Ishihara T. (Ed.) Perovskite Oxide for Solid Oxide Fuel Cells (Fuel Cells and Hydrogen Energy)
Подождите немного. Документ загружается.

and protons are the representative conducting ions in perovskite oxide. Also,
the lithium ion is mobile in some perovskite-type oxides. In addition, several
non-oxide perovskite compounds such as halides are known to be ionic
conductors. Furthermore, some kinds of antiperovskite non-oxide compounds
are good silver ion conductors. Regarding oxide ion conduction and proton
conduction, detailed descriptions are given in Chapters 4 and 11, respectively.
In this chapter, an outline of ionic conduction in perovskite compounds and a
brief history of the resear ch in this field are described , introducing some
examples of the studies.
3.2 Conduction Behavior of Perovskite-Type Compounds
Ionic species that contribute to high conductivity of perovskite-type compounds
are rather limited. They are listed in Table 3.1 with representative compounds,
their conductivities, and distinctive features. The mobile ionic species, except
hydrogen, are host components of the compounds, whereas protons are unique
in that they are incorporated from water vapor or hydrogen gas in the ambient
atmosphere at elevated temperature. Of these, oxide ion conductors are best
known, and oxide ionic conduction in various kinds of perovskite and perovskite-
related oxides has been studied.
Confirmation of ionic conduction in the electrically conductive oxides can be
made in different ways. One of the most convenient methods is to examine the
electromotive force (emf ) of an electrochemical oxygen concentration cell:
Pt; O
2
ðP
O2
ð1ÞÞ=oxide specimen=O
2
ðP
O2
ð2ÞÞ; Pt cell ½1
using the oxide sample as an electrolyte diaphragm at elevated temperature.
The concept of the oxygen concentration cell is schematically shown in Fig. 3.1.
Table 3.1 Examples of ionic conduction in perovskite-type compounds
Mobile
ions
Examples of ionic
conductor
Conductivity /
Scm
1
(at8C) Remarks
O
2
La
0.9
Sr
0.1
Ga
0.8
Mg
0.2
O
2.85
1.5x10
1
(8008C) Doped single perovskite oxide
H
+
SrCe
0.95
Yb
0.05
O
3a
1x10
2
(9008C) Doped Single perovskite oxide
under hydrogen-containing
atmosphere
Li
+
La
0.51
Li
0.34
TiO
2.94
1.4x10
3
(278C) Host oxide: La
2/3
TiO
3
A-site
deficient perovskite
Cl
CsPbCl
3
1.2x10
3
(5008C) Non-oxide perovskite
Br
CsPbBr
3
8x10
4
(5008C) Non-oxide perovskite
Ag
+
Ag
3
SI 1 x 10
2
(258C) Anti-perovskite-type
structure. Non-oxide
anti-perovskite Averaged
structure for Ag
46 H. Iwahara

If the observed emf is close to the theoretical value E
o
calculated from Nernst’s
equation given by Eq. (3.1), the oxide can be regarded as an ionic conductor.
E
0
¼
RT
4F
ln
P
O2
1ðÞ
P
O2
2ðÞ
(3:1)
If no emf is observed, the charge carriers in the oxide would be electrons or
electron holes. When emf is not zero but smaller than E
o
in Eq. (3.1), conduc-
tion in the oxide would be partially ionic and partially electronic. However,
readers sho uld note that this method does not give any information about
which ions in the oxide are mobile and that the rati o of observed to theoretical
emf, E/E
o
, does not always give the correct value of ionic transp ort number. To
determine which ions are mobile, additional experiments are necessary, such as
electrochemical mass transport measurement or tracer technique.
Transport behavior of mobile ions in perovskite-type compounds has been
investigated by many researchers using various experimental methods and
computer modeling. It is acceptable that the transport mechanism of mobile
host ions in the perovskite is based on hopping via vacant sites of the ionic
species. In that case, tolerance factor t and free vo lume v of the crystal are
thought to be important factors affecting the mobility of the ions. The toler ance
factor is a measure of symmetry of the crystal structure of the perovskite and is
written as follows:
t ¼
r
A
þ r
X
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
2 r
B
þ r
X
ðÞ
p
(3:2)
where r
A
, r
B
, and r
X
are ionic radii of A, B, and X ions, respectively, in ABX3.
Empirically and qualitatively, good ionic conductors of perovskite compounds
Ion conductor
P
O2
(1) O
2
O
2
O
2
O
2
P
O2
(2)
P
O2
(1) > P
O2
(2)
+
E
ದ
Electromotive force
Porous electrodePorous electrode
Fig. 3.1 Concept of an
electrochemical oxygen
concentration cell
3 Ionic Conduction in Perovskite-Type Compounds 47
have a relatively large value of t. The free volume is a measure of degree of
packing in a unit cell, which is given by the difference between measured unit
cell volume and the sum of ionic volume in the unit cell calculated from known
ionic radii. In many cases, the free volume of the good ionic conductors is large,
although quantitative studies have not yet been completed. Of course, other
factors such as the concentration of vacancy, ionic radius, and polarizability are
also important for good ionic conduction. Detailed discussions are presented in
the following chapters.
As described in the Introduction section, many perovskite-type compounds
can deviate from their stoichiometric composition to a considerable extent
because of the strong stability of the perovskite-type structure. This deviation
results in the formation of electronic defects such as excess electrons or electron
holes, which cause n-type or p-type electronic conduction. Thus, it should be
noted that, in general, perovskite-type compounds are have facile electronic
conduction, and that the ionic conduction is often accompanied by electronic
conduction. As the concentration of the electronic defects depends on the
deviation of A/B and/or (A + B)/X from their stoichiometric ratio, the content
of impur ity, atmosphere and temperature, and contribution of electronic con-
duction varies with tho se conditions.
In general, electronic conductivity of an oxide electrolyte at elevated
temperature is influenced by partial pressure of oxygen, P
O2
, in the atmosphe re;
n-type electronic conductivity s
n
increases with decreasing P
O2
, whereas p-type
electronic conductiv ity s
p
increases with increasing P
O2
. It is known that P
O2
dependence of s
n
or s
p
is given by the following:
s
n
¼ s
o
n
exp P
1=n
O2
(3:3)
and
s
p
¼ s
o
p
exp P
1=n
O2
(3:4)
where n is some natural number, and s
n
˚
and s
p
˚
are the constants that are
independent of the partial pressure of oxygen [1]. It is accepted that the ionic
conductivity s
i
itself is independent of P
O2
for many oxide electrolytes. Accord-
ingly, the total conductivity s is expressed as follows:
s ¼ s
i
þ s
o
n
exp P
1=n
O2
þ s
o
p
exp P
1=n
O2
(3:5)
The relationship between each logarithm of conductivity and logarithm of
P
O2
is illustrated in Fig. 3.2. The hatched regions indicate the mixed conduction
domains, and between them there exists an ionic conduction domain where
electronic conductivity is negligibly low. The outer sides of the mixed conduction
domains are electronic conduction regions. An example of the experimental
48 H. Iwahara

results on s P
O2
plots is shown in the following section for oxide ion–electron
mixed conductors.
3.3 Early Studies on Ionic Conduction in Perovskite-Type Oxides
Most of the possible combinations of large A cations and smaller B ions, which
is needed to form perovskite-type oxides ABO
3
, had been tried by 1955, as
described by F.S. Galasso in his famous book [2] entitled Structure, Properties
and Preparation of Perovskite-Type Compounds, published in 1969. This book
compiled almost all available data at that time concerning structure, properties,
and preparation of perovskite-type compounds. In this book, although lattice
defects in the perovskite-type crystal were described, the author did not touch
on ionic conduction in the perovskite except for a very brief description of
BaTiO
3
. However, in the 1960s, several pioneering studies on ionic conduction
in perovskite-type oxides were performed.
Ionic conduction in perovskite-type oxides was first a source of interest in
ferroelectric materials. S. Swanson showed that DC conductivity of BaTiO
3
ceramics was significantly influenced by their fabrication history, which suggests
that there would be an intimate relationship between the solid-state reactions of
raw materials and ionic conduction [3]. In the 1960s, when research and devel-
opment of perovskite-type oxides as a dielectric or ferroelectric material such as
BaTiO
3
and PbTi
1x
Zr
x
O
3
had become active, some of the researchers paid
attention to the conduction behavior of these perovskite-type oxides. They
log σ
log P
O2
Ionic domain
Mixed
cond.
domain
σ
p
σ
i
σ
n
Mixed
cond.
domain
Mixed
cond.
domain
Fig. 3.2 Dependence of
conductivity on partial
pressure of oxygen
3 Ionic Conduction in Perovskite-Type Compounds 49
considered that ionic conduction in the ferroelectric materials would be affected
by their manufacturing and the characteristics of pyroelectric properties [4, 5].
In 1961, Stephenson and Flanagan thought that the anomalous pyroelectric
behavior revealed in lead zirconate titanate (PZT) would probably be caused by
ionic conduction in the oxides [5]. To test the existence of ionic conduction in
this oxide, they adopted the electrochemical oxygen concentration cell method
that had been reported by Kuikkola and Wagner [6] 4 years previously for
stabilized zirconias and which subsequently became a very familiar method to
solid-state electrochemists.
Stephenson and Flanagan constructed the concentration cell using PZT as a
diaphragm of two oxygen electrode chambers.
Pt; Pb; PbO=PbZ
r0:53
Ti
0:47
O
3
=Cu; Cu
2
O=Pt Cell ½2
The electromotive force (emf) of this cell in purified nitrogen gas has been
measured. The observed emf was 0.205 0.005 V at 2508C, which is close to the
theoretical value calculated from Nernst’s equation given in Eq. (3.1), where
P
O2
(1) and P
O2
(2) were thermodynamic oxygen partial pressures in each gas
chamber. This result means that conduction was purely ionic, as described in
the previous section. The authors reported that there was no conclusive evi-
dence as to whether the ionic conduction is caused by a cation or an anion
migration. However, from the observed cell behavior, the authors considered
that a t least some of the conduction is contributed by oxygen ion migration.
Heckman et al. [7] studied the conduction properties of polycrystalline lead
zirconate titanates Pb(Zr
x
Ti
1x
)O
3
+ 1 w% Nb
2
O
5
, constructing electroche-
mical oxygen concentration cells
Pt ð1:0 atm O
2
Þ = PTZ = Pt ð0:01 atm O
2
ÞCell ½3
in the temperature range from room temperature (r.t.) to 6008C, and they
published the results 2 years after Stephenson’s report. The cells sh owed a
stable emf, suggesting that the specimens exhibit ionic conduction. However,
in contrast to Stephenson’s report that the conduction was entirely ionic, the
results indicated that the conduction was partly assigned to an ionic state and
partly an electronic one, which is dependent on temperature and the composi-
tion of specimens. The difference in the ionic contribution in the conduction
between the two experiments would be caused by the differences in the sample
composition and the condition of oxygen concentration cells. Stephenson’s cell
[2] had a low er average partial pressure of oxygen than that of Heckman’s cell
[3]. In any case, the absolute values of ionic conductivity are low; the specific
resistivity of 1 w% Nb
2
O
5
-contained Pb(Zr
x
Ti
1x
)O
3
is reported to be 5 10
8
to 1 10
10
ohm cm at 3008C.
Subsequently, Ezis et al. studied the dependence of the transport number of
ions on composition x in Pb(Zr
x
Ti
1x
)O
3
in detail using an electrochemical
oxygen concentration cell [8]. Their experiment showed that the transport
50 H. Iwahara
number decreased with increasing value of x over the range 0.05 < x < 0.35
and that electronic conduction became dominant above x ¼0.40. Ezis et al.
considered that the reduction of T
i
to the trivalent state might be a mechanism
for defect formation, which tended to counter the effects of Nb
5+
included in all
specimens investigated.
In addition to the studies on PZT, Glower et al. examined the ionic conduc-
tion in a ferroelectric material ,Ca
0.1
Ba
0.9
TiO
3
, by means of the same method as
that used for PTZ [9]. They showed that the conduction in this oxide is ionic
below 3008C, but electronic conduction appears sharply near 3008Cand
becomes predominant above 5008C. They identified the mobile ions as calcium
ions by using so-called activation analysis, which was a kind of irradiation
analysis of the surface of the solid after passing DC current across the solid
conductor.
An important study of charge carrier in a typical ferroelectric perovskite-type
oxide BaTiO
3
was published in 1964 by Glower and Heckman [10]. The title
of the paper ‘‘Conduction – Ionic or Electronic in BaTiO
3
’’ was attractive for
materials researchers in those days. In their paper, they reported about conduc-
tion species in barium titanate as follows: ‘‘Usual practice of authors has been to
make the tacit assumption that conduction is exclusively electronic. There is no
priori assurance that this is true.’’ Also, they wrote ‘‘ In fact, to our knowledge,
no one has to date addressed himself to the primary problem of transport,
i.e., does conduction occur via the motion of electrons or of ions?’’ The purpose
of their study was to answer this essential question from experimental results.
They applied Cell [1] to BaTiO
3
specimens for different oxygen partial pressures
p
1
and p
2
:
Pt; O
2
ðp
1
Þ = BaTiO
3
specimen = O
2
ðP
2
Þ; Pt Cell ½4
The cell using a pure single crystal showed no significant emf in an y cell
examined, indicating that its conduction was not ionic but electronic. On the
other hand, the single crystal containing 0.1 at% of iron showed strong emf
dependency on temperature and oxygen partial pressures at two electrodes. The
values were less than the theoretical one calculated from Nersnt’s equation,
suggesting that the conduction was partially ionic. BaTiO
3
showed theoret ical
emf below 2508 C, indicating that the conduction was purely ionic but at 5408C,
emf of the concentration cell was far lower than the theoretical one, suggesting
that contribution of ionic conduction to total one is rather small. Thus, it was
clarified that the contribution of ionic conduction in BaTiO
3
depends on its
purity and morphology, single crystal or polycrystal ceramic. The conduction
in a pure single crystal is electronic and that in impure single crystal and
polycrystalline ceramic is partially ionic, but the ionic contribution decreases
with increasing temperature.
However, gas concentration cell experiments give essentially no information
about which kind of ion among the constituent ions is mobile in the solids. To
investigate this, Glower and Heckman have also applied the activation analysis
3 Ionic Conduction in Perovskite-Type Compounds 51
method to the surface of an iron-containing single crystal of BaTiO
3
before and
after applying DC voltage of 22 V/mil for 52 h. The result showed that the iron
ions were mobile and contributed to ionic conduction but that titanium was not
mobile.
These studies were not intended to seek a good ionic conductor but to
confirm the conduction species to clarify the phenomena characteristic to the
ferroelectric or pyroelectric materials. It should be noted that the conductivity
of ferroelectric materials mentioned above is very low and most of the research-
ers in those days took no notice of the value of conductivity itself. Studies on
highly conductive ionic conductors of perovskite-type compounds were started
in the second half of the 1960s to search for a good oxide ion-conducting
electrolyte for fuel cells and oxygen sensors. These are described in the following
sections.
3.4 Oxide Ion Conduction
When a cation A or B in a perovskite-type oxide ABO
3
is partially replaced by
a cation M of lower valence, it sometimes gives rise to a relatively large
number of oxygen ion vacancies in the lattice so as to maintain the electrical
neutrality of the crystal. Chemical composition of the oxide can be expressed as
A
1x
M
x
BO
3a
or AB
1x
M
x
O
3a
, where a is an average number of oxygen
deficiencies per unit formula. In such crystals, appreciable oxide ion conduction
may be expected at elevated temperatures if the energy needed for the oxygen
ions to jump from their original sites to adjacent vacant sites is not so high.
In this case, similarly to the case of stabilized zirconia, oxide ions can migrate
through the crystal lattice with the assistance of oxide ion vacancies.
There are several methods to confirm oxide ion conduction in the oxide
specimens. One of the most convenient methods is to examine the discharge
performance of the oxygen concentration cell. If the conduction in the sample is
oxygen ionic, a steady and stable current with a reasonable value can be taken
from the oxygen concentration cell, which is composed of the specimen oxide as
a solid electrolyte. Therefore, for example, one can regard the conduction as the
oxide ion conduction, if the oxygen concentration cell shown in Cell [1] gives
rise to stable emf and a steady and reasonable current can be taken from the cell.
Historically, oxygen ion conduction in the perovskite-type oxide was reported
for the first time by Stephenson and Flanagan [4], as described in the previous
section (3.2). They tried to construct a fuel cell using modification of oxygen
concentration cell shown in Cell [3]. In the experiment, PZT was used as an
electrolyte, and hydrogen gas and oxygen gas were supplied to each side of the
electrolyte separately. They observed a steady terminal voltage of 0.81 V at 3258C
and 0.41 V at 7008C. The current output was estimated to be a few mA/cm
2
.The
authors wrote as follows: ‘‘These results would indicate that at least some of the
conduction is by oxygen ion migration.’’ This study would historically be the first
52 H. Iwahara
experiments to confirm oxygen ion conduction in perovskite-type oxide and be
the first experiment of SOFC using a perovskite-type oxide as a solid electrolyte,
although their intention was not to develop a fuel cell and its solid electrolyte.
The research for developing the good ionic conductors with perovskite-type
structure was first considered by van Gool, who was known as the first to
propose a one-chamber solid oxide fuel cell. In 1965, he published a paper about
one-chamber fuel cells entitled ‘‘The possible use of surface migration in fuel
cells and heterogeneous catalysis’’ [11]. In this paper, he touched on the oxygen-
deficient perovskite-type oxides as a candidate for an oxide ion conductor
applicable to a fuel cell electrolyte. However, he thought that the perovskite
structure seemed to be less favorable because A and O in ABO
3
would make a
closed packing structure in which the ion migration might be difficult.
Studies on highly conductive ionic conductors of perovskite-type compounds
were started in the second half of the 1960s to find a superior electrolyte for fuel
cells and sensors. From the analogy of oxygen ion conduction in fluorite-type
oxides such as stabilized zirconias, it was thought that considerable concentration
of oxygen vacancy would be essential for high oxygen ion conductivity. The
present author and coworker have paid attention to the solid solution based on
LaAlO
3
that is composed of large-sized trivalent cation La and a small-sized
trivalent cation Al. In this oxide, calcium ions are partially substituted for
lanthanum ions and, as a result, oxygen ion vacancies are formed to compensate
charge neutrality in the crystal [12]; i.e., the composition is expressed as
La
1x
Ca
x
AlO
3a
. Having studied the behaviors of oxygen concentration cells
and fuel cells with La
1x
Ca
x
AlO
3a
(x = 0.1, 0.2, and 0.3) ceramics as a solid
electrolyte, they confirmed that the conduction was partly oxygen ionic and partly
electronic (due to electron holes) in air at elevated temperatures and that, under
the fuel cell condition, the conduction is predominantly oxide ionic [13]. The
CaTiO
3
can take aluminum to form a solid solution CaTi
1x
Al
x
O
3a
(x 0.5) in
which almost stoichiometric amounts of oxygen vacancies are generated [14].
It was confirmed that this solid solution exhibits conduction behavior similar to
that of La
1x
Ca
x
AlO
3a
[15], and that the oxide ion conductivity is rather higher
than that of the latter. These studies were reported in a Japanese journal in 1967 and
1979, and these results were summarized in English and published in 1971 [16].
Steele et al. also reported oxygen ionic conduction in CaTiO
3
-based ceramic [17].
CaTiO
3
is a typical 2:4-type perovskite composed of large-sized divalent
cation Ca and small-sized tetravalent cation Ti, whereas the aforementioned
LaAlO
3
is a typical 3:3 type composed of large trivalent cation La and small
trivalent cation Al. The excellent high oxide ion conductor that was discovered
by Ishihara and reported in 1994 [1 8] is also based on 3:3-type perovskite
LaGaO
3
, as described in detail in Chapter 4.
Oxide ion conductors with the perovskite structure mentioned above belong
to so-called single perovskites, which can be expressed as the simple form,
ABO
3
. Besides these, there are different types of perovskite-related oxides and
some of them are known to show oxide ion conduction. One of them is
Brownmillerite, Ba
2
In
2
O
5
. This composition can be written as BaInO
2.5
, and
3 Ionic Conduction in Perovskite-Type Compounds 53
it is regarded that the ½O per unit formula of a perovskite is deficient; i.e., it can
be written as BaInO
2.5
h
0.5
, where hexpresses oxygen vacancy. At temperature
lower than 9308C, the arrangement of oxygen vacant sites is ordered [19].
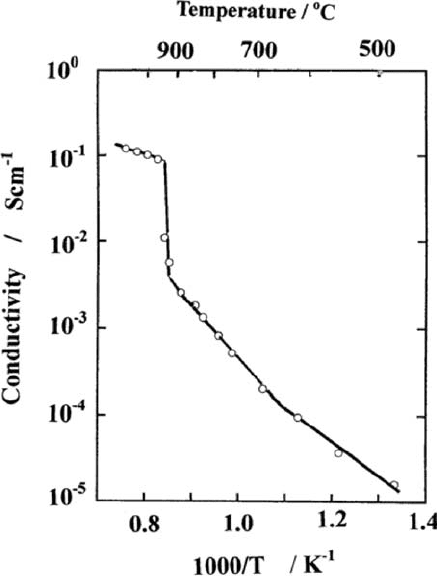
Goodenough et al. found that the oxide ion conductivity in this Brownmillerite
oxide jumps up by more than one order of magnitude above 9308C, as shown in
Fig. 3.3 [20]. Above this temperature, the arrangement of oxide ion vacanci es
becomes disordered, and oxide ions can move easily in assistance of disordered
vacancies.
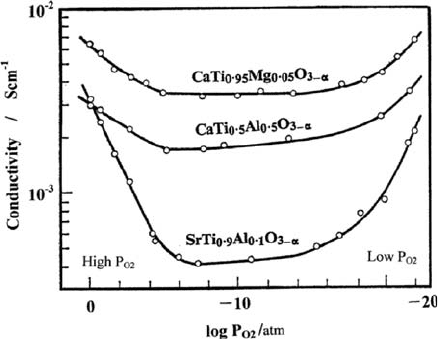
A characteristic feature of perovskite-type oxide ion conductors is that they
are often accompanied with p-type electronic conduction under an oxidizing
atmosphere such as air at elevated temperatures. As described in Section 3.2,
the contribution of electronic conduction depends on P
O2
in the atmosphere
and temperature. As a typical example, Fig. 3.4 shows the P
O2
dependence
of conductivities of CaTiO
3
- and SrTiO
3
-based solid solutions at 8008C [16].
P-type electronic conduction appears in the region of high P
O2
and n-type one
under low oxygen partial pressure, i.e., a reducing atmosphere. The shape of the
curve lns lnP
O2
is essentially the same as that shown schematically in Fig. 3.1.
In many fluorite-type oxide ion conductors such as stabilized zirconias and
Fig. 3.3 Conductivity of
Ba
2
In
2
O
5
as a function of
temperature [20]
54 H. Iwahara
doped cerias, p-type electronic conduction is rarely observed under oxidizing
atmosphere even under p(O
2
) = 1 atm, whereas many perovskite-type oxide
ion conductors become the mixed conductors (O
2
þh
+
) under oxidizing
atmosphere at elevated temperature. Hole conduction arises from the defect
equilibrium with oxygen in gas phase:
V
O
þ 1=2O
2
k
1
!
O
x
O
þ 2h
:
(3:6)
In many perovskite-type oxide ion conductors, the equilibrium constant K
1
is
so large that hole conduction appears even at a relatively weak oxidizing atmo-
sphere such as air, whereas in the case of fluorite-type oxides such as stabilized
zirconias, the equilibrium constant K
1
is too small to cause p-type electronic
conduction in air at elevated temperatures.
Some perovskite-type oxides having transition elements at B sites exhibit
mixed conduction at elevated temperatures. A typical example is doped lantha-
num cobaltite, in which oxide ions and holes are charge carriers. The electronic
conductivity is a few orders of magnitudes higher than that of the oxide-ionic
although the ionic conductiv ity itself is sufficiently high (>10
1
Scm
1
at
several 1008C). This kind of mixed conductor is a promising candidate for the
electrode materials of SOFCs and ceramic membrane reactors and is described
in Chapters 7 and 8 in detail.
3.5 Proton Conduction
Some perovskite-type oxides exhibit proton conduction under hydrogen-
containing atmosphere at elevated temperatures. Cerates or zirconates of
alkaline earth elements in which some trivalent cations are partially substituted
Fig. 3.4 Dependence of
conductivities of CaTiO
3
-
based and SrTiO
3
-based
solid solutions on oxygen
partial pressure at 8008C [16]
3 Ionic Conduction in Perovskite-Type Compounds 55
