Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

458 Williams
1.2. Primer on General Lipoprotein Biology and Methods
Lipoproteins are noncovalent complexes of lipid and protein that allow the body to
transport many hydrophobic substances through the aqueous environment of blood.
All normally occurring mammalian lipoproteins have the same basic structure, known
as the oil-drop model (14): a central core of hydrophobic lipid, chiefly triacylglycerols
and esterified cholesterol, surrounded by a layer of amphipathic molecules, chiefly
phospholipids, unesterified cholesterol, and proteins known as apolipoproteins or
apoproteins (Fig. 1). The most widely used nomenclature defines mammalian lipopro-
teins by their densities: high-density lipoprotein (HDL, 1.063 g/mL < d < 1.21 g/mL),
low-density lipoprotein (LDL, 1.019 < d < 1.063 g/mL), and very low-density lipopro-
tein (VLDL, d < 1.006 g/mL). Sometimes, intermediate-density lipoproteins are
referred to as a separate class (IDL, 1.019< d < 1.019 g/mL). In addition, there are two
lipoproteins specifically associated with meals: the chylomicron (d < 0.96 g/mL),
which appears in plasma in the post-prandial state and transports lipids, chiefly
triacylglycerols, that have been ingested and absorbed; and the chylomicron remnant,
which can appear in the VLDL or IDL density ranges and is the particle that remains
after peripheral tissues have extracted most of the triglycerides from circulating
chylomicrons. An abnormal particle, β-VLDL, which appears in individuals with
certain genetic abnormalities in apolipoprotein (apo) E or after prolonged administra-
tion of high-cholesterol diets to experimental animals, is commonly used as an experi-
mental substitute for chylomicron remnants. Importantly, the different classes of
lipoproteins have distinct lipid compositions and apoprotein constituents (Table 1)
and hence distinct metabolic roles.
LDL and β-VLDL are the lipoproteins most commonly studied with proteoglycans.
They are abundant, easy to isolate, easy to label either radioactively or fluorescently, and
they exhibit important interactions with proteoglycans in vivo. Both contain apoB, a
large amphipathic protein that cannot move between lipoproteins and hence serves as an
excellent anchor for tags to track entire particles. In contrast, all other known apoproteins
readily exchange between lipoprotein particles, and surface and core lipids are moved
between lipoproteins by lipid transfer proteins in human plasma. Both LDL and β-VLDL
bind cell-surface LDL receptors in vitro, but are also known to undergo substantial
catabolism in vitro (1,15) and in vivo (16,17) independent of LDL receptors. Both of
these particles have been implicated in atherosclerotic vascular disease.
Isolation of these particles from plasma requires sequential ultracentrifugation at
accelerations sufficient to overcome Brownian motion. Several companies in the U.S.
sell human LDL, such as Sigma Chemical Company (St. Louis, MO), CalBiochem (La
Jolla, CA), Molecular Probes (Eugene, OR), and Academy Bio-Medical (Houston,
TX), but as with most materials, it can be less expensive to isolate it yourself, particu-
larly for long-term needs [ultracentrifugal isolation is described in detail in (18,19)].
Radioactive labeling is best performed by the iodine monochloride method (19–22),
which covalently attaches
125
I to tyrosyl residues in apoproteins, with minimal disrup-
tion of double bonds in the fatty acyl side chains of lipoprotein lipids. Fluorescent
labeling usually employs DiI or DiO, which are generally nontoxic lipophilic com-
pounds that insert into cell membranes or lipoprotein surfaces, but then are poorly
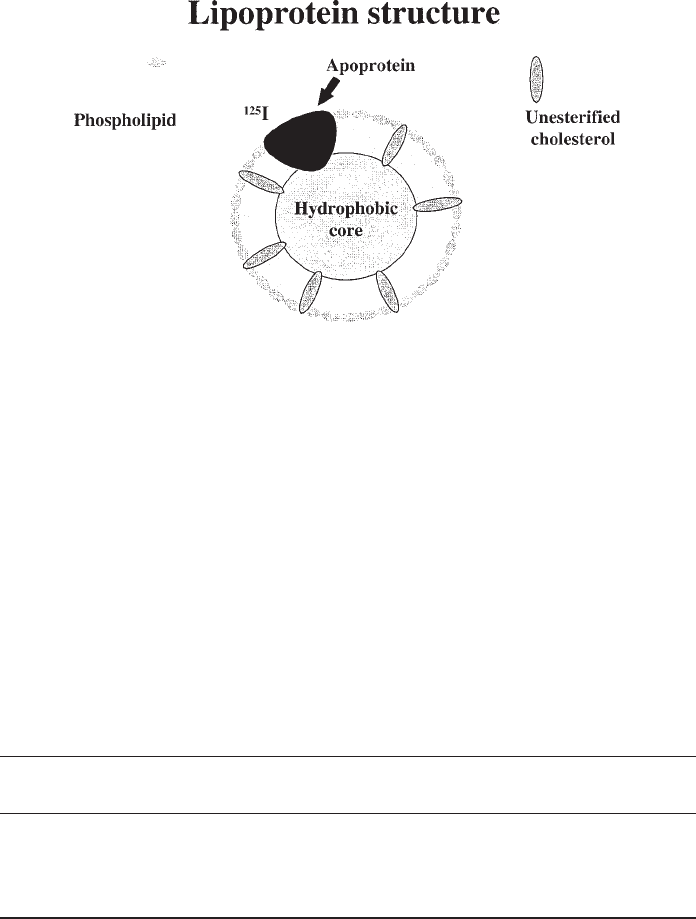
Lipoprotein Proteoglycan Interactions 459
Fig. 1. General schematic of normal lipoprotein structure. All normal human plasma lipopro-
teins exhibit the same basic structure, known as the oil-drop model, which consists of a single
layer of amphipathic molecules (phospholipids, apoproteins, and unesterified cholesterol)
surrounding a hydrophobic core (cholesteryl ester or triacylglycerides). The fatty acyl side chains
of each phospholipid molecule point inward toward the hydrophobic core, while the polar head
group is exposed on the particle surface. Apoproteins are similarly oriented, with a hydrophobic
face associating with lipid, and the hydrophilic face directed outward. Owing to its alcohol group,
unesterified cholesterol is associated mainly with the phospholipid molecules of the particle
surface, although some partitions into the core. This overall arrangement keeps hydrophobic
molecules and domains shielded from the surrounding aqueous environment of blood and
interstitial fluid. Only one schematic apoprotein has been drawn, although many species of
lipoproteins have several protein molecules per particle. Apoproteins are often convenient sites to
place radioactive tags, shown here as an
125
I label (see Subheading 1.2. for more details).
Table1
Physical Characteristics of the Major Plasma Lipoproteins in Humans
Diameter Core Principal
Particle (nm) lipid Apoproteins
Chylos 80–1000 Tg (diet) B
48
, AI,AII,CII,E
VLDL 30–80 Tg (liver) B
100
, CII,E
LDL 18–28 ChE B
100
HDL 5–12 ChE AI, AII, occasionally E
Abbreviations: Chylos, chylomicrons and remnants; VLDL, very low-density lipoprotein; LDL,
low-density lipoprotein; HDL, high-density lipoprotein; diet, originating from dietary intake and
secreted by the intestines; liver, synthesized and secreted from the liver; Tg, triacylglycerol; ChE,
cholesteryl ester; B
100
, the full-length apolipoprotein-B, which is originates primarily from the liver;
B
48
, a truncated version of apolipoprotein-B that contains the N-terminal 48% of the full molecule and
originates primarily from the intestine.
460 Williams
exchangeable to other particles or surfaces. These moieties emit at 571 nm (orange)
and 501 nm (green), respectively, and can therefore be used with standard optical fil-
ters for rhodamine and fluorescein during multicolor imaging. Fluorescently labeled
lipoproteins are available commercially (Molecular Probes, Eugene, OR, and Leiden,
The Netherlands), and so protocols from the literature for preparing these particles
(22,23) are not reproduced here. Quantitative methods using fluorescently labeled
lipoproteins have been developed (24), but
125
I-labeled lipoproteins remain the stan-
dard in catabolic studies and will be the focus of the rest of this chapter.
The parameters most commonly measured after incubation of
125
I-lipoproteins with
cells are surface binding, intracellular accumulation, and lysosomal degradation of
the particles. Typically, monolayers of essentially confluent cells are used, usually
fibroblasts, macrophages, or hepatocytes, that have been preincubated overnight in
cholesterol-poor medium to stimulate expression of LDL receptors (19). Cells are then
incubated at 37°C for 2–6 h in the continuous presence of
125
I-labeled LDL or β-VLDL
(usually 1–10 µg lipoprotein protein per millilliter of medium, although wider concen-
tration ranges are used for formal assessment of the K
d
and V
max
). During this time,
particles bind to the cell surface, become internalized, and are then delivered to lysos-
omes. In the lysosomes,
125
I-labeled protein is degraded to individual amino acids, but
[
125
I]monoiodotyrosine, the radiolabeled form of tyrosine, does not charge the tyrosine-
specific tRNA (25), and so it simply leaks out of the cells back into the media. At the end
of the incubation, cells are chilled to 4°C to stop further metabolism. The culture media
are harvested for isolation of [
125
I]monoiodotyrosine as an indication of lysosomal
degradation (see Subheading 3.1.) (19,26). The cell monolayers are rinsed twice for 10
min with chilled phosphate- or Tris-buffered saline, pH 7.4, supplemented with 2 mg of
fatty acid-free bovine serum albumin per milliliter, then twice rapidly with chilled buff-
ered saline without albumin. Surface-bound particles are released from LDL receptors
and other sites by a 30-min incubation at 4°C in buffered saline with 10 mg of heparin/
mL. The cell monolayers are rinsed rapidly once more in buffered saline at 4°C, this
rinse is pooled with the heparin wash, and the radioactivity in an aliquot is determined
by gamma counting. The cell monolayers are then dissolved in 0.1 M NaOH. One ali-
quot of dissolved cells is used for gamma counting to assess intracellular accumulation
of labeled material, and another aliquot is used to assess total cellular protein content.
Radioactivity results are converted to mass of lipoprotein protein using the known
specific activity of the particular radiolabeled preparation, and results are expressed as
nanograms of catabolized lipoprotein protein per milligram of total cell protein. To verify
that particle degradation involves lysosomes, cells are treated with chloroquine (150
µM), an inhibitor of lysosomal proteases (19), beginning 0–45 min before the incubation
with
125
I-labeled lipoproteins, and continuing until the end of the experiment. Typically,
ligand degradation is inhibited by 85–90%, and there is a corresponding increase in the
accumulation of intracellular radioactive material.
1.3. Background for Methods Used
to Study Lipoprotein-HSPG Interactions
Several modifications in the general methods outlined above are necessary for the
study of lipoprotein–HSPG interactions. First, there are several distinct genetic
Lipoprotein Proteoglycan Interactions 461
families of cell-surface HSPGs, prinicpally syndecans, perlecan, and glypicans, and it
is most informative to use cellular preparations that have one predominant cell-surface
HSPG, or else a limited and known combination of these HSPGs. For the study of
syndecans (Fig. 2A, left schema), we have used mainly Chinese hamster ovary (CHO)
cells that we transfected with an expression construct for the human syndecan-1 core
protein (27). For perlecan, we have used the WiDr colon carcinoma line, which
expresses perlecan but no other proteoglycans (American Type Culture Collection
[ATCC], Manassas, VA, cat. no. CCL 218, also known as HT-29) (28–32). For
glypicans, there are reports of transfected mammalian cells in the literature (33).
Second, the binding of lipoproteins to HSPGs in vivo is facilitated by bridging mol-
ecules, such as lipoprotein lipase (LpL), apoE, defensins, and hepatic lipase, each of
which has a hydrophobic face that adheres to the lipoprotein surface and a cationic face
that binds HS. Thus, studies in vitro typically involve examination of cellular catabo-
lism of lipoproteins in the absence and in the presence of one of these molecules, and
the arithmetic increase in each of the catabolic parameters is calculated (27,32,34). We
have preferred to use LpL because, unlike apoE, it does not bind LDL receptors, and
unlike hepatic lipase, it is relatively easy to isolate in large quantities [see Subheading
3.2., adapted from references (35,36)]. Typically, we add 5 µg of
125
I-labeled lipoprotein
protein per milliliter of medium, without or with 5 µg LpL/mL. Alternatively, cells that
naturally secrete these bridging molecules (37) or cells transfected to express them [e.g.,
(38,39)] can be used. The role of HSPGs in the increased catabolism upon addition of
LpL is verified by heparitinase digestion of the cells, which typically abolishes ~90% of
LpL-dependent catabolism (27,34,37,40,41); by the use of HS-negative CHO mutants
(34,40,42,42a); by addition of very low concentrations of heparin (<100 µg/mL) that are
insufficient to interfere with LDL receptor binding but are able to displace surface-bound
LpL and other bridging molecules (34,42a); or by pre-incubation of cells in chlorate to
block sulfation of glycosaminoglycan side chains (43–45). Heparitinase digestion usu-
ally involves preincubation of cells in serum-free medium at 37°C for several hours to
allow the cells to clear surface-bound serum-derived molecules, then an initial digestion
with heparitinase for 60–90 min at 37°C before addition of ligand, and finally an incuba-
tion of cells with ligand, but in the continuous presence of heparitinase, to avoid
rapid regeneration of side chains (34). Apoproteins normally found on LDL, VLDL,
and β-VLDL include apoB and apoE, both of which bind HS, although lipoprotein con-
centrations around 100 µg/mL are usually required before this binding becomes a sig-
nificant contributor to total cellular catabolism in the absence of added bridging
molecules (15,45). These higher concentrations may be physiological, and LDL recep-
tor-independent clearance of lipoproteins is substantial in vivo (16,17). Interestingly,
two common, naturally occurring polymorphisms of apoE, one of which has been asso-
ciated with Alzheimer’s diease (46), show substantial differences in their catabolism by
cells through a pathway mediated by HSPGs (47), particularly syndecan HSPGs (48).
Third, careful attention must be paid to catabolic contributions by members of the
LDL receptor family. These contributions take the form of direct internalization, which
appears simply as background in measurements of lipoprotein catabolism in the
absence of LpL, and synergistic interactions, in which cell-surface HSPGs and LDL
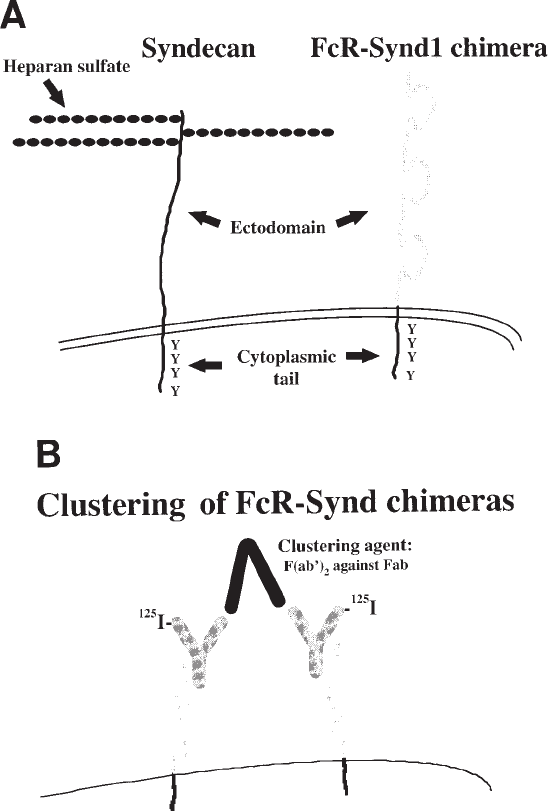
462 Williams
Fig. 2. The FcR-Synd1 chimera, a molecular tool to study the syndecan transmembrane and
cytoplasmic domains. (A) Comparison of the syndecan-1 HSPG (left schema) and the FcR-
Synd1 chimera (right schema). As indicated, the chimera contains the ectodomain of the IgG
Fc receptor-Ia linked to the highly conserved transmembrane and cytoplasmic domains of the
human syndecan-1 core protein (27). (B) Clustering of FcR-Synd chimeras. Two chimeric mol-
ecules are shown, each bound to an
125
I-labeled nonimmune human IgG. Clustering is accom-
plished by adding the clustering agent, that is, goat F(ab')
2
fragments against the Fab domain of
human IgG. This clustering agent cannot interfere with the binding of whole IgG to the chime-
ras, because it has no Fc domain to bind to the chimeras, nor does it interact with the Fc domain
of the IgG ligands.
Lipoprotein Proteoglycan Interactions 463
receptor family members co-operate in ligand catabolism. Direct internalization via
LDL receptor family members is easier to deal with. LDL can be 35% reductively
methylated (mLDL; see Subheading 3.3.), which abolishes its ability to bind LDL
receptors (49) while having no effect on the binding to cell-surface HSPGs in the
presence of LpL (27,32). In an experimental tour de force, a series of transgenic mice
were created that express different site-directed mutants of human apoB, including
one variant with defective LDL receptor binding but unaltered affinity for
proteoglycans, and another that binds LDL receptors but not proteoglycans (9,10,10a).
Monoclonal antibodies against specific domains of apoB and apoE have been reported
and used in studies of lipoprotein–proteoglycan interactions (50,51) (many of these
antibodies are available for purchase from the University of Ottawa Heart Institute,
Ottawa, Ontario, Canada). Cellular LDL receptors can be blocked with a polyclonal
antibody (52) or with a monoclonal antibody produced by a clone available from the
ATCC (#CRL 1691), but this monoclonal antibody must already be bound to cells
before the lipoproteins are added (53,54). Cellular LDL receptors are readily
suppressed in most cell types by an overnight incubation in medium supplemented
with 1–2 µg of 25-hydroxycholesterol, which potently downregulates LDL receptors,
plus 20-40 µg of cholesterol per milliliter to protect the cells from the toxicity of
25-hydroxycholesterol (19), although cholesterol-induced alterations in glycosami-
noglycan synthesis have been reported (55). Suppression of LDL receptors must be
verified by a substantial reduction in
125
I-LDL binding: some macrophage-like cell
lines (56) and cells expressing cholesterol 7α-hyroxylase (57), such as hepatic paren-
chymal cells, maintain significant LDL receptor expression despite the presence of
abundant sterol. Unlike proteoglycans, LDL receptor family members depend on
calcium ions for binding (19), which can be chelated by EDTA (45,51). Finally, LDL
receptor-negative human fibroblasts (the ATCC has several lines) (19) and CHO cells
(58), as well as an LDL receptor knockout mouse (Jackson Laboratory, Bar Harbor,
ME, cat. no. 002207) (59), are available. Owing to their simplicity, our methods of
choice for controlling ligand binding to the LDL receptor in vitro have been LDL
methylation or cellular supplementation with sterols.
The LDL receptor-related protein (LRP), a cell-surface LDL receptor family member
that has been reported to bind LpL, apoE, and hepatic lipase, is readily blocked by the
39-kDa receptor-associated protein (RAP), which is available as a recombinant protein
(60). Unfortunately, RAP is the human homolog of mouse heparin-binding protein 44
(61), readily binds heparin (61), and therefore might also compete for binding to HS side
chains, particularly ones that are rich in heparin-like domains. In the experience of our
laboratory (27,32), RAP has had small or no effects on the binding of LpL-enriched
125
I-
labeled mLDL to the HSPGs of CHO or WiDr cells, although the binding of RAP to cell-
surface HSPGs remains controversial (62,63). To our knowledge, no studies have
systematically examined the binding of RAP to hepatic parenchymal cells, which syn-
thesize HS with abundant heparin-like domains (64). LRP-deficient cell lines (65–67)
and liver-specific knockout mice (68) have been reported, although the status of their
proteoglycans is unknown.
125
I-labeled RAP is prepared using Iodobeads (Pierce Chemi-
cal Company, Rockford, IL) instead of the iodine monochloride method, owing to the
464 Williams
lack of unsaturated lipid in RAP, and this radiolabeled molecule can serve as a convenient
ligand for coated pit-mediated internalization, provided it is known to bind poorly to the
particular HSPGs that are expressed by the cell type of interest (27).
125
I-RAP can be
released from cell-surface LRP by a protease cocktail, to distinguish surface-bound from
internalized material (27,69).
Synergistic interactions between cell-surface HSPGs and LDL receptor family
members are thought to involve either the transfer of ligand from HSPGs to LDL
receptor family members or the formation of ternary complexes of HSPGs,
HSPG-bound ligands, and LDL receptor family members (40,41,70,71). No direct
evidence to date has distinguished these possibilities. Quantification of synergy
between cell-surface HSPGs and LDL receptors requires measurement of the catabo-
lism of
125
I-labeled native LDL and
125
I-mLDL, each in the absence and presence of
LpL. Four catabolic components can then be computed (32): (1) the LDL receptor-
independent, LpL-independent component, which is usually referred to as nonspecific
uptake or assay background and is measured by the catabolism of
125
I-mLDL in the
absence of LpL; (2) the LDL receptor-dependent, LpL-independent component, which
is the classical LDL receptor pathway and is computed by the difference between the
catabolism of
125
I-LDL vs
125
I-mLDL; (3) the LDL receptor-independent, LpL-depen-
dent component, which involves cell-surface HSPGs and is computed by the differ-
ence between the catabolism of
125
I-mLDL in the presence vs the absence of LpL; and
(4) a synergistic component, which requires cooperation between LDL receptors and
LpL and is computed as the increase in
125
I-LDL catabolism upon addition of LpL
minus the increase in
125
I-mLDL catabolism upon addition of LpL. Total catabolism
of
125
I-labeled native LDL in the presence of LpL equals the sum of these four compo-
nents, reflecting the ability of LpL-enriched
125
I-LDL to participate in these four
potential uptake mechanisms (32). Similar information can be obtained using cellular
sterol enrichment instead of LDL methylation to manipulate binding to the LDL
receptor, or heparitinase digestion instead of omission of LpL to manipulate binding
to cell-surface HSPGs. These alternative methods tend to be more cumbersome and
somewhat less effective and, as noted above, cellular sterol enrichment has been
reported to affect glycosaminoglycan synthesis (55). In WiDr cells under our experi-
mental conditions, we have found that the four components respectively account for
approximately 4%, 15%, 57%, and 23% of total LpL/
125
I-LDL catabolism. In other
words, most LpL/
125
I-LDL enters these cells via perlecan directly (component 3) with-
out any assistance from LDL receptors (31,32).
Fourth, careful attention must be paid to the unusual characteristics of HSPG-medi-
ated ligand internalization. To assess the efficiency of endocytosis, we calculate ligand
internalization as the sum of intracellular accumulation plus degradation (27). Internal-
ization calculated in this fashion takes into account ligand still within the cells, as well as
ligand that had been taken up by cells but then degraded into amino acids, which the
cells release to the culture medium. To examine in detail the kinetics of ligand internal-
ization and degradation, we incubate labeled ligands with cells in
serum-free medium for 1 h at 4°C, to allow surface binding without further catabolism,
and then the cells are washed at 4°C to remove unbound material. Fresh media at 37°C
Lipoprotein Proteoglycan Interactions 465
with no ligands are added, and incubations are continued at 37°C for various times,
usually 15 min to 24 h (27,32). Assays for surface-bound, intracellular, and degraded
ligand are then performed, and ligand internalization is calculated. In some experiments,
TCA-precipitable radioactivity in the media is also quantified (Subheading 3.1.), as an
indication of retroendocytosis or desorption from the cell surface during the incubation
at 37°C. In addition, because the principle method under Subheading 3.1. for assessing
lysosomal degradation requires complete breakdown of labeled apoproteins into indi-
vidual amino acids, supplementary tests for partial breakdown products can also be
informative (also given under Subheading 3.1.). From these studies, we have found that
syndecan-mediated endocytosis of LpL/
125
I-mLDL proceeds with a t
1/2
of ~1 h (27), and
that perlecan-mediated internalization exhibits a t
1/2
of ~5 h (31,32). By contrast, the t
1/2
for coated pit-mediated internalization is ~10 min (27,32,72,73).
HSPG-mediated pathways for ligand internalization can also be distinguished through
the use of metabolic inhibitors, particularly genistein (0–400 µM), a tyrosine kinase
inhibitor (74), and cytochalasin D (0–2 µM), which disrupts the actin cytoskeleton (75).
Typically, ligands are bound to the cell surface at 4°C, unbound ligand is washed away,
and then specific inhibitors are added simultaneously with fresh medium at 37°C. Cells
are incubated at 37°C until ~30–50% of surface-bound ligand has been internalized in
the absence of inhibitors, that is, 45 min for syndecan and 2 h for perlecan (incubations
longer than 2 h can encounter fading of the genistein effect) (27,76). To allow compari-
son with coated pit-mediated internalization, we employ a 30-min pre-incubation at 37°C
in the presence of these inhibitors then a 10- to 15–min incubation in the presence of
inhibitors plus
125
I-RAP (27) or surface-bound
125
I-LDL (32). Coated pit-mediated
endocytosis is insensitive to genistein, whereas both syndecan- and perlecan-mediated
internalization are inhibited (27,32). Coated pit endocytosis exhibits limited sensitivity
to cytochalasin (27,32,77), whereas syndecan-mediated endocytosis is readily inhibited
(27), and perlecan-dependent internalization is slightly enhanced by this agent (32). Thus,
the three pathways exhibit distinctive kinetics of intenalization and different dependence
on tyrosine kinases and the actin cytoskeleton.
1.4. Background for Molecular Methods
to Study HSPG-Mediated Endocytosis
The underlying model is that each cell-surface HSPG, like other receptors, is
organized into domains that mediate specific functions. Portions of the HS side chains
serve as the ligand-binding domains, while regions within the core protein are respon-
sible for recruiting the specific cellular machinery used for internalization of that
particular species of HSPG [see (1,33,78)]. In this light, the distinct pathways for
cellular uptake of ligands bound to syndecan HSPGs versus the perlecan HSPG can be
understood as a consequence of the differences between the two core proteins, which
lack any significant homology to each other.
To examine this model, we constructed the FcR-Synd1 chimera, which consists of
the ectodomain of the IgG Fc receptor-Ia linked to the highly conserved transmem-
brane and cytoplasmic domains of the human syndecan-1 core protein (Fig. 2A, right
schema) (27). Thus, this chimera contains the portions of the syndecan-1 core protein
466 Williams
that contact the plasma membrane and cellular interior. The ligand for the chimera is
125
I-labeled nonimmune human IgG, which we prepare using Iodobeads. We have
made this chimera and cells that express it available to other investigators, so the pro-
tocols for construction and transfection are not reproduced here.
The FcR-Synd1 chimera offers many advantages, primarily by providing a simple and
easily controlled experimental system. First, contributions to ligand catabolism from other
cell-surface molecules, such as LDL receptor family members and HSPGs, are completely
eliminated if the chimera is expressed in a cell type with no endogenous Fc receptors.
Thus, we stably express FcR-Synd1 in CHO cells, which we have already used to study
syndecan-mediated endocytosis (27). Second, ligand clustering, which appears to trigger
efficient syndecan-mediated endocytosis, can be induced at will through the use of a clus-
tering agent (see Fig. 2B and Subheading 3.4.) (27). Third, the ligand,
125
I-IgG, does not
deliver any lipids to the cells, nor is it broken apart by detergents. Thus, the role of cold
Triton-insoluble, cholesterol-rich membrane rafts in this endocytic pathway can be more
easily examined than when relying on lipoproteins as ligands (see Subheading 3.5.)
(79,80). Fourth, in terms of kinetics and the dose-responses to different metabolic inhibi-
tors, the cellular uptake of surface-bound, clustered
125
I-IgG is identical to the uptake of
LpL/
125
I-mLDL, which supports the model described above (27).
Most important, the syndecan domains within the FcR-Synd1 chimera are readily
deleted or mutated by standard molecular techniques, thereby allowing identification
of molecular determinants for each stage of this endocytic pathway (81).
2. Materials
2.1. Purification of [
125
I]Monoiodotyrosine from Culture Media,
as an Assay of Degradation of
125
I-Proteins in Lysosomes
1. Two sets of borosilicate glass tubes, 12 × 75 mm, with one tube in each set for each
sample of medium.
2. One set of 12 × 75-mm plastic tubes (e.g., Sarstedt, Newton, NC, cat. no. 55.476) with
push-in stoppers (e. g., Sarstedt cat. no. 65.809).
3. 50% (w/v) solution of trichloroacetic acid, kept at 4°C, 0.25 mL for each sample of medium.
4. Freshly made solution of 40% (w/v) of KI, 5 µL for each sample of medium.
5. 30% H
2
O
2
, 20 µL for each sample of medium.
6. Chloroform, analytic grade, 1–2 mL for each sample of media.
7. Table-top refrigerated centrifuge (e.g., a Beckman GS-6R).
8. Fume hood.
9. Mixing platform for test tubes, such as a Vortex Genie.
10. Gamma counter.
11. Additional materials for Subheading 3.1., step 8 (optional):
a. 20% (w:v) solution of trichloroacetic acid, kept at 4°C.
b. 0.1 N NaOH solution.
12. Additional materials for Subheading 3.1, step 9 (optional):
a. Buffered saline with 10 mg heparin/mL, kept at 4°C.
b. 1% solution of sodium dodecyl sulfate (SDS).
c. SDS-polyacrylamide gel and electrophoresis apparatus.
d. Materials for autoradiography, preferable a PhosphorImager (Molecular Dynamics,
Sunnyvale, CA).
Lipoprotein Proteoglycan Interactions 467
2.2. Isolation of Bovine Milk LpL
1. 3.6 L of raw, fresh whole cows’ milk (it is best to milk the cows on the morning the
isolation begins, and the milk must be promptly chilled and kept cold).
2. 160 mL of heparin-agarose beads (Bio-Rad cat. no.153-6173 is suitable and inexpensive).
3. One 4-L vacuum Erlenmyer flask with a large scintered glass funnel.
4. One 4-L beaker.
5. One 250-mL beaker.
6. A refrigerated table-top centrifuge with centrifuge bottles, to accommodate about 4 L of
liquid (Four Beckman 750-mL centrifuge bottles in a GS-6R centrifuge work well).
7. Cheesecloth or any other sort of fine mesh that is lint-free.
8. Seven 700-mL plastic culture flasks with canted necks.
9. A magnetic stir plate, kept at 4°C.
10. A rocking platform, kept at 4°C.
11. A fraction collector, kept at 4°C.
12. A glass chromatography column, 2.5 cm × 30 cm (e.g., Bio-Rad cat. no. 737-2532), kept
at 4°C, and connected to the fraction collector.
13. A small funnel for the glass chromatography column (e.g., Bio-Rad cat. no. 731-0003).
14. A spectrophotometer, to measure absorbance at 280 nm.
15. Distilled water, chilled to 4°C.
16. Clean, powder-free gloves or a large, clean spatula.
17. Buffers, chilled to 4°C:
a. 2 L of 0.4 M NaCl, 10 mM Tris/HCl, pH 7.4
b. 2 L of 0.75 M NaCl, 10 mM Tris/HCl, pH 7.4
c. 1 L of 1.5 M NaCl, 10 mM Tris/HCl, pH 7.4
d. 3 L of phosphate-buffered saline, pH 7.4
e. 200 mL of phosphate-buffered saline, pH 7.4, with 0.02% sodium azide
18. Air-tight 2-mL freezer vials: these must have screw caps that close on an O-ring, to avoid
sublimation of H
2
O during storage in a freezer (e.g., Sarstedt cat. no. 72692005).
19. 8 M urea, 1.5 M NaCl, in phosphate buffer, pH 7.4, at room temperature (optional).
2.3. Reductive Methylation of LDL
1. 1.5 L of lipoprotein buffer (150 mM NaCl, 0.3 mM Na
2
EDTA, pH 7.4) at 4°C.
2. A preparation of LDL or
125
I-LDL of known protein concentration, in lipoprotein buffer
at 4°C.
3. 0.1 M NaCNBH
3
(sodium cyanoborohydride; Aldrich) in saline at 4°C.
4. 133.2 mM HCHO in distilled water (mix 10 µL of Fisher F79-500 ACS-grade formalde-
hyde, which is 37% w/w, i.e., 13.32 M, with 990 µL of distilled water) at 4°C.
5. Dialysis membranes, such as 30-kDa cutoff dialysis tubes (e.g., Spectra/Por, carried by
Fisher Scientific, Pittsburgh, PA) or 10-kDa membrane cassettes (e.g., Slide-A-Lyzer,
Pierce), which are very convenient.
6. 0.45-µm syringe filter, such as from Millipore (Bedford, MA, USA), and a syringe.
2.4. Special Methods Allowed by the Use of the FcR-Synd1 Chimera:
Control of Ligand Clustering
1. CHO cells expressing the FcR-Synd1 chimera, grown in Ham’s F-12 medium with 10%
fetal calf serum to a confluent monolayer.
2. Ice tray or cold room.
3. Phosphate- or Tris-buffered saline, pH 7.4, at 4°C.
