Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

500 Langford and Sanderson
g. Centrifuge the tubes containing the noninvasive cell population at 300g for 10 min
and resuspend the cell pellet in an appropriate amount of buffer for cell counting (i.e.,
hemocytometer or Coulter counter).
2. Collection of the invasive cell population:
a. Add 0.5 mL of collagenase solution to each well and incubate at 37°C until the gels are
completely digested (approximately 1.5 h).
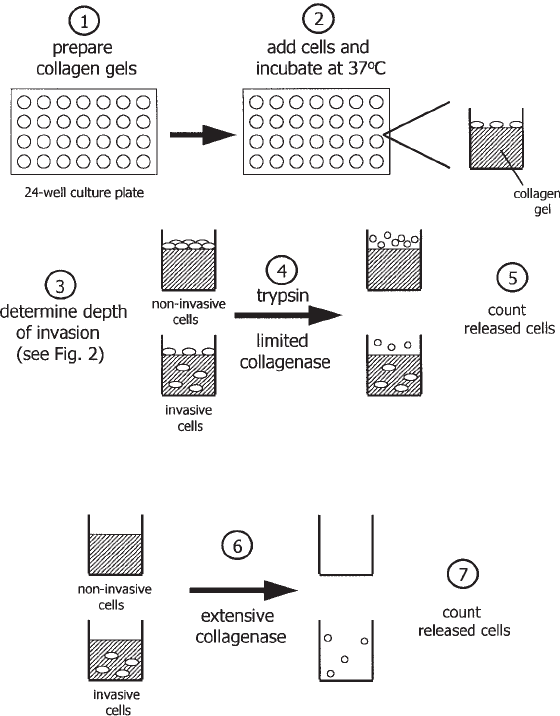
Fig. 3. Diagram of cell invasion assay. Collagen gels are prepared and seeded with cells.
After the appropriate incubation period, first the depth of cell invasion is determined, followed
by determination of the percent of cell invasion. Noninvading cells are recovered from the
surface of the gels by trypsin and limited collagenase digestions and counted. Next, following
complete digestion of the remaining collagen with collagenase, the invading cells are collected
and counted. The percentage of cell invasion for each gel is calculated by dividing the number
of invading cells by the total number of cells recovered from the collagen gel (noninvading +
invading cells) and multiplying by 100.

Syndecans in Cell Adhesion and Invasion 501
b. Collect the solution by aspiration into individual tubes. Place all solutions collected
from each well into the same 15-mL culture tube.
c. Wash the wells and collect the solution by aspiration as in step b.
d. Centrifuge the cells and count as in g above.
3. Determine percentage of invasive cells:
invasive cells
× 100 = % invasive cells
noninvasive cells + invasive cells
4. Notes
1. Because the buffer used for this assay is highly conducive to bacterial growth, care must
be taken to use fresh and uncontaminated solutions. The buffer should be sterile filtered,
stored at 4°C, and handled using aseptic techniques in order to reduce contamination.
2. The heparin solution is used to disrupt cell aggregation and thus obtain single cells that are
counted and used in the calculation of the percentage of cell aggregation. This is possible
because aggregation, in our cell lines, is mediated via the heparan sulfate chains of syndecan-
1. It may be necessary to use another disrupting agent for other cell types.
3. The force used to resuspend the cells prior to counting is the only subjective step in the entire
assay. Considering this, it has been helpful to gain the assistance of a colleague to code the
tubes containing the cells and thus “blind” the study.
4. A disposable transfer pipet (5-mL capacity) works well for resuspending the cells prior to
counting. Holding the opening of the pipet at the 0.5-mL line of a 1.5-mL Eppendorf tube
and gently applying pressure to the bulb has yielded consistent results when resuspending
cells. Also, this pipet has a large enough opening so cell aggregates are not mechanically
dispersed as they would if forced through a narrow aperture.
5. When counting cells using a hemocytometer, for each sample, count the cells within the four
1-mm
2
corners and divide by 4 to obtain an average cell number. This is done for cells in
buffer containing heparin and cells in heparin-free aggregation buffer. Cells in groups of 2
and 3 cells may not represent aggregated cells but may simply be cells passively associated.
Therefore, cells in these groups are counted individually.
6. Because of the duration of the invasion assay, care must be taken to ensure the sterility of the
solutions and collagen gels during gel preparation.
7. A major consideration in the preparation of the gels is the collagen concentration best
suited to yield cell invasion. The concentration of the collagen dramatically affects the
rate of cell invasion. At high collagen concentrations, invasion may be inhibited all
together; whereas at low concentration, with little to no resistance, all cells may simply
fall through the large spaces of the gel. Therefore, a concentration of collagen that
allows suitable invasion in an appropriate period of time and allows experimental
manipulation of invasion (i.e., inhibition) must be determined empirically for each cell line.
For example, although we use a collagen concentration of 0.5 mg/mL and an assay time of
48 h for myeloma cells (5) for breast cancer cells, the time was extended to
72 h (6).
8. Although a collagen stock may be obtained from several sources, we have found that the
collagen produced by Collaborative Biomedical Products yields consistent results and
reproducible gels.
9. Prepare the collagen gel solution on ice, making certain that the solution remains cold during
setup, to prevent premature polymerization of the collagen.
502 Langford and Sanderson
10. The pH of the collagen gel is a variable that influences the integrity of the gel and thus the
invasive extent of the cells. The pH is adjusted by varying the amount of sodium bicarbon-
ate added to the collagen gel solution. At slightly more acidic pH (i.e., 6.8–7.0) the gels do
not polymerize as well, and are unstable. While this allows cells to invade faster, the gels
become fragile, making it difficult to add media or wash solutions to the gels without damag-
ing the surface. Conversely, a more basic pH (i.e., 7.6–8.0) creates a gel through which cells
have difficulty invading. With experience, one can determine the optimal pH by carefully
examining the color of the solution. The appropriate pH results in a “salmon pink” color. For
initial experiments, a small amount of collagen gel solution can be applied to pH test strips for
accurate pH determination. If the pH of the collagen gel solution becomes too basic, discard
the solution.
11. If the collagen gel solution is not properly degassed, trapped air bubbles will compromise
the integrity of the gel and allow cells to invade at an artificially rapid rate.
12. While washing the gels and changing the media, care must be taken not to damage the gels.
If a hole appears during the quantification process, the gel must be discarded. When pipetting,
slowly allow each drop of liquid to spread over the surface of the gel or liquid surface rather
than drop from a distance. The impact of a large droplet of liquid may damage the gel.
13. The density of cells growing in culture before to the start of the invasion assay may also
affect the results. For myeloma cells, the most consistent results are obtained from cells
growing between 50% and 75% confluency. Myeloma cells at low density in fresh media,
or overconfluent cells in exhausted media, do not invade well.
14. Cells placed on the collagen gels should not have extensive cell–cell contact. This applies at
the beginning and end of the experiment. Thus, the size and mitotic rate of the cells to be
used dictate the density at which they are added to each collagen well. For myeloma cells, we
use 5.0 × 10
4
cells per collagen gel.
15. One of the most necessary requirements for obtaining interpretable results is the inclusion of
positive and negative controls (i.e., invasive and noninvasive cells respectively). This ensures
that the collagen gel is permissive for invasive cells yet still able to prevent noninvasive cells
from passively falling through the spaces between collagen fibers. By including these two
controls, data can be reported either as raw data (5) or as the percent invasion relative to the
controls (3). The latter method compensates for interassay variability.
16. The most critical step in the quantification process is the collagenase treatment used to
remove the noninvasive cell population from the gel surface. Overdigestion of the gel will
result in removal of many of the invasive cells, while underdigestion will fail to remove the
cells at the surface. Both situations produce inaccurate data.
17. Because the majority of cells in the noninvading controls are attached at the surface of the gel,
they form a “landmark” that can be used to determine the time required to remove the
noninvasive cells from the surface of all gels. After 5 min at 37°C of the first collagenase
digestion, the plate can be removed from the incubator and the cells on the surface examined
under an inverted phase microscope. If the majority of cells in the noninvading control are in
suspension, proceed to the next step. If many cells remain attached at the gel surface, return
the plate to the incubator for 1-min intervals and examine until the noninvading cells are
released from the gel surface.
References
1. Bernfield, M., Gotte, M., Park, P. W., Reizes, O., Fitzgerald, M. L., Lincecum, J., and
Zako, M. (1999) Functions of cell surface heparan sulfate proteoglycans. Annu. Rev.
Biochem. 68, 729–777.
Syndecans in Cell Adhesion and Invasion 503
2. Stanley, M. J., Liebersbach, B. F., Liu, W., Anhalt, D. J., and Sanderson, R. D. (1995)
Heparan sulfate-mediated cell aggregation. Syndecans-1 and -4 mediate intercellular
adhesion following their transfection into human B lymphoid cells. J. Biol. Chem.
270, 5077–5083.
3. Langford J. K., Stanley M. J., Cao D., and Sanderson R. D. (1998) Multiple heparan sulfate
chains are required for optimal syndecan-1 function. J. Biol. Chem. 273, 29,965–29,971.
4. Mareel, M. M., Baetselier, P. D., and Van Roy, F. M. (1991) Mechanisms of Invasion and
Metastasis, CRC Press, Boca Raton, FL.
5. Liebersbach, B. F. and Sanderson, R. D. (1994) Expression of syndecan-1 inhibits cell
invasion into type I collagen. J. Biol. Chem. 269, 20013–20019.
6. Kelly, T., Yan, Y., Osborne, R. L., Athota, A. B., Rozypal, T. L., Colclasure, J. C., and
Chu, W. S. (1998) Proteolysis of extracellular matrix by invadopodia facilitates human
breast cancer cell invasion and is mediated by matrix metalloproteinases. Clin. Exp.
Metastasis. 16, 501–512.

Protein–Polysaccharide Interactions 505
505
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
49
Optical Biosensor Techniques to Analyze
Protein-Polysaccharide Interactions
David G. Fernig
1. Introduction
Networks of interacting molecules, operating from the outside of the cell to the cell
nucleus, regulate cell behavior. Optical biosensors provide a means of analyzing
these interactions and possess key advantages over other methods: posttranslationally
modified proteins, secondary gene products such as polysaccharides, chemically
synthesized molecules, and nucleic acids are all equally susceptible to analysis; a
quantitative description of an interaction is obtained; the structural rules and the
kinetics governing the formation of multimolecular assemblies can be probed.
The principles of measurement underlying optical biosensors have been reviewed
in detail elsewhere (1), and only a basic description will be given here. Optical
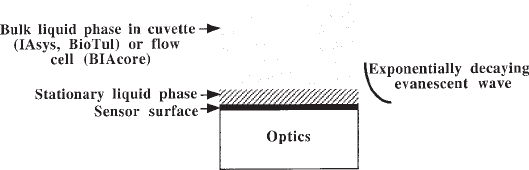
biosensors consist of a sensor surface, on one side of which reside the optics that
enable measurements to be made and on the other side of which resides the liquid
phase (see Fig. 1). The optical system generates an exponentially-decaying evanes-
cent wave that penetrates into the liquid phase. The greatest sensitivity occurs where
the evanescent wave is strongest, that is, closest to the surface. In general, useful
measurements can be made within about 200 nm of the surface. The optics essentially
measure changes in refractive index, so the signal obtained depends on the refractive
index within this 200 nm.
The essence of experiments using optical biosensors is that one partner of a
molecular interaction is immobilized on the surface and the interaction of the other
partner(s) is then followed in real time by adding them to the liquid phase. It is usual
to refer to the immobilized molecule as the immobilized ligand or ligand and the soluble
partner(s) as the soluble ligate(s) or ligate(s). The signal obtained from proteins and
nucleic acids depends solely on the amount of material at the surface. Hence with
these macromolecules, the optical biosensor acts as a very sensitive mass sensor. How-
ever, glycosaminoglycans have a low refractive index, which may vary with the degree
of sulfation (Fernig, D. G., unpublished). Therefore, compared to proteins, glycosami-
506 Fernig
noglycans give a small signal on a mass basis, and this signal cannot be related directly
to the amount of material, since it may depend on composition. For these reasons,
experiments should always be designed such that the proteoglycan, its glycosami-
noglycan chains or oligosaccharides derived from the latter are immobilized on the
surface and the protein partner(s) are used as soluble ligate. Unfortunately, pro-
teoglycans often contain more than one glycosaminoglycan chain, and each chain may
contain more than one binding site for the protein partner of interest. Multivalent
ligands usually have high avidity. Molecules with high avidity exacerbate the major
artifacts encountered in optical biosensors (see Subheading 3.5.5.).
There are currently four commercial instruments on the market, BIAcore
(Pharmacia Biosensor, Uppsala, Sweden), BIOS-1 (Artificial Sensing Instruments,
Zurich, Switzerland), BioTul (BioTul, Munich, Germany), and IAsys (Affinity Sen-
sors, Cambridge, UK). Two of these instruments, the BIAcore and the BioTul, use
surface plasmon resonance to produce the evanescent wave in order to probe the liquid
phase and thus have a gold film at the sensor surface. The ability of thiol groups to
bond gold is used to modify the surface so as to make it amenable to the attachment of
biological macromolecules. Available surfaces include carboxymethyl dextran for
BIAcore and BioTul, and for BIAcore only, carboxymethyl dextran derivatized with
streptavidin and nitrilotriacetic acid, short carboxymethyl dextran, dextran with a low
degree of carboxylation, planar hydrophobic, carboxylated, and gold. In contrast, the
BIOS-1 and IAsys use an evanescent wave generated by waveguiding to probe the
liquid phase. The IAsys has a layer of metal oxide at the sensor surface, which enables
molecular deposition of a variety of groups, and, in addition to carboxymethyl dext-
ran, hydrophobic, amino, carboxyl, and biotin surfaces are available. There are two
issues to bear in mind regarding the surfaces. First, they will possess holes at the
atomic level and thus some gold or metal oxide may be exposed to the experiment.
Second, carboxymethyl dextran gels possess hydrophobic pockets, which may either
cause nonspecific binding artifacts or be a useful functionality.
The liquid phase, in which experiments take place (see Fig. 1), consists of two
components, a homogenous bulk phase that is mixed by a vibrational stirrer (IAsys) or
by flow (BioTul and BIAcore), and a thin stationary phase next to the sensor surface,
whose constituents exchange by diffusion with the bulk phase. The ligand immobilized
on the sensor surface is within the stationary phase. The depth of the stationary phase
Fig. 1. Schematic of an optical biosensor.
Protein–Polysaccharide Interactions 507
depends on the efficiency of mixing, which in turn determines the likelihood of diffu-
sion artifacts (see Subheading 3.5.5.). The vibrational stirrer in IAsys should always
be set to maximum. Since it induces chaotic mixing at the interface between the bulk
phase and the stationary phase, the latter is reduced to a minimum and exchange
between the two phases is efficient. In flow systems, mixing depends on the rate of
flow (BioTul and BIAcore), and this should always be set to the maximum. The issue
of mixing is compounded with laminar flow systems (BIAcore), since, as the bulk
phase approaches the stationary phase, the rate of flow decreases, thus reducing the
efficiency of mixing at the bulk phase–stationary phase interface.
2. Materials
2.1. Biotinylation
1. N-hydroxysuccinimide (NHS) amino caproate (LC) biotin (Pierce) or hydrazide-LC-biotin
(Pierce), 50 mM, dissolved in dimethyl sulfoxide. It is essential to use biotin with the
aminocaproate spacer arm. Stored in 10-µL aliquots at –70°C, the NHS-LC-biotin and
hydrazide-LC-biotin are stable for at least 4 mo.
2. Proteoglycan or peptidoglycan chains, normally desalted and freeze-dried.
3. 3 M Tris-HCl, pH 7.2.
4. Sephadex G-25 desalting column (1 × 25 cm).
2.2. Biotinylation of Amino Groups
1. Add 10 µL of a 50 mM solution of NHS-LC-biotin in dimethyl sulfoxide to 100 µg of
proteoglycan in 100 µL of distilled water, mix, and allow the reaction to proceed at room
temperature for 24 h.
2. Two further additions of 10 µL of NHS-LC-biotin may be made, either over the initial 24 h
or over a subsequent 48 h. Blocking of unreacted NHS groups on biotin is achieved by the
addition of 10 µL of 3 M Tris-HCl, pH 7.2, followed by a 10 min incubation at room
temperature.
2.3. Biotinylation of Reducing Ends
1. Add 10 µL of a 50 mM solution of hydrazide-LC-biotin in dimethyl sulfoxide to 100 µg of
oligosaccharide in 100 µL of distilled water, mix, and allow the reaction to proceed at room
temperature for 24 h. Two further additions of 10 µL of hydrazide-LC-biotin may be made
either over the initial 24 h or over a subsequent 48 h.
2.4. Removal of Free Biotin
1. Free biotin is removed by fractionation on a Sephadex G-25 column (1 × 25 cm, flow rate
0.5 mL/min), equilibrated in distilled water, and calibrated with high-molecular-weight
blue dextran and potassium dichromate. The biotinylated proteoglycans or peptidoglycans
elute in the void volume and are then lyophilized.
2.5. Capture of Biotinylated Ligands, Surface Regeneration
and Storage
1. PBST (phosphate-buffered saline, pH 7.2 with 0.02 % [v/v] Tween-20).
2. Biotinylated ligand between 1 µg/mL and 1 mg/mL dissolved in PBST (see Subhead-
ing 3.4.).
3. Instrument stirrer setting: 100 (maximum).
508 Fernig
2.6. Regeneration
1. 2 M NaCl, 10 mM NaH
2
PO
4
, pH 7.2.
2. 20 mM HCl.
3. 2 M guanidine-HCl, pH 7.0, freshly made and the highest grade available, for example,
Aristar from BDH, (Poole, UK).
3. Methods
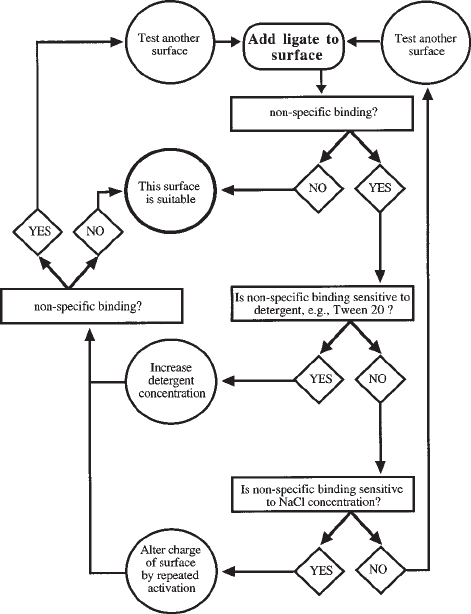
3.1. Background Binding and Choice of Surface
Optical biosensors are extremely sensitive and precise instruments. Since all
instruments offer parallel/multiple channels, the temptation is to subtract any
nonspecific binding by using a control surface. There are two drawbacks to this
approach. When nonspecific binding is considerable, the signal is a fraction of the
noise, which is always a poor basis for any type of measurement. In addition, a true
control surface is almost impossible to generate, since, by definition, only the experi-
mental surface has immobilized ligand. If an unrelated ligand is used on the control
surface, the potential effects of immobilization of this ligand on the ligate should be
explored. For example, commonly used “blocking” proteins such as bovine serum
albumin (2) bind weakly a large number of protein ligates and are often not suitable.
The search for a suitable control surface for nonspecific binding rapidly becomes
recursive. It is far more profitable to spend time identifying a surface that maximizes
the signal-to-noise ratio. In the author’s experience, protocols that have no background
binding can be identified with a little effort (see Fig. 2). Ideally, two different sur-
faces, at least one of which should be planar (see Subheading 3.5.5.) should be
identified.
If a capture system, such as streptavidin for biotinylated ligands, is to be used,
following the identification of suitable surfaces, the tests for nonspecific binding
should be repeated on a surface with the capture system immobilized. If nonspecific
binding occurs, then, in addition to the procedures outlined in Fig. 2, alternative cap-
ture systems should be explored. In the case of streptavidin, substitution with avidin or
neutravidin often reduces background binding to zero.
3.2. Immobilization of Proteoglycans, Glycosaminoclycan Chains
and Oligosaccharides
The immobilization of ligands is accomplished by chemically activating a func-
tional group on the surface, e.g., carboxyl on carboxymethyl dextran or amino on
aminosilane. Since excellent protocols for ligand immobilization are supplied by the
manufacturers, only points specific to proteoglycans will be considered here. There
are major difficulties in the efficient direct coupling of proteoglycans, glycosaminogly-
can chains, and oligosaccharides to surfaces, due to the highly anionic character of
these molecules, which is a consequence of the carboxyl and sulfate groups present on
the saccharides. Thus, electrostatic uptake strategies, which are used to concentrate
ligand on carboxymethyl dextran surfaces to increase the efficiency of immobiliza-
tion, will not work, since the isoelectric point of the polysaccharide chains is lower
than that of the carboxyl groups on the matrix. Moreover, due to the high negative
Protein–Polysaccharide Interactions 509
charge of the glycosaminoglycan chains, proteoglycans and their saccharide
components will not penetrate readily into the carboxymethyl dextran gel in the absence
of a counterion. To obtain reasonable levels of immobilized ligand in the absence of
electrostatic uptake requires a high concentration of ligand (>1 mg/mL) during the
coupling reaction. Samples of proteoglycan, glycosaminoglycan chains, and oligosac-
charides are usually fairly precious. A capture system such as biotin–streptavidin
avoids the problems inherent to the direct coupling of proteoglycans and derived
polysaccharides, and so is the preferred method for the immobilization of these ligands.
In addition, the capture of biotinylated proteoglycans and polysaccharides allows
the oriented immobilization of the ligand, which optimizes ligate binding (see Sub-
heading 3.5.5.).
Fig. 2. Identification of zero background surfaces. Ligate is added in the buffer chosen for
binding assays at the highest concentration likely to be used in these assays. The most commonly
used detergent is Tween-20. In 0.5% (v/v) Tween-20 no background binding is observed with
crude lysates of tissues or cells on streptavidin-derivatized IAsys aminosilane surfaces (Wain-
wright, G. and Fernig, D.G., unpublished observations). Changing the charge of the surface at
physiological pH, e.g., negative charge of carboxymethyl dextran, is accomplished by repeated
activation of the charged groups of the surface. An elegant example is provided in ref. (8).
510 Fernig
Proteoglycans and glycosaminoglycan chains isolated as peptidoglycans can be
conveniently biotinylated on amino groups. Free amino groups may also occur along
the polysaccharide chain. If required, the relative level of biotinylation of peptide and
polysaccharide can be estimated, with respect to a specific ligate, e.g., basic fibroblast
growth factor (bFGF) (3). Oligosaccharides produced by enzymatic cleavage of gly-
cosaminoglycan chains, e.g., heparinase, will contain a reducing end. Again this pro-
vides a unique functionality that can readily be biotinylated.
3.3. Capture of Biotinylated Ligands, Surface Regeneration and Storage
3.3.1. Capture
Streptavidin, avidin, or neutravidin should be immobilized on the appropriate sur-
face according to the instructions of the instrument manufacturer. The following pro-
tocol is suitable for the IAsys instrument. It will have to be adapted for the other
instruments. In particular, high flow rates (100 µL/min) should be maintained through-
out the capture reaction in BIAcore and BioTul to ensure efficient mixing and hence
an even capture of biotinylated ligand over the sensor surface.
The cuvet is equilibrated in 50 µL of PBST. Replace the PBST with 20 µL
biotinylated proteoglycan, peptidoglycan chains, or oligosaccharides, usually at con-
centrations between 1 µg/mL and 1 mg/mL in PBST. The contact time of the
biotinylated ligand is generally 30 min.
If required, the unbound biotinylated material may be recovered by pausing the
experiment, withdrawing the cuvet and removing the bulk phase with a pipet.
Wash the cuvette 5 × 50 µL PBST.
Before using the cuvet for measurements, perform two cycles of regeneration, fol-
lowed by one binding reaction and one cycle of regeneration.
Before using a stored cuvet, perform one cycle of regeneration, followed by one
binding reaction and one cycle of regeneration.
3.3.2. Regeneration
Regeneration of the surface serves to remove all bound ligate, thus returning the
ligand to its original state. The importance of efficient regeneration cannot be over-
stated, since for optical biosensors to be useful the same surface, (see e.g., Subhead-
ing 3.5.), must be used for multiple, comparable measurements. It is essential that
regeneration protocols do not remove immobilized ligand or chemically alter the struc-
ture of the immobilized ligand. Luckily, glycosaminoglycans are robust ligands and
the biotin–streptavidin bond is extremely strong, resistant to 2 M NaCl, 20 mM HCl,
and 2 M guanidine. Most protein-glycosaminoglycan interactions depend heavily on
ionic bonding between the sulfate groups of the polysaccharide and the amino groups
of the protein. Therefore 2 M NaCl is usually sufficient to regenerate the surface.
When 2 M NaCl fails to remove all the bound the ligate, additional regeneration steps
with 20 mM HCl and, in extreme cases, with 2 M guanidine-HCl, may be required.
3.3.3.Storage
Cuvettes (BioTul, IAsys) and sensor chips (BIAcore) can be stored wet or dry at
4°C. For wet storage (<1 wk) of cuvets, put 70 µL PBST with 0.02 % (w/v) NaN
3
in the
