Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

Protein–Polysaccharide Interactions 511
cuvet and wrap with Parafilm to prevent evaporation. For dry storage, cuvets should
be washed 5 times with water, emptied, and stored upside down. Sensor chips
(BIAcore) can be stored in a similar fashion in a screw-top tube. Immobilized
proteoglycans, peptidoglycan chains, and oligosaccharides are very stable and will
retain their full ligate-binding capability after months of storage. The major cause of
deterioration of sensor surfaces is the effect of incomplete regeneration, which results
in a gradual loss of binding sites.
3.4. Control of the Amount of Immobilized Ligand
In some applications it is important to control the amount of immobilized ligand,
and in general, it is advisable to repeat experiments with different levels of immobi-
lized ligand. In addition, the interaction of proteins with the glycosaminoglycan
chains of proteoglycans is dependent on the presence of specific sequences of saccha-
rides. Therefore equal amounts of proteoglycan isolated from different sources will
not necessarily contain the same number of binding sites for a given ligate. The
difficulty of reliably detecting the amount of immobilized proteoglycan means that
this quantity must be determined empirically by calculating the total number of bind-
ing sites on the surface, B
max
(see Subheading 3.5.5.). To vary the amount of immobi-
lized ligand, vary the concentration of biotinylated ligand during ligand capture (see
Subheading 3.3.).
3.5. Binding Assays
A single binding assay is illustrated in Fig. 3, and the schematic (see Fig. 4) shows
the changes occurring at the surface in the bulk phase.
3.5.1. Footprinting and Multimolecular Complexes
In all footprinting experiments, it is essential that the ligates are used at
concentrations > K
d
for the ligand, when the ligate will occupy >50% of the binding
sites on the ligand. The main measurement made in footprinting experiments is the
extent of ligate bound, which requires the binding reaction to be at or near equilib-
rium. Consequently, these experiments take a relatively long time. The extent of
binding is calculated in two ways, the results of which should be identical. First, the
extent of ligate binding can be determined directly from the binding curve, where it is
equal to the response at equilibrium minus the response before the addition of ligate,
minus the bulk shift. Second, the nonlinear curve-fitting software supplied by the
manufacturer is used to calculate the extent of binding from the association curve.
The protocols described in Figs 5 and 6 can be adapted for the other instruments,
though the high flow rates required for efficient mixing will require quite extensive
use of ligates.
3.5.2. Footprinting with a Competitor of the Ligate
These experiments test the hypothesis that two ligates bind to sites on the ligand
that are the same, overlap, or interfere with each other. In the example shown (see
Fig. 5), the two ligates are bFGF and a synthetic peptide bFGF(127–140). The experi-
ment illustrates how the dissociation rate constant of a ligate determines the experi-
512 Fernig
mental protocol. The bFGF dissociates slowly from heparin, whereas the peptide
bFGF(127-140) dissociates more rapidly (4).
In the first footprinting experiment (Fig. 5, 7.9–104 min), once bFGF binding has
reached a maximum, dissociation is initiated (Fig. 5, 29.7 min). At 43.8 minutes, the
dissociation of bound bFGF from the immobilized heparin is negligible compared to
the association of the peptide bFGF(127–140), so the peptide is added. In the second
experiment (116.9–206.1 min) the order of addition of the ligates is reversed. This
experiment has to contend with the relatively fast dissociation rate of the peptide
from heparin. After the binding of the peptide has reached a maximum, 1 µL of the
bulk phase is replaced with 1 µL of an identical solution (PBST containing 100 µg/
mL bFGF (127–140) and 90 µg/mL bFGF. In this way the equilibrium between the
soluble and bound peptide is only perturbed by the bFGF binding to the immobilized
heparin.
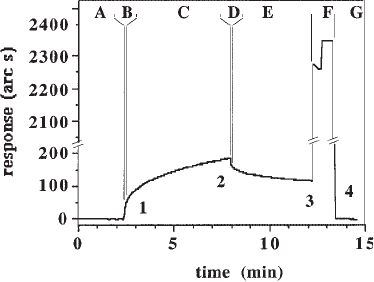
Fig. 3. A binding assay. Binding of bFGF (final concentration 3 µg/mL or 167 nM) to
biotinylated heparan sulfate from Rama 27 cell culture medium, immobilized on a streptavidin
derivatized carboxymethyl dextran cuvet. A response of 200 arc seconds is equal to 1 ng/mm
2
of
protein in the carboxymethyl dextran gel. The binding curve is described by a series of events
(arabic numerals) and regions (letters). Event 1: Addition of ligate . Binding is initiated by the
addition of 1 µL of 90 µg/mL bFGF to the 29 µL of PBST in the cuvet. Event 2: PBST washes.
At the end of the association reaction, the cuvet is washed 3 times with 50 µL of PBST to
initiate the dissociation reaction. Event 3: Regeneration. To regenerate the surface, the cuvet is
washed 3 times with 2 M NaCl, 10 mM NaHPO
4
, pH 7.2, and left in this solution for 1 min.
Event 4: Return to starting conditions. The cuvet is washed 3 times with 50 µL of PBST and
then once with 29 µL of PBST. Region A is the baseline, 29 µL of PBST in the cuvet. Region
B is the 3–5 s region immediately after the addition of ligate (event 1), where mixing and bulk
shifts occur—the latter result from the difference in refractive indices of PBST and PBST con-
taining 3 µg/mL bFGF. Region C is the association phase from which the k
on
and the extent of
binding are calculated. Region D is the 3–5 s region immediately following the PBST washes
(event 2), where mixing and bulk shifts occur. Region E is the dissociation phase from which
k
diss
is calculated. Region F is regeneration, which is initiated by the addition of 2 M NaCl
(event 3). Region G is the new baseline with 29 µL of PBST in the cuvet (event 4).
Protein–Polysaccharide Interactions 513
The concentration of the ligates and their molecular weight have a considerable
effect on this type of footprinting experiment. The concentrations of peptide and bFGF
relative to their K
d
for heparin (4) were chosen such that the peptide in the first experi-
ment (43.8 min) could displace bFGF, but in the second experiment bFGF was unlikely
to displace the peptide. The signal produced by proteins and nucleic acids in optical
biosensors is directly proportional to molecular weight. In this example the
molecular weights of bFGF and bFGF(127–140), 18 and 1.6 kDa, respectively,
differ by over 10-fold. Thus displacement of bFGF in the first experiment (43.8
min) by the peptide would cause a large decrease in signal, whereas bFGF is un-
likely to displace the peptide in the second experiment and cause an increase in signal.
Other experiments, however, may be set up differently due to the type of ligates, and
the results not be so clearcut. In these cases, if ligate dissociation is slow, antibodies
can be used at the end of the experiment to quantify the amount of each ligate that is
bound. Thus an antibody to bFGF could be added at 94.1 min, and once this has bound
maximally, antibody to the peptide would be added.
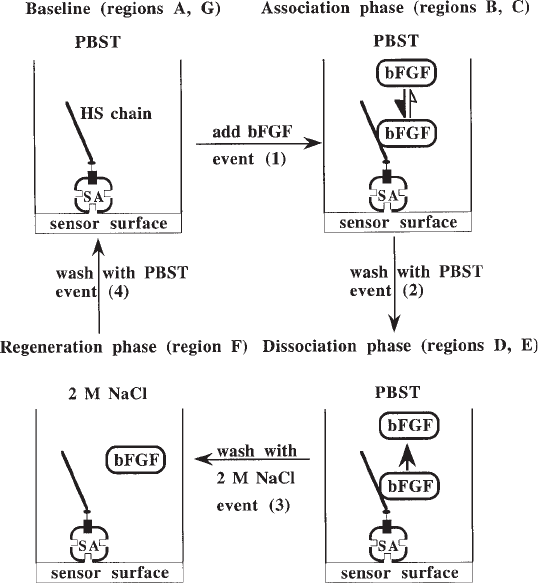
Fig. 4. Schematic of molecular changes occurring at the sensor surface in the course of the
experiment in Fig. 3. SA, streptavidin.
514 Fernig
Time (min) Event
0 Establish a baseline with 29 µL of PBST.
7.9 Add 1 µL of 90 µg/mL bFGF to the cuvet.
29.7 Maximal binding of bFGF, wash 3 × with 50 µL of PBST; leave the
cuvet in 29 µL of PBST.
43.8 Add 1 µL of 3 µg/mL bFGF(127–140) peptide.
77.7 Maximal binding of bFGF(127–140).
77.7 Wash 3 × with 50 µL of PBST; leave the cuvet in 29 µL of PBST.
94.1 Regenerate with multiple washes of 2 M NaCl.
104 Establish a baseline in 29 µL of PBST.
116.9 Add 1 µL of 3 mg/mL bFGF(127–140) peptide.
156.8 Maximal binding of bFGF(127–140); remove 1 µL from the cuvet.
156.8 Add 1 µL of 90 µg/mL bFGF in PBST containing 100-µg/mL
bFGF(127–140).
184.6 Wash 5 × times with PBST and allow dissociation to proceed.
206.1 Regenerate with multiple washes of 2 M NaCl to recover the baseline.
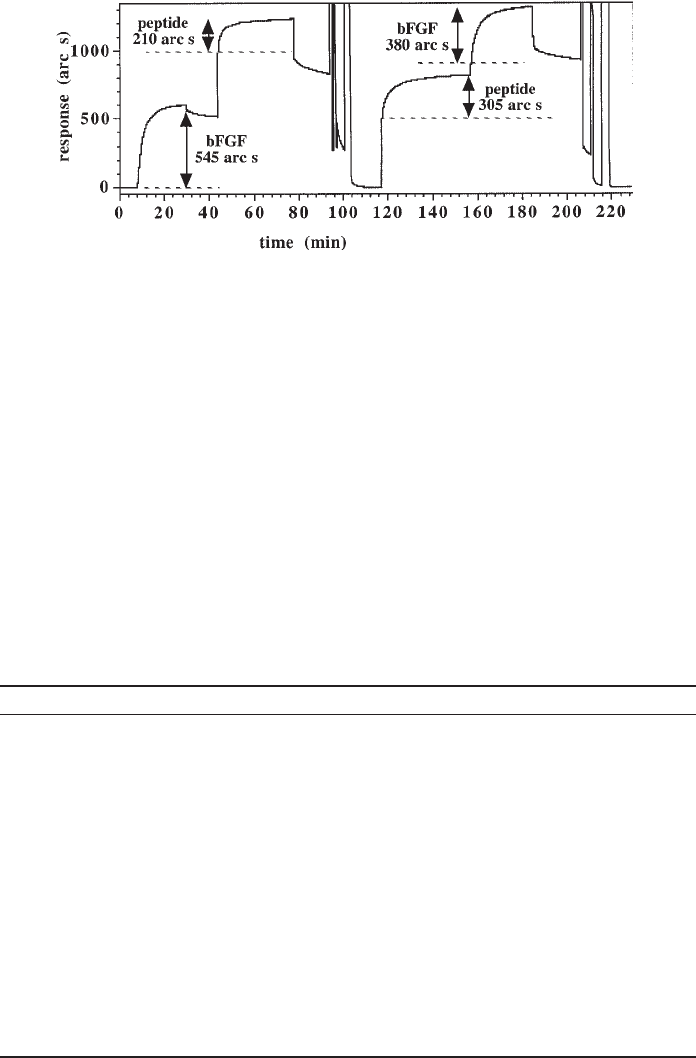
Fig. 5. Footprinting with a competitor of the ligate in an IAsys optical biosensor. In the first
experiment, the extent of binding of bFGF to biotinylated porcine mucosal heparin, captured on
streptavidin immobilized on a carboxymethyl dextran surface, is measured. Then the synthetic
peptide bFGF(127–140) is added to determine the extent of binding of the peptide to porcine mu-
cosal heparin in which over half the binding sites or bFGF are occupied. In the second experiment
the order of addition of the ligates is reversed, so the synthetic peptide is added first, followed by
bFGF. This experiment measures the extent of binding of the synthetic peptide to porcine mucosal
heparin and the extent of binding of bFGF to porcine mucosal heparin in which over half the
peptide binding sites are occupied. The table below is a detailed description of experiment.
Calculation of extent of binding: In the first experiment the bulk shifts do not have to
be explicitly subtracted, since the two binding reactions start and finish in PBST. The
extent of binding of bFGF and the bFGF(127-140) peptide is denoted by the vertical two
headed arrows. In the second experiment, the situation is a little more complex, since the
cuvette is not returned to PBST after the bFGF(127-140) peptide has bound, due to the
relatively fast rate of dissociation of the peptide-heparin complex. In this case the bulk
shift is taken as the first 5 s of the binding reaction and an offset baseline (horizontal
dotted lines) is used to calculate the extent of binding (two headed arrow).

Protein–Polysaccharide Interactions 515
3.5.3. Footprinting with a Competitor of the Ligand
These experiments test the hypothesis that the region of the ligate that recognizes the
ligand can also recognize the soluble competitor. In the example shown (see Fig. 6),
the effect of heparin on the binding of heparin affin regulatory peptide (HARP) (5,6) to
immobilized heparin is determined.
3.5.4. Multimolecular Complexes
The analysis of multimolecular complexes, for example where ligate (1) binds to
heparan sulfate and ligate (2) binds to ligate (1), is a simple extension of the above
footprinting experiments. The only constraint is that the dissociation rate of ligate (1)
from the ligand is sufficiently slow (as in the case of bFGF in Fig. 5) to allow the
cuvette to be washed and returned to binding buffer prior to the addition of ligate (2).
3.5.5. Kinetics
One of the key uses of optical biosensors is the rapid determination of the kinetics
of a molecular interaction. The instrument manufacturers provide ample information
on the theoretical considerations behind the measurements of binding kinetics. This
section will therefore deal only with artefacts.
The major artefact associated with the determination of kinetics in optical
biosensors is the generation of second phase binding kinetics by diffusion limitations
(the so-called mass-transport artefact) and steric hindrance (1,7). Diffusion limitations
arise when the rate of diffusion of the soluble ligate (dependent on its diffusion
coefficient, D) from the bulk, stirred solution, through the boundary layer of immobile
Fig. 6. Footprinting with a competitor of the ligand. In the control binding reaction, 3 µL
HARP (20 µg/mL in PBST) is added to a cuvet containing 27 µL of PBST. In the subsequent
competitions, 3 µL of PBST containing 20 µg/mL HARP and 50-ng/mL, 5 µg/mL, or 50 µg/mL
heparin is added. The signal from the last experiment is indistinguishable from the background.
The fourth binding curve, which is again indistinguishable from background, controls for any
signal that might be generated by the competitor alone; in this case 3 µL of PBST containing no
HARP and 50 µg/mL of heparin is added.
516 Fernig
solution, which exists next to the surface of the sensor, is equivalent or slower than the
apparent on rate, k
on
. In this case, after the initial rapid depletion of soluble ligate from
the solution near the surface, the observed association kinetics reflect diffusion rates
rather than association rates. The diffusion artefact also affects the measurement of the
dissociation rate constant, k
diss
. k
diss
should be independent of ligate concentration. If
k
diss
is found to increase with increasing ligate concentration, it is likely that dissociated
ligate is rebinding to unoccupied ligand faster than it is diffusing into the bulk phase.
Multivalent ligates, which possess a high avidity, represent a special case of this effect.
The steric hindrance of binding sites arises when the immobilized ligand is at a high
density and/or randomly oriented, and is most prominent on 3-dimensional carboxy-
methyldextran surfaces (7).
There are two ways to investigate whether diffusion is indeed rate-limiting:
1. Decreasing the rate of flow or of stirring to determine the rate at which diffusion becomes
limiting. A drawback is that this is rather insensitive and in stirred systems the relationship
between diffusion and the rate of stirring may not be linear.
2. Increasing the viscosity of the binding buffer (diffusion is inversely proportional to viscos-
ity) by the addition of glycerol. This is the more sensitive method.
To avoid these possible artefacts, experimental design should always include the
following:
1. The minimum amount of ligand required to give a useful signal should be immobilized.
This is determined empirically.
2. Oriented immobilization of ligand rather than random reduces steric hindrance between
the surface and the binding site on the immobilized ligand.
3. Since k
on
depends directly on concentration, keeping the concentrations of ligate as low
as possible reduces the possibility of the rate of diffusion controlling the binding reaction.
4. To avoid rebinding during dissociation, k
diss
should be determined at ligate concentra-
tions where the majority of the binding sites are occupied. If rebinding is still a problem,
soluble ligand can be added as a competitor to the dissociation buffer [e.g., (8)].
5. Diffusion into the bulk phase from the stationary phase is faster with planar than three-
dimensional, e.g., carboxymethyl dextran, surfaces, so some experiments should always
use a planar surface (7).
3.6.6. Microaffinity Chromatography
Optical biosensors provide the opportunity to carry out microaffinity chromatogra-
phy. In these experiments, the immobilized ligand is used to fish for a specific target
in a mixture. Once the target is bound, the regeneration step is used to elute the target
into the bulk phase. Recovery of the target is simplest in cuvet-based instruments.
IAsys cuvets come in analytical and preparative formats. Analytical surfaces have a
mask that covers all but 4 mm
2
of the surface, so the total amount of ligand is low, thus
preventing depletion of ligate from the bulk phase during the binding reaction. Pre-
parative surfaces (“Select”) do not have the mask and the area of the surface is 16
mm
2
, which allows the immobilization of fourfold more ligand and the capture of a
correspondingly larger amount of ligate. Microaffinity chromatography can be used
either as a means to identify the steps required to prepare an efficient conventional
Protein–Polysaccharide Interactions 517
chromatography column or as the preparative chromatography step itself. The only
constraint on the use of optical biosensors as microchromatography systems is that
nonspecific binding is undetectable.
The example shown is an experiment that used the optical biosensor to isolate hep-
arin-binding phage from a library that displayed a constrained 7-amino acid peptide
(see Fig. 7 and Chapter 50). Twenty clones were isolated from the second-generation
phage and, compared to the initial starting library, the DNA sequences encoding the
peptide library in second-generation phage always contained codons for basic amino
acids, illustrating the success of the method. The clear advantage of using the optical
biosensor as a microaffinity chromatography system is that the instrument provides a
readout in real time of the binding events. It is therefore possible to troubleshoot
protocols in real time and thus save considerable amounts of time and precious mate-
rials. In addition, since nonspecific binding is negligible, the fold-purification achieved
is dramatic. The major inconvenience is that only small amounts of material are recov-
ered, which require either a simple amplification step (as in the case of the phage) or
suitable downstream microanalytical facilities.
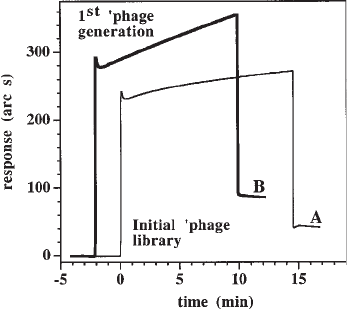
Fig. 7. Selection of phage that bind heparin. Biotinylated porcine mucosal heparin was immo-
bilized on a biotin select surface. Wild-type phage do not bind to this surface at NaCl concentra-
tions as low as 10 mM (Rahmoune, H., and Fernig, D. G. unpublished). The cuvet is cleaned with
phenol prior to the addition of phage, to eliminate contaminating wild-type phage from the envi-
ronment and all solutions are autoclaved prior to use. Phage from the initial library (70 µL at 10
11
pfu/mL) are added to the cuvette (0 min, thin line). After a large bulk shift due to the high concen-
tration of phage, a low but significant amount of binding is observed (about 20 arc s). After
washing the surface with PBST, bound phage (A, the first generation) are eluted (equivalent to
surface regeneration) with either 2 M NaCl or 1 mg/mL heparin in PBST, both of which were
equally effective. These first-generation phage are grown up and added (70 µL at 10
11
pfu/mL) to
the same surface (–2 min, thick line). Clearly the first generation is enriched in phage that are able
to bind to the heparin compared to the initial library, since the extent of binding is double and the
k
on
is considerably faster. Bound phage (B, the second generation) were collected as before and
grown up.
518 Fernig
Acknowledgements
The author would like to thank the Association for International Cancer Research,
the Biotechnology and Biological Sciences Research Council, the Cancer and Polio
Research Fund, the Leverhulme Trust, the Medical Research Council, the Mizutani
Foundation for Glycoscience, the North West Cancer Research Fund, and The Royal
Society for financial support, and his colleagues in Liverpool for help and advice.
References
1. Schuck, P. (1997) Use of surface plasmon resonance to probe the equilibrium and dynamic
aspects of interactions between biological macromolecules. Annu. Rev. Biophys. Biomol.
Struct. 26, 541–566.
2. Peters, T. (1996) All About Albumin : Biochemistry, Genetics, and Medical Applications.
Academic Press, San Diego, CA.
3. Rahmoune, H. J., Gallagher, T., Rudland, P. S., and Fernig, D. G. (1998) Interaction of
heparan sulphate from mammary cells with extracellular regulatory proteins. Acidic and
fibroblast growth factor: regulation of the activity of bFGF by high and low affinity binding
sites in heparan sulphate. J. Biol. Chem. 273, 7303–7310.
4. Kinsella, L., Chen, H.-L., Smith, J. A., Rudland, P. S., and Fernig, D. G. (1998) Interactions
of putative heparin-binding domains of basic fibroblast growth factor and its receptor, FGFR-
1, with heparin using synthetic peptides. Glycoconjugate J. 15, 419–422.
5. Courty, J. M., Dauchel, C., Caruelle, D., Perderiset, M., and Barritault, D. (1991). Mitoge-
nic properties of a new endothelial-cell growth-factor related to pleiotrophin. Biochem.
Biophys. Res. Commun. 180, 145–151.
6. Vacherot, F., Delbe, J., Heroult, M., Barritault, D., Fernig, D. G. and Courty, J. (1999)
Glycosaminoglycans differentially bind HARP and modulate its biological activity. J. Biol.
Chem. 274, 7741–7747.
7. Edwards, P. R., Gill, A., Pollardknight, D. V., Hoare, M., Buckle, P. E., Lowe, P. A., and
Leatherbarrow, R. J. (1995) Kinetics of protein-protein interactions at the surface of an
optical biosensor. Anal. Biochem. 231, 210–217.
8. Sadir, R., Forest, E., and LortatJacob, H. (1998) The heparan sulfate binding sequence of
interferon-gamma increased the on rate of the interferon-gamma-interferon-gamma receptor
complex formation. J. Biol. Chem. 273, 10919–10925.

Phage Display Antibodies to Heparan Sulfate 519
519
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
50
Phage Display Technology
to Obtain Antiheparan Sulfate Antibodies
T. H. van Kuppevelt, G. J. Jenniskens, J. H. Veerkamp,
G. B. ten Dam, and M. A. B. A. Dennissen
1. Introduction
Antibodies have proven to be valuable tools in research on proteoglycans. They
have been used extensively to study the tissue expression patterns of proteoglycans at
the light as well as at the electron microscopical level. In addition, they have been
frequently applied as (immuno)precipitating agents, in immunoaffinity chromatogra-
phy, and—in some cases—as blocking agents. Most antibodies available are directed
against the core protein of proteoglycans. Only a few are reactive with the glycosami-
noglycan moiety. This is due to the largely nonimmunogenic character of glycosami-
noglycans. Those antibodies that have been raised to glycosaminoglycans were
obtained using proteoglycans as antigen, rather than the glycosaminoglycan chains as
such. Here, we describe the use of phage display technology to obtain antibodies to
glycosaminoglycans, as exemplified by heparan sulfate. Phage display allows the gen-
eration of antibodies to “self” antigens. The antibody is “displayed” at the surface of the
phage by fusion to a coat protein (1,2). In the protocol described here, a semisynthetic
antibody phage display library [“synthetic scFv library # 1”, (3)] was used, consisting of
>10
8
different clones, each expressing one unique antibody. In principle, any anti-
body phage display library can be used. The synthetic library #1 contains 50 different
V
H
genes with synthetic complementarity-determining region 3 segments (CDR3),
which contain a random sequence, encoding 4–12 amino acid residues. Only one light-
chain gene is present. In the library, only the variable parts of the heavy and light chains
are expressed, joined to each other by a linker sequence to form so-called single-chain
variable fragments (scFv). All antibodies contain a cMyc tag for identification with
anti-cMyc antibodies.
520 van Kuppevelt et al.
A major advantage of the phage display system is that once a phage expressing the
antibody has been selected, the DNA encoding the antibody is available. This opens
the realm of molecular-biological techniques (e.g., large-scale production in bacteria,
easy purification using His-tags, fusion of the antibodies to other proteins). Phage
display-derived antibodies may be of value in characterizing the structural heteroge-
neity of heparan sulfate and other glycosaminoglycans.
This chapter describes the selection and characterization of anti-heparan sulfate
antibodies and their coding genes using antibody phage display technology.
2. Materials
2.1. Selection of Phages Displaying Antibodies Reactive with Heparan
Sulfate Using Biopanning
1. To avoid any carryover of phages during selections, several precautions need to be taken.
The use of sterile disposable plastic ware and devoted pipetes is highly recommended.
Nondisposable plastic ware should be soaked for 1 h in 2% (v/v) hypochlorite, followed
by thorough washing and autoclaving. Glassware should be baked at 200ºC for at least 4
h. Use aerosol-resistant pipet tips (Molecular Bio-Products) when working with bacteria
or phages. It is recommended to work in a laminar-flow cabinet or in a fume cabinet.
Clean the workplace (benchtops, etc.) with 10% (v/v) hypochlorite before and after each
working day. Clean pipetes etc., daily by wiping the outside with 0.1 M NaOH.
2. Bacterial strain: Escherichia coli TG1 (3) suppressor strain (K12, ∆(lac-pro), supE, thi,
hsd∆(5/F'traD36, proAB, lacI
q
, LacZ∆(M15) (see Note 1).
3. VCS-M13 helper phages (Stratagene) (see Note 2) used at a titer of 1 × 10
12
CFu/mL.
Alternatively, M13 KO7 helper phages (Pharmacia) can be used.
4. Glycerol stock of the (semi)-synthetic scFv Library #1 [Dr. G. Winter, Cambridge Uni-
versity, Cambridge, UK (3)], stored at –80ºC.
5. 2XTY: 1.6% (w/v) Bacto-Trypton, 1.0% (w/v) Bacto-Yeast extract (Gibco BRL), and
0.5% (w/v) NaCl.
6. 40% (w/v) glucose (Sigma) in H
2
O sterilized by filtering using a 0.2-µm filter (Schleicher
& Schuell).
7. Ampicillin (Sigma) and kanamycin (Gibco BRL).
8. 2XTY containing 100 µg of ampicillin/mL and 1% (w/v) glucose.
9. 2XTY containing 100 µg of ampicillin/mL and 25 µg kanamycin/mL.
10. Minimal medium: Autoclave 450 mL of 2.2% (w/v) Bacto-Agar (Gibco BRL) in H
2
O.
Cool the solution down to 60°C and add, after sterile filtering with a 0.2-µm filter
(Schleicher & Schuell), 50 mL of 10XM9, 0.5 mL of 20% (w/v) MgSO
4
, 2.5 mL of 40%
(w/v) glucose, and 0.25 mL of 1% (w/v) thioamine.
a. 10 × M9 medium: 0.60 M K
2
HPO
4
, 0.33 M KH
2
PO
4
, 76 mM (NH
4
)
2
SO
4
, 17 mM
trisodium citrate · 2H
2
O, pH 7.4 (adjust with phosphate component).
11. Polyethylene glycol (PEG)/NaCl: 20% (w/v) PEG 6000 (Serva) containing 2.5 M NaCl.
12. Phosphate-buffered saline (PBS): 0.14 M NaCl, 8.1 mM Na
2
HPO
4
and 1.5 mM
NaH
2
PO
4
· 2H
2
O, 2.7 mM KCl, pH 7.4 (adjust with phosphate component).
13. Microlon immunotubes, 12/55 mm, 4 mL (Greiner).
14. Heparan sulfate from bovine kidney (Seikagaku).
15. Marvel: dried skimmed milk (Premier Beverages, Stafford, UK).
16. PBS containing 2% (w/v) Marvel.
17. PBS containing 4% (w/v) Marvel.
