Ishihara T. (Ed.) Perovskite Oxide for Solid Oxide Fuel Cells (Fuel Cells and Hydrogen Energy)
Подождите немного. Документ загружается.

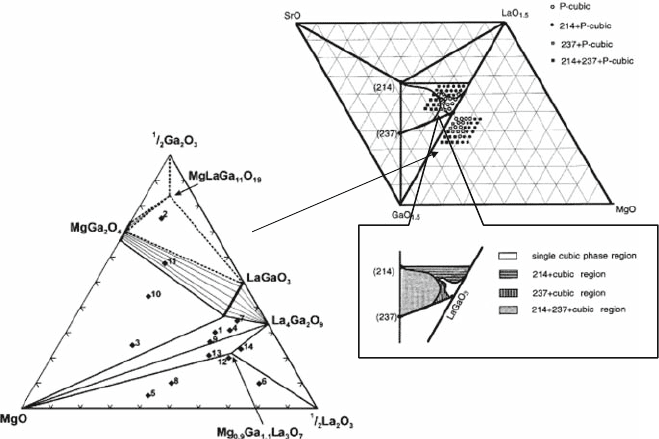
4.5 Basic Properties of the LSGM Electrolyte System
4.5.1 Phase Diagram of La-Sr-Ga-Mg-O
The phase diagram of the quaternary system LaO
1.5
–SrO–GaO
1.5
–MgO has
been reported for LaGaO
3
-based oxides (Fig. 4.12) [18, 25, 26]. Several impur-
ity phases, i.e., LaSrGa
3
O
7
(237), LaSrGaO
4
(214) phases, are reported for this
LaGaO
3
perovskite oxide, and the single, two-phase, and three-phase regions
appeared in phase diagrams. However, no phase containing Mg was found in
the compositional range of Fig. 4.12, which implies a higher so lubility of Mg in
the perovskite phase and related compound. As already discussed in a previous
section, doping with Mg is also effective for expanding the solubility of Sr in the
La site and expands the perovskite regions compared to the non-doped La
2
O
3
–
Ga
2
O
3
binary phase diagrams; thus, doping Mg is effective not only for the
introduction of vacancies but also in expanding the perovskite regions.
4.5.2 Reactivity with SOFC Component
The reactivity of this LaGaO
3
oxide has been also investigated by several
groups. The reactivity of LaGaO
3
-based oxide [27] with La(Sr)CoO
3
perovskite
oxide or a Pt electrode [28] is important. In particular , platinum seems to react
Fig. 4.12 Phase diagram of pseudo-quaternary LaO
1.5
–SrO–GaO
1.5
–MgO system
4 Oxide Ion Conductivity in Perovskite Oxide for SOFC Electrolyte 77
easily with gallium oxide to redu ce Ga
3þ
to Ga
þ
to form Ga
2
O, which is volatile
[28]. Therefore, for the practical application of this material to the SOFC, one
should pay attention to the choice of the electrode mate rial and/or its condi-
tions of use, such as temperature or atmosphere. Another undesirable reaction
of LSGM for the electrolyte of a SOFC is that with an Ni-based anode [27].
Because LaNiO
3
is one of the typical perovskite oxides, Ni is easily substituted
with Ga on the B site of the perovskite phase to form a highly resistive phase
between the LSGM electrolyte and NiO anode during cell preparation [29]. To
prevent the reaction between the components, man y buffer layers are used for
the current SOFC, even for the case of YSZ electrolyte. In the case of the LSGM
perovskite electrolyte, it is reported that CeO
2
doped with La (LDC) shows low
reactivity when the amount of La is in a narrow range, around 40 mol%.
Therefore, by insertion of an LDC layer between an NiO anode and an
LSGM electrolyte, a SOFC with high power density can be achieved [30]. It is
also reported that insertion of an LDC layer is effective for preparing an LSGM
thin film by a conventional method such as slip casting. However, sintering
LDC is rather difficult, and also the electrical conductivity of this compound is
low. Therefore, this LDC buffer layer makes a significant contribution to the
total resistance of the cell. Further suitable buffer layer compound for LSGM
electrolyte is still needed to prevent the reaction between NiO and LSGM. One
of the useful compounds is Sm-doped CeO
2
(SDM), which also exhibits high
oxide ion conduction.
In contrast, the reactivity of the LSGM electrolyte with the cathode perovs-
kite oxide is not extensive. When YSZ is used for the electrolyte, Co-based
perovskite oxides such as LaCoO
3
cannot be used, in spite of the high surface
activity to oxygen dissociation; this is because the reaction between YSZ and
LaCoO
3
forms the La
2
Zr
2
O
7
pyrochlore phase with high resistivity [31]. How-
ever, in case of LSGM electrolyte, compatibility with LaCoO
3
is high enough to
use it as a cathode of SOFCs. Horita et al. report ed that no reaction products
were observed after a reasonably long period of operation [32]. As a result, Co-
based perovskites can be used as cathodes with low values of cathodic over-
potentials. High compatibility with Co-based perovskites is one of the main
advantages of LaGaO
3
perovskites as the electrolyte of SOFCs. It is also noted
that La
2
NiO
4
has low reactivity toward LSGM; however, in the case of
LaMnO
3
, which is the most popular cathode material, some interactions
between La
2
NiO
4
and LaMnO
3
were observed with the formation of the
insulator layer.
4.5.3 Thermal Expansion Behavior and Other Properties
Thermal expansion is another important property for the application of mate-
rials to SOFC. The thermal expansion increased with the increa se in the dopant
concentration. Anomalies in thermal expansion behavior for LaGaO
3
and
78 T. Ishihara

La
0.9
Sr
0.1
GaO
3
were observed around 400 K and were assigned to the phase
transition from orthorhombic to rhombohedral structure. On the other hand,
Sr- and Mg-doped materials show a monotonic expansion; the average thermal
expansion coefficient measured was around 11.5 10
6
K
1
within the tem-
perature range from 298 to 1273 K. Therefore, the average thermal expansion
coefficient is slightly larger than but close to that of Y
2
O
3
-stabilized ZrO
2
.
Thermal conductivity of this material has also been studied. Table 4.1
summarizes the thermal conductivity, specific heat, and fracture strength of
LaGaO
3
perovskite. The average thermal conductivity of this LSGM is slightly
smaller than that of YSZ. Therefore, SOFCs using LaGaO
3
-based perovskites
are more desirable from the point of view of uniform temperature distribution.
4.5.4 Behavior of Minor Carrier
Since the concentration of the minor carriers (electrons and/or holes) deter-
mines the chemical leakage of oxygen when oxide ion conductor is used as the
electrolyte in SOFCs [33], analysis of the performance of electron and hole
conduction is an important subject for the electrolyte materials. Partial electro-
nic conduction is commonly analyzed by the ion blocking method, the so-called
Wagner polarization method. Partial electronic conductivity is the sum of
electronic and hole contribution to the total conductivity, and each conductiv-
ity is proportional to a carrier density. Therefore, the total electronic conduc-
tivity can be expressed as follows:
s ¼ ILF=RT ¼ s
n
þ s
p
¼ n
0
f1 expð FEðLÞ=RTÞg
þsp
0
fexpðFEðLÞ=RTÞ1g (4:3)
Here, I, L, E(L),F, R, and T are current, length of the sample, applied
voltage, the Faraday constant, the gas constant, and temperature, respectively.
When the hole cond uction is dominant, the second term in the above equation is
dominant and the current increases exponentially with applied potential. Since
the predominant charge carriers change from holes to electrons with decreasing
P
O2
and p–n transition occurs at intermediate P
O2
, current, I, shows a typical
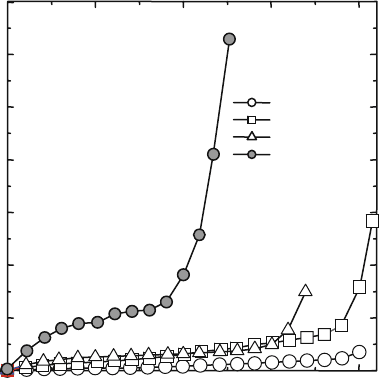
‘‘S’’-shaped curve against potential, E(L). Figure 4.13 shows the typical I–E(L)
curve observed in Ni-doped LSGM by the ion blocking method [34].
Table 4.1 Thermal properties of LaGaO
3
oxide
Temperature
(K)
Specific heat
(J/g k)
Thermal conductivity
(W/m K)
Fracture
strength (MPa)
298 0.410 1.55 220
673 0.464 1.55 180
1073 0.556 1.77 136
4 Oxide Ion Conductivity in Perovskite Oxide for SOFC Electrolyte 79
Differential of the observed current with respect to potential, which corre-
sponds to the P
O2
in the sample, gives the dependence of the partial electronic
conductivity on P
O2
.
Determination of hole and electron conductivities and transport numbers of
oxide ion in LaGaO
3
-based oxides were performed by the polarization method
by Baker et al. [21], Yamaji et al. [35], and Kim and Yoo [36]. Kim et al. reported
that P
O2
dependence of hole and electron conductivity is proportional to P
O2
1/4
and P
O2
1/4
, respectively, and well obeys the Hebb–Wagner theory. The results
of the polarization method clearly indicate that LaGaO
3
-based oxides exhibit
almost pure oxide ion conductivity over a wide oxygen partial pressure range
(10
5
> P
O2
> 10
25
atm). Compared with CeO
2
-based oxides or Bi
2
O
3
oxide,
this is a major advantage of the LaGaO
3
-based oxides as well as the redox
stability comparable to that of ZrO
2
-based oxide. Consequently, LaGaO
3
-
based oxides are highly promising as an electrolyte for SOFCs, particularly
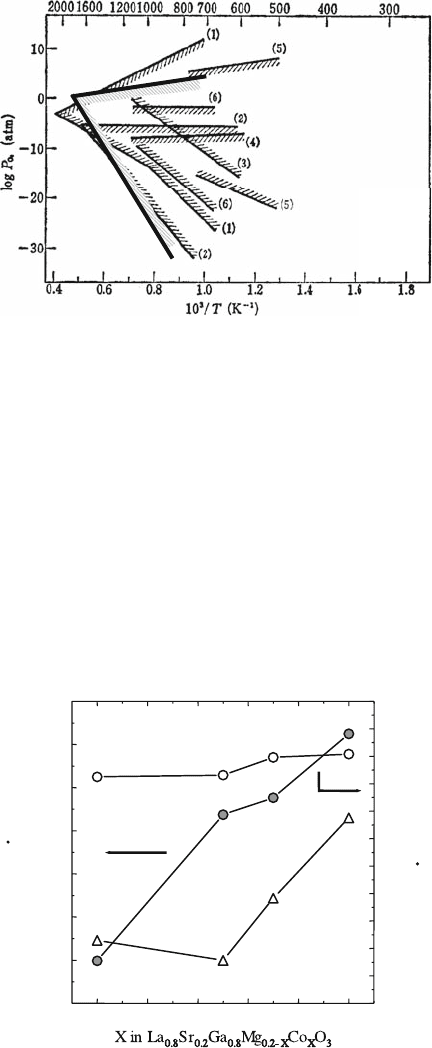
when compared with ceria-based oxides. Kim et al. [36] also investigated the
temperature dependence of hole and electronic conductivity in Mg-doped
gallate, La
0.9
Sr
0.1
Ga
0.8
Mg
0.2
O
3
, with the polarization method. Figure 4.14
shows the evaluated boundaries of the electrolytic domain in La
0.9
Sr
0.1-
Ga
0.8
Mg
0.2
O
3
plotted in the axis of log (P
O2
/atm) versus reciprocal tempera-
ture. The lower boundary of the electrolytic domain (defined as t
ion
> 0.99) for
LSGM is 10
23
atm at 1273 K. This pressure is even lower than that of CaO-
stabilized ZrO
2
and that of YSZ, which is also plotted in Fig. 4.14. Conse-
quently, it is clear that the electrolytic domain covers the P
O2
range required for
the operation of SOFCs and that the LSGM can be successfully used as
electrolyte in SOFCs.
0 500 1000 1500 2000
0
5
10
15
20
25
30
35
600°C
700°C
800°C
900°C
le(mA)
V(mV)
Fig. 4.13 Typical I–E (L)
curves observed in Ni-doped
LSGM by the ion blocking
method
80 T. Ishihara
On the other hand, doping by a small amount of Co is effective in increasing
the electrical conductivity, as discussed in Section 4.2. However, with the increas-
ing Co concentration, the partial hole conductivity also increases. As a result,
hole, electron, and oxide ion conductivities in Co-doped LSGM have also been
studied by the polarization method [34], and the estimated conductivities at
1073 K as a function of Co content are shown in Fig. 4.15. It is seen that both
the electronic and hole conductivities become significant with the increasing Co
concentration; however, at Co amount less than 5 mol% on the Ga site, the oxide
ion conductivity is much higher than partial hole conductivity. Furthermore, the
oxide ion conductivity also increases as Co amount increases. Although the hole
conduction becomes significant and the oxide becomes a mixed electronic and
ionic conductor when the amount of Co is excess, doping Co is preferable for
improving the oxide ion conductivity in LaGaO
3
-based oxide.
(7)
(7)
CaO-ZrO
2
2) Y
1)
2
O
3
-ThO
2
3) (Y
2
O
3
)
0.05
(CeO
2
)
0.95
4) (CaO)
0.15
(La
2
O
3
)
0.85
5) (Y
2
O
3
)
0.27
(Bi
2
O
3
)
0.73
6) (Y
2
O
3
)
0.5
(TiO
2
)
0.5
7) La
0.9
Sr
0.1
Ga
0.8
Mg
0.2
O
3
Temperature /°C
Fig. 4.14 Boundaries of the
electrolytic domain of
La
0.9
Sr
0.1
Ga
0.8
Mg
0.2
O
3
in
the plane of log (P
O2
/atm)
versus reciprocal
temperature
–0.55
0.00 0.02 0.04 0.06 0.08 0.10
– 0.90
–0.85
–0.80
–0.75
–0.70
–0.65
–0.60
log(σ
i
/S cm
–1
)
–11
–10
–9
–8
–7
–6
–5
–4
–3
–2
–1
0
1073K
σ
e
σ
i
σ
h
Po
2
=1atm
log(σ
h
σ
e
/Scm
–1
)
Fig. 4.15 Estimated ionic
and electronic conductivity
in LSGM at 1073 K as a
function of Co content
under P
O2
¼ 10
5
atm
4 Oxide Ion Conductivity in Perovskite Oxide for SOFC Electrolyte 81
4.5.5 Diffusivity of Oxide Ion
The diffusiv ity of ox ide ions in LSGM was further studied by
18
O tracer
diffusion measurements [37]. Diffusion of oxide ion in perovski te oxide is
explained in detail in Chapter 5. LSGM exhibits large values of diffusion
coefficient, and the observed fast diffusion in LSGM originates from the higher
mobility of oxide ions in the perovskite structure as compared with the fluorite
structure (Table 4.2), presumably due to a large free volume in the lattice.
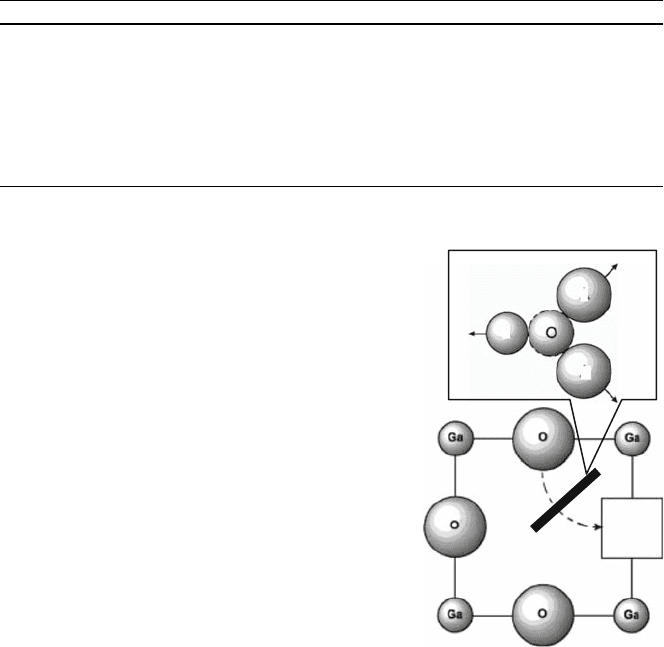
Recently, atomic simulation of the oxygen transport in the perovskites, in
particular, LaGaO
3
, were performed based on quantum chemistry [38, 39]. In the
case of perovskite oxides, the migrating oxygen ion must pass through the
triangular orifice defined by two A-site (La
3þ
) ions and one B-site ion. As a result
of lattice relaxation during oxygen ion migration, it was suggested that there is a
small deviation from the direct path for oxygen migration, as illustrated schema-
tically in Fig. 4.16. Indeed the calculations predicted a curved path around the
Table 4.2 Comparison of mobility of oxide ion in selected fluorite and LSGM oxide at
1073 K (Cited from Ref. 54)
Dt /em
2
/s Ea /eV d ½V
o
/cm
3
Dcm
2
/s m cm
2
/Vs
Zr
0.81
Y
0.19
O
2d
6.2x10
8
1.0 0.10 2.95x10
21
1.31x10
6
1.41x10
5
Zr
0.858
Ca
0.142
O
2d
7.54x10
9
1.53 0.142 4.19x10
21
1.06x10
7
1.15x10
6
Zr
0.85
Ca
0.15
O
2d
1.87x10
8
1.22 0.15 4.43x10
21
2.49x10
7
2.69x10
6
Ce
0.9
Gd
0.1
O
2d
2.70x10
8
0.9 0.05 1.26x10
21
1.08x10
6
1.17x10
5
La
0.9
Sr
0.1
Ga
0.8
Mg
0.2
O
3d
3.24x10
7
0.74 0.15 2.53x10
21
6.4x10
6
6.93x10
5
La
0.8
Sr
0.2
Ga
0.8
Mg
0.2
O
3d
4.13x10
7
0.63 0.20 3.34x10
21
6.12x10
6
6.62x10
5
La
0.8
Sr
0.2
Ga
0.8
Mg
0.125
4.50x10
7
0.42 0.1645 2.78x10
21
8.21x10
6
8.89x10
5
Co
0.085
O
3d
D
t
: Tracer diffusion coefficient, D: Self diffusion coefficient
Ga
La
La
Fig. 4.16 Calculated path
for oxygen vacancy
migration. h: Vacancy
82 T. Ishihara
octahedron edge with the saddle point located away from the adjacent B-site
cation. Similar experimental results are discussed in Chapter 6. Further detailed
molecular dynamic calculations were also done for the LSGM system. Figure 4.17
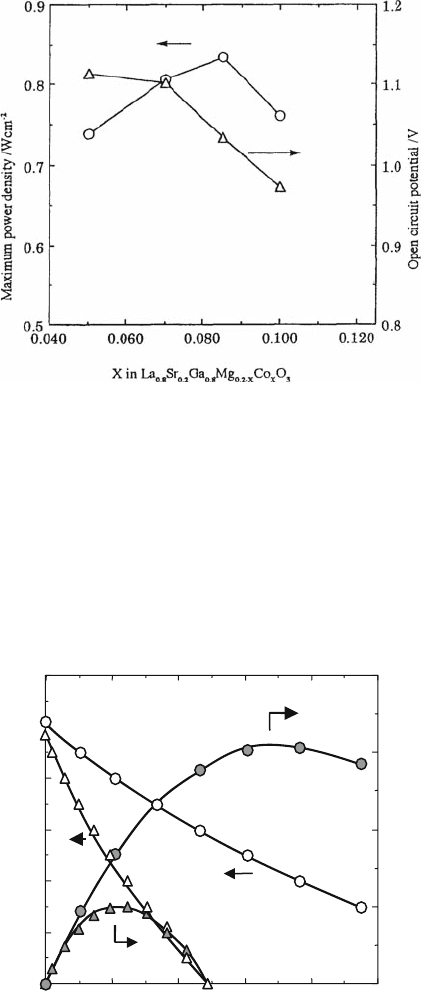
shows the calculated mean square displacement of constituent atoms as a func-
tion of time. It is evident that the mean displacement between O–O ions expanded
with time; however, the positions of other ions remain essentially constant, which
suggests only diffusion of oxygen but not of cations in LSGM. The diffusion
coefficients were calculated according to the random walk theory by using the
slope in Fig. 4.17, and the results are compared in Fig. 4.18 with the diffusion
O-O
Sr-Sr
La-La
Ga-Ga
Mg-Mg
0.0
1.0
2.0
3.0
4.0
5.0
0.40
0.30
0.20
0.10
0.0
Time /
p
s
Mean square displacement /
2
Α
°
Fig. 4.17 Mean
displacement of constituent
atoms as a function of time
in LSGM
0.8 0.9 1.0 1.1 1.2 1.3
10
–6
10
–7
10
–8
from Conductivity
from Tracer diffusion
from MD Calculation
Diffusion constant /cm
2
s
–1
1000/T /K
–1
Fig. 4.18 Comparison of diffusion coefficient in LSGM estimated from ionic conductivity
and tracer diffusion measurements and calculated by molecular dynamic calculations
4 Oxide Ion Conductivity in Perovskite Oxide for SOFC Electrolyte 83

coefficients measured during the tracer diffusion experiments. A good agreement
of the values of oxygen diffusion coefficients estimated from the results of the
computer simulation and experimentally measured in tracer diffusion measure-
ments is observed, suggesting that the mobile oxygen vacancies and substituted
ions in this oxide behave as ideal noninteracting point defects.
Furthermore, the defect binding energy in LaGaO
3
was also calculated by
Islam et al. [38, 39 ], and Table 4.3 summarizes the calculated cluster binding
energies (E
b
) for some selected dopants on the La and Ga sites. The low binding
energy calculated for the Sr substitution on the La site could be assigned to the
similarity in ionic size of Sr with that of La, leading to smaller local stresses in
the lattice. In contrast, rather large cluster binding energies of about 0.9 eV are
estimated for B-site dopants, suggesting that the oxygen vacancies tend to be
trapped around dopants in the Ga site. Although dopants on the Ga sites are
effective for expanding the unit cell volume and improving the solubility of Sr
on the La site, observed binding of oxygen vacancies may de crease the ox ygen
vacancy diffusivity. The results of computer calculations presented in Table 4.3
suggest that doping of Cu on the Ga site has the smallest binding energy;
however, the electrical conductivity of LSGM decreased when doped with
Cu
2þ
, presumably because of the low chemical stability of Cu
2þ
. Consequently,
from the aspect of chemical stability, Co
2þ
/Co
3þ
doping is more desirable
experimentally. In any case, due to the high crystal symmetry, it is evident
that the perovskites are interesting subjects for a computer simulation of ion
conductivity, in particular, oxygen ion diffusivity.
4.6 Performance of a Single Cell Using LSGM Electrolyte
The applications of LSGM as the electrolyte in fuel cells are now commonly
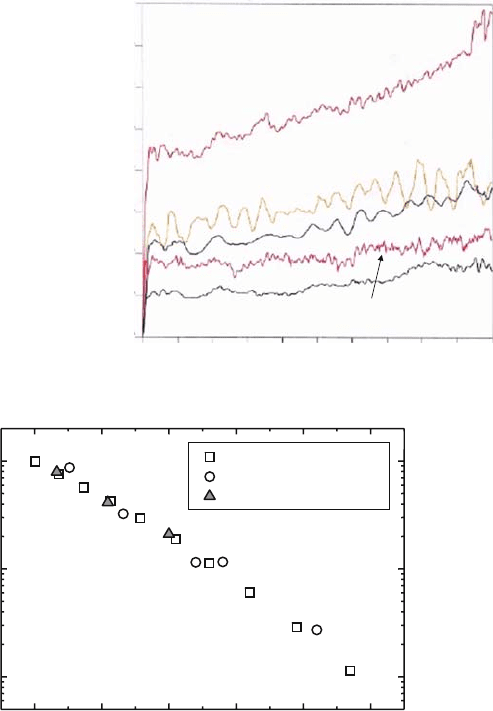
investigated. Figure 4.19 shows the temperature dependence of the maximum
power density and the open circuit potential (OCV) of the cell with Sm
0.5
Sr
0.5
CoO
3
cathode and Ni anode [40]. It is seen that open circuit potential (OCV)
increased with the decrease in the operating temperature; the results are in good
Table 4.3 Calculated cluster binding energies (E
b
) for some selected dopant on the La and Ga
sites in LaGaO
3
Location Cluster pair Cluster binding energy (E
b
/eV/defect)
La site Sr
2þ
-oxygen vacancy –0.01
Ca
2þ
-oxygen vacancy 0.10
Ga site Mg
2þ
-oxygen vacancy 0.90
Co
2þ
-oxygen vacancy 0.87
Ni
2þ
-oxygen vacancy 0.91
Cu
2þ
-oxygen vacancy 0.65
84 T. Ishihara
agreement with the theoretical values estimated from the Nernst equation.
Furthermore, the power density was improved by using Sm
0.5
Sr
0.5
CoO
3
as
cathode at all temperatures studied, comparing with that of LaCoO
3
-based
conventional cathode. The maximum power density was higher than 1.0 and
0.1 W/cm
2
at 1273 and 873 K, respectively, in spite of the 0.5-mm thickness of the
electrolyte [40]. In comparison with the power densities of the cell utilizing YSZ
electrolyte, a reasonably high power density was achieved at 873 K. Goodenough
and coworkers also investigated the application of LaGaO
3
-based oxides as the
electrolyte in fuel cells [41]. Similar large values of power density were reported at
intermediate temperatures with La
0.6
Sr
0.4
CoO
3
cathode and Ni-La-doped CeO
2
cermet anode [42]. Due to high power density, LSGM has been attracting much
interest as a promising SOFC electrolyte for intermediate temperature SOFC.
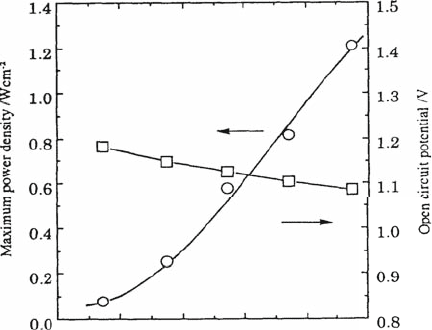
Oxide ion conductivity in LSGM is further improved by doping Co to Ga
site, albeit the hole conductivity increases. Figure 4.20 shows the open circuit
potential as well as the maximum power density at 1073 K as a function of Co
content in LaGaO
3
-based oxide electrolyte [40]. The open circuit potential (OCV)
decreases monotonically with increasing Co concentration. In particular, the
decrease in OCV is significant when the Co content on the Ga site is higher
than 10 mol%; this is caused by the increased hole conductivity due to electronic
compensation of doped Co ions. The dependence of OCV on the amount of
doped Co is in good agreement with that of the transport number of oxide ion
(see Fig. 4.10). On the other hand, the power density increases with increasing Co
content and attains a maximum value when 8.5 mol% Co is doped on the Ga
sites. Improvement in the power density is simply explained by the enhanced
oxide ion conductivity resulting from Co doping. When the amount of doped Co
further increases, the hole conduction becomes significant, short-circuiting the
900 1000 1100 1200
Operating temperature /K
2 2 0.9 0.1 0.8 0.2 3 0.5 0.5 3
H +3vol%H O,Ni/La Sr Ga Mg O /Sm Sr CoO , O
2
Fig. 4.19 Temperature
dependence of the maximum
power density and the open
circuit potential (OCV) of
the cell with Sm
0.5
Sr
0.5
CoO
3
cathode o and Ni anode.
Thickness of electrolyte was
0.5 mm
4 Oxide Ion Conductivity in Perovskite Oxide for SOFC Electrolyte 85
cell and decreasing the power density. Consequently, the maximum power den-
sity is obtained when 8.5 mol% Co-doped LSGM electrolyte is used.
On the other hand, it is expected that the power density of the cell i ncreased
with decreasing thickness of the electrolyte, since the main internal resistance
is due to IR losses. Figure 4.21 shows the power density of a n H
2
-O
2
cell with
0.18-mm-thick LSGMC-8.5 electrolyte at 1073 and 873 K. As expected, the
power density of the cell increases with decreasing the thickness of the
LSGMC electrolyte. However, the open circuit potential exhibited a tendency
1073K
H
2
+3vol%H
2
O,Ni/La
0.8
Sr
0.2
Ga
0.8
Mg
0.2-X
Co
X
O
3
/Sm
0.5
Sr
0.5
CoO
3
, O
2
(0.5mm thickness)
Fig. 4.20 Open circuit
potential as well as the
maximum power density at
1073 K as a function of Co
content in LaGaO
3
-based
oxide electrolyte
54321410
0.0
0.5
1.0
1.5
2.0
4
0.0
0.2
0.4
0.6
0.8
1.0
1.2
Terminal voltage /V
Current density /A cm
–2
Power density /W cm
–2
1073K
873K
0.183mm thickness
Fig. 4.21 Power-generating
property of H
2
-O
2
cell at
1073 and 873 K using 0.18-
mm thickness LSGMC-8.5
as the electrolyte
86 T. Ishihara
