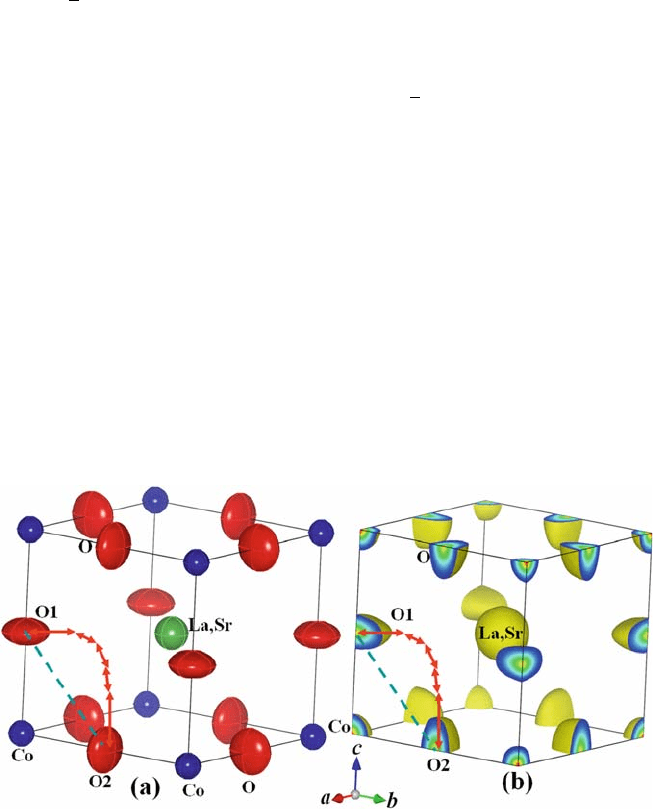
Fig. 6.9 (a) Refined crystal structure and (b) isosurface of nuclear density at 2 fm A
˚
–3
for
La
0.6
Sr
0.4
CoO
3–d
at 1531 K [12]. The arrows denote possible diffusion paths of oxide ions. The
dashed straight line is the edge of the CoO
6
octahedron
132 M. Yashima
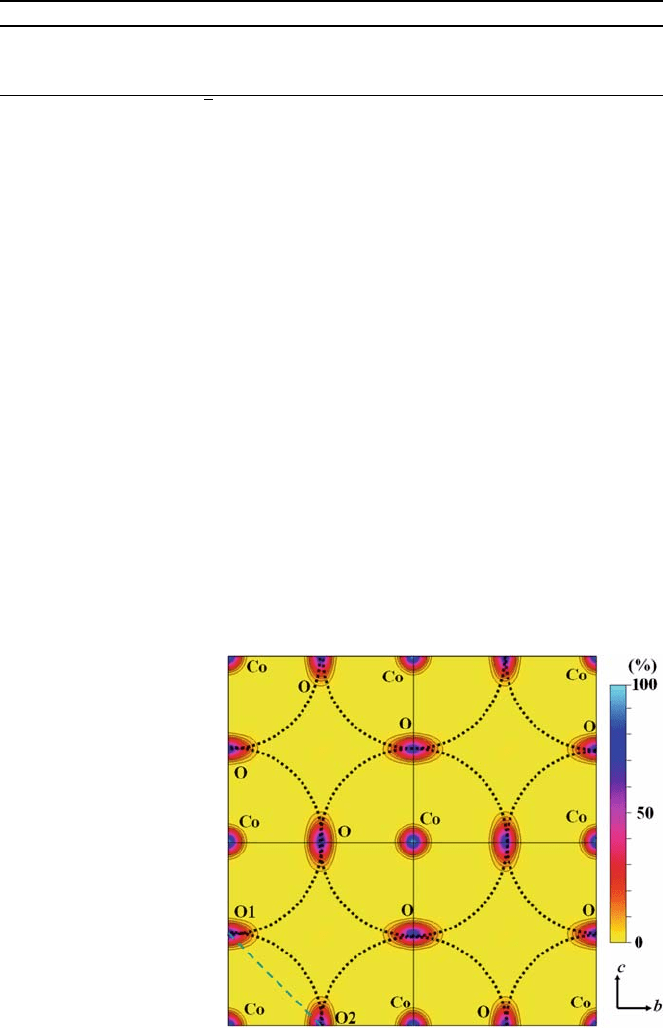
Rietveld analysis. A total of 16 structure factors were obtained. The 100
reflection appearing at the lowest 2y position (26.78) was included, as this
peak provides information on the disorder of the oxide ions. MEM calcula-
tions were performed with the unit cell divided into 64 64 64 pixe ls. The R
factor based on the Bragg intensities (R
I
) was improved from 2.33% (Rietveld
analysis) to 1.71% (MPF), and that based on the structure factors (R
F
)was
improved from 1.72% to 1.25%. The MEM nuclear density distribution map
for the (100) plane is shown in Fig. 6.10. The map reveals that the oxide ions in
cubic Pm
3m La
0.6
Sr
0.4
CoO
3–d
exhibit a large thermal motion perpendicular to
the Co–O bond, corresponding to the large anisotropy in the atomic displace-
ment parameters (Figs. 6.9 and 6.10). The arrows in Fig. 6.9 and dotted circles
in Fig. 6.10 indicate possible diffusion paths of oxide ions in La
0.6
Sr
0.4
CoO
3–d
.
Table 6.3 Refined crystallographic parameters and reliability factors obtained from Rietveld
and MPF analysis for La
0.6
Sr
0.4
CoO
3–d
at 1531.4 K (d ¼0.34(2)) [12]
Site and atoms Wyckoff position gxyzU(A
˚
2
)
La
0.6
Sr
0.4
1b 1.0 1/2 1/2 1/2 0.0425(9)
Co 1a 1.0 0 0 0 0.025(2)
O3d 0.886(6) 1/2 0 0 0.066*
Note: Cubic space group Pm
3m (No. 221). Number of formula units of La
0.6
Sr
0.4
CoO
3–d
in a
unit cell: Z ¼1. Unit-cell parameters: a ¼b ¼c ¼3.9496 (3) A
˚
, a ¼b ¼g ¼908; unit-cell volume:
61.612(9) A
˚
3
; g, occupancy; x, y, z, fractional coordinates; U, isotropic atomic displacement
parameters; *equivalent isotropic atomic displacement parameters; anisotropic atomic displa-
cement parameters of O atom: U
11
¼0.027(2) A
˚
2
, U
22
¼U
33
¼0.085(1) A
˚
2
,
U
12
¼U
23
¼U
31
¼0A
˚
2
.
Reliability factors from Rietveld analysis: R
wp
¼3.73%, R
p
¼2.68%, R
e
¼1.86%, R
wp
/R
e
¼2.00,
R
I
¼2.33%, R
F
¼1.72%. Reliability factors from first MPF analysis: R
I
¼1.71%, R
F
¼1.25%.
Fig. 6.10 Nuclear density
distribution in the (100)
plane for La
0.6
Sr
0.4
CoO
3–d
,
measured at 1531 K, with
black contours in the range
from 2 to 10 fm A
˚
–3
(2 fm
A
˚
–3
steps) [12]. The color
scale of 100% corresponds
to the maximum density of
46.4 fm A
˚
–3
. The dotted
circles indicate possible
oxide-ion diffusion paths.
The dashed straight line
indicates the edge of the
CoO
6
octahedron. The solid
straight lines indicate the
unit cell. The figure shows
four unit cells
6 Perovskite-Type Oxides and Related Materials 133

The diffusion path does not follow the edge of the CoO
6
octahedron (shown as
straight dashed lines between the ideal O1 and O2 positions in Figs. 6.9 and
6.10), but displays an arc shape (curved solid arrows in Fig. 6.9 and dotted
circles in Fig. 6.10), avoiding the Co cation. This possible diffusion path is
similar to that observed for (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
[10]. Compu-
ter simulations have also indicated a similar curved path f or oxide ion migra-
tion [40].
6.6.3.2 Crystal Structure and Disorder of La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
Neutron diffraction data for La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
(LSCF6482) at 667 K
indicated that the specimen consisted of a single trigonal R
3c phase. All the
peaks in the neutron diffraction pattern of LSCF6482 at 1533 K were indexed
by the cubic perovskite-type structure with Pm
3m symmetry, indicating a phase
transformation from the low-temperature trigonal to high-temperature cubic
phase between 667 and 1533 K, which is consistent with the literature [67].
Rietveld analysis of LSCF6482 was performed using the neutron diffraction
data taken at 667 K in the 2y range of 208–1538 by a trigonal R
3c perovskite-
type structure. La and Sr atoms were placed at the special position 6a 0, 0, 1/4 of
the R
3c symmetry. Co and Fe atoms were put at the 6b 0, 0, 0 site. O atom was
placed at the 18ex, 0, 1/4. In a preliminary analysis, the refined occupancy
factor of O atoms at the 18e site g(O) was unity within the estimated standard
deviation in the Rietveld analysis Thus, the g(O) was fixed to be unity in the
final refinement. Isotropic and anisotropic atomic displacement parameters
were used for the cations and anions, respectively. The calculated profile agreed
well with the observed one [13]. The refined crystal parameters and reliabil ity
factors are shown in Table 6.4 [13]. The averaged valence of the Co and Fe
cations was estimated to be 3.4 from the occupancy factor at 667 K, which is
consistent with the calculated bond valence sum (BVS) value of 3.3. Here the
average value of the bond valence parameter of 1.7118 was used for the
Table 6.4 Refined crystal parameters and reliability factors in Rietveld and MPF analyses for
La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
at 667 K (d ¼0) [13]
Site and atoms Wyckoff position gx yzU(A
˚
2
)
La
0.6
Sr
0.4
6a 1.0 0 0 1/4 0.0100(6)
Co
0.8
Fe
0.2
6b 1.0 0 0 0 0.0061(9)
O18e 1.0 0.5202(3) 0 1/4 0.0228*
Note: Trigonal space group R
3c (hexagonal setting); number of formula units of
La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
in a unit cell: Z ¼6. Unit-cell parameters: a ¼b ¼5.46974(18) A
˚
,
c ¼13.3693 (5) A
˚
, a ¼b ¼908, g ¼1208; unit-cell volume: 346.40(2) A
˚
3
; g, occupancy; x, y, z,
fractional coordinates; U, isotropic atomic displacement parameters; *equivalent isotropic
atomic displacement parameter.
Reliability factors in the Rietveld analysis: R
wp
¼7 .64%, R
p
¼6.00%, R
e
¼4.75 %,
R
wp
/R
e
¼1.26, R
I
¼3.76%, R
F
¼2.49%. Reliability factors in the first MPF analysis:
R
I
¼1.04%, R
F
¼0.82%.
134 M. Yashima
calculation [66]. Although the BVS is usually applied to the crystal structure at
room temperature, we can use it at high temperatures because of small change
of unit-cell parameters between different temperatures.
MPF analysis of R
3c LSCF6482 was conducted using diffraction data in the
2y range from 208 to 1538 with the structure factors obtained from Rietveld
analysis. The R factors for the Bragg intensities, R
I
, and for the structure factors,
R
F
, were improved from 3.76% in the Rietveld analysis to 1.04% in the MPF,
and from 2.49% to 0.82%, respectively. The resultant nuclear density indicated
that the oxide ions are localized near the stable positions (Fig. 6.11(a)).
Rietveld analysis was performed using the diffraction data of LSCF6482
taken at 1533 K in the 2y range of 208–1538 by a cubic Pm
3m perovskite-type
structure (Table 6.5). La and Sr atoms were placed at the special position 1b 1/2,
1/2, 1/2 of the Pm
3m symmetry. Co and Fe atoms were put at the 1a 0, 0, 0 site,
whereas the O atom was placed at the 3d 1/2, 0, 0 posit ion. The atomic
displacement parameters of the O atom exhibited large anisotropy
(Fig. 6.11(b) and Table 6.5), which reflects the rotational motion of O atoms
in the rigid (Co,Fe)O
6
octahedron. Similar anisotropy has been observed in
other cubic perovskite-type compounds [10, 12, 68, 69]. The atomic displace-
ment parameters at 1533 K were higher than those at 667 K. The equivalent
isotropic displacement parameter of O atom is larger than those of cations
(Tables 6.4 and 6.5), suggesting the higher diffusivity of O atoms. The occu-
pancy factor of the O atom at the 3d site was estimated to be 0.904(6), indicating
an oxygen deficiency of d ¼0.288(15) in La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
at 1533 K. The
change of oxygen deficiency from d ¼0 at 667 K to 0.288 at 1533 K, which was
obtained in the Rietveld analyses, is reasonably consistent with the weigh t loss
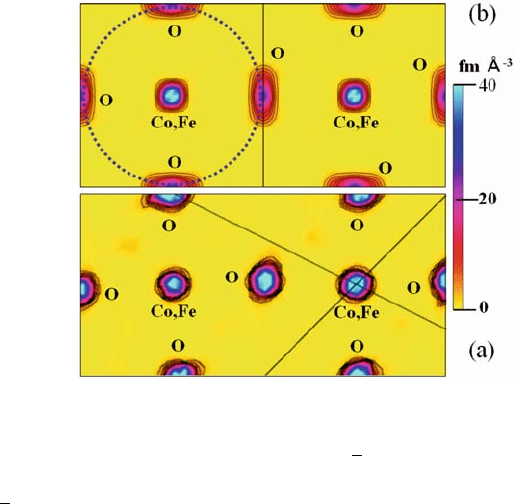
Fig. 6.11 (a) Nuclear
density distribution on the
(012) plane of trigonal
La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3-d
measured at 667 K. (b)
Nuclear density distribution
on the (100) plane of cubic
La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
measured at 1533 K. Black
contours in the range from 3
to 13 fm A
˚
–3
(3 fm A
˚
–3
step).
The dotted circle indicates
the possible oxide-ion
diffusion path. The straight
solid line indicates the unit
cell
6 Perovskite-Type Oxides and Related Materials 135
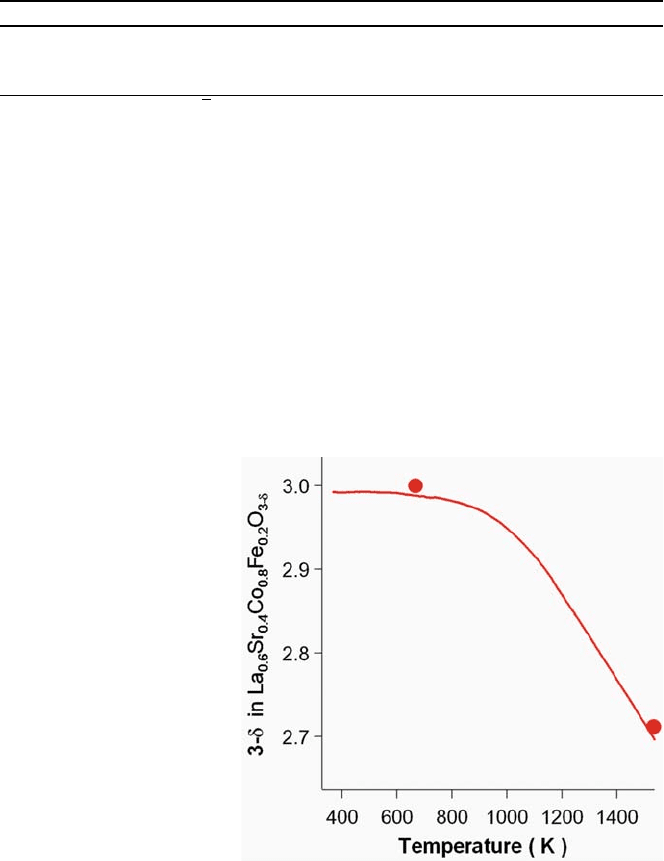
observed in the TG curve (Fig. 6.12). The average d valence of the B-site Co and
Fe cations was estimated to be 2.8 from the refined occupancy of O atoms at
1533 K, which is consistent with the calculated bond valence sum value of 2.9.
Here, the average value of the bond valence parameter of 1.7118 was used for
the calculation [66].
MPF analysis of LSCF6482 was conducted using diffraction data taken at
1533 K in the 2y range from 208 to 1538, with the structure factors obtained
from Rietveld analysis. The R factors for the structure factors, R
F
was improved
from 3.20% in the Rietveld analysis to 2.32% in the MPF. To visualize the
structural disorder, the MEM nuclear density distribution map on the (100)
Table 6.5 Refined crystal parameters and reliability factors in Rietveld and MPF analyses for
La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
at 1533 K (d ¼0.288(15))
Site and atoms Wyckoff position gxyzU(A
˚
2
)
La
0.6
Sr
0.4
1b 1.0 1/2 1/2 1/2 0.0416(9)
Co
0.8
Fe
0.2
1a 1.0 0 0 0 0.0294(11)
O3d 0.904(6) 1/2 0 0 0.071*
Note: Cubic space group Pm
3m, number of formula units of La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
in a unit
cell; Z ¼1. Unit-cell parameters: a ¼b ¼c =3.9540(3) A
˚
, a ¼b ¼g ¼908; unit-cell volume:
61.815(8) A
˚
3
; g, occupancy; x, y, z, fractional coordinates; U, isotropic atomic displacement
parameters. *Equivalent isotropic atomic displacement parameters, anisotropic atomic displa-
cement parameters of O atom: U
11
¼0.0290(13) A
˚
2
; U
22
¼U
33
¼0.0921(12) A
˚
2
;
U
12
¼U
23
¼U
31
¼0A
˚
2
. Reliability factors in the Rietveld analysis: R
wp
¼4.82%,
R
p
¼3.50%, R
e
¼1.70%, R
wp
/R
e
¼2.83, R
I
¼3.56%, R
F
¼3.20%. Reliability factor in the
first MPF analysis: R
F
¼2.32%.
Fig. 6.12 Temperature
dependence of 3–d in
La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
,
where d is the concentration
of oxygen vacancy. Solid
line was obtained using
the weight loss from the
TG data of
La
0.6
Sr
0.4
Co
0.8
Fe
0.2
O
3–d
where no oxygen vacancy is
assumed at room
temperature. Circle was
calculated from the refined
occupancy at the oxygen site
in the Rietveld analyses of
high-temperature neutron
diffraction data
136 M. Yashima
plane in LSCF6482 is shown in Fig. 6.11(b). The nuclear density map reveals that
the oxide ions in the cubic Pm
3m LSCF6482 exhibit a large thermal motion
perpendicular to the (Co,Fe)-O bond, corresponding to large anisotropy of the
atomic displacement parameters (Table 6.5). The dotted circle in Fig. 6.11(b)
indicates possible diffusion paths of the oxide ions in LSCF6482. The diffusion
path does not follow the edge of the (Co,Fe)O
6
octahedron, but displays an arc
shape away from the Co,Fe cation. The nuclear density of O atoms in LSCF6482
did not connect with that of nearest neighbor O atoms (Fig. 6.11). On the contrary,
the (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
perovskite exhibited connected diffusion
paths (Figs. 6.5 and 6.6) [10]. This finding strongly suggests that the diffusivity of
oxide ions in LSCF6482 is lower than that in (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
.
Similarly, the nuclear density around an O site in La
0.6
Sr
0.4
CoO
3–d
did not connect
with that around the nearest neighbor O site (Figs. 6.9 and 6.10) [12].
It has been commonly assumed that the migr ating anion in the AB O
3–d
perovskite-type structure takes a direct linear path along the <110> edge of
the BO
6
octahedron. However, based on the results of this work, we suggest a
curved diffusion path of the oxide ions in the electrode material LSCF6482 at
1533 K. The oxide ions migrate in the <100> directions near the stable 3d
position, while they move along the <110> directions around the center of the
diffusion path. Similar diffusion paths have been observed in the MEM nuclear
density maps of (La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
(Figs. 6.5 and 6.6), of
La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
(Figs. 6.7 and 6.8), and of La
0.6
Sr
0.4
CoO
3–d
(Figs. 6.9
and 6.10) [10–12]. Computer simulations have also indicated a similar curved
path for the oxide ion migration in perovskite-type compounds [40]. The diffu-
sion paths of the cubic perovskite-type LSCF6482, La
0.6
Sr
0.4
CoO
3–d
,and
(La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
form a three-dimensional network. As
described previously, Ali et al. [11] reported a similar but two-dimensional curved
diffusion path of oxide ions in an oxide ion conductor, La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
,
with a double perovskite-type structure. All four ABO
3–d
perovskite-type com-
pounds LSCF6482, La
0.6
Sr
0.4
CoO
3–d
,(La
0.8
Sr
0.2
)(Ga
0.8
Mg
0.15
Co
0.05
)O
2.8
,and
La
0.64
(Ti
0.92
Nb
0.08
)O
2.99
exhibit a curved migration path of the mobile oxide
ions, keeping the B–O distance constant to some degree. Thus, this curved feature
should be common in perovskite-type ionic and mixed conductors.
6.7 Structural Disorder and Diffusion Path of Oxide Ions in a
Doped Pr
2
NiO
4
-Based Mixed Ionic-Electronic Conductor
(Pr
0.9
La
0.1
)
2
(Ni
0.74
Cu
0.21
Ga
0.05
)O
4+d
with a K
2
NiF
4
-Type
Structure [15]
6.7.1 Introduction
A
2
BO
4
-based oxides with K
2
NiF
4
-type structure have extensively been stu-
died as new mixed ionic-electronic conductors [23, 70–80], where A and B are
6 Perovskite-Type Oxides and Related Materials 137
