Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

438 Patel and Iozzo
changes are difficult to distinguish from actual changes in [Ca
2+
] when using
intensiometric indicators. For example, in many cells, dye can be actively extruded
from the cytosol or undergo photobleaching (see Note 1), which results in a decrease
in fluorescence intensity. This decrease in fluorescence would appear as a decrease in
[Ca
2+
] when measured at a single wavelength. For ratiometric dyes such as fura-2,
however, nonreciprocal changes in the fluorescence intensities are cancelled out after
calculation of the fluorescence ratio (Fig. 2B). Ratiometric recording is also less prone
to artefacts arising from cell movement, uneven dye distribution, or inhomogeneities
in cell thickness. Fura-2, then is the dye of choice for single cell Ca
2+
imaging.
1.2. Loading Fluorescent Ca
2+
Indicators Into Cells
Most Ca
2+
indicators are available as acetoxymethyl (AM) esters, rendering them
cell permeable (15). Once inside the cell, endogenous esterases cleave the ester bonds,
releasing the negatively charged dye and essentially trapping it within the cell. Thus,
simple incubation of the cells in a physiological medium supplemented with the AM
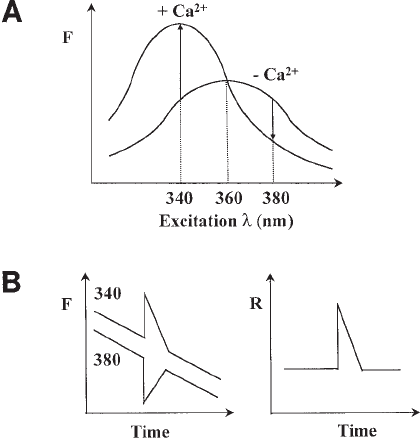
Fig. 2. (A) Excitation spectra of fura-2 in the presence of zero and saturating Ca
2+
. The
graph shows fluorescence (F) of the dye measured at 510 nm at a range of excitation wave-
lengths. Binding of Ca
2+
reduces the excitation peak from 360 to 340 nm. Fluorescence of the
dye therefore increases at 340 nm and decreases at 380 nm in response to Ca
2+
. No change in
fluorescence occurs at 360 nm. (B) Ratio measurements cancel out nonreciprocal changes in
fura-2 fluorescence. A schematic fura-2 timecourse recording showing a change in [Ca
2+
] manifest
as a transient increase and decrease in fluorescence at excitation wavelengths of 340 and 380
nm, respectively (left). The response is superimposed on a progressive slow decrease in fluo-
rescence at both wavelengths. These non-reciprocal (Ca
2+
-independent) changes in fluores-
cence are eliminated after calculation of the fluorescence ratio (340/380, right).
Principles of Ca
2+
Imaging 439
ester for a defined period of time results in significant accumulation of the dye within
the cell (see Subheading 3.1.). The ease by which these dyes can be introduced into
cells is a major advantage of using these dyes for monitoring [Ca
2+
].
1.3. Instrumentation
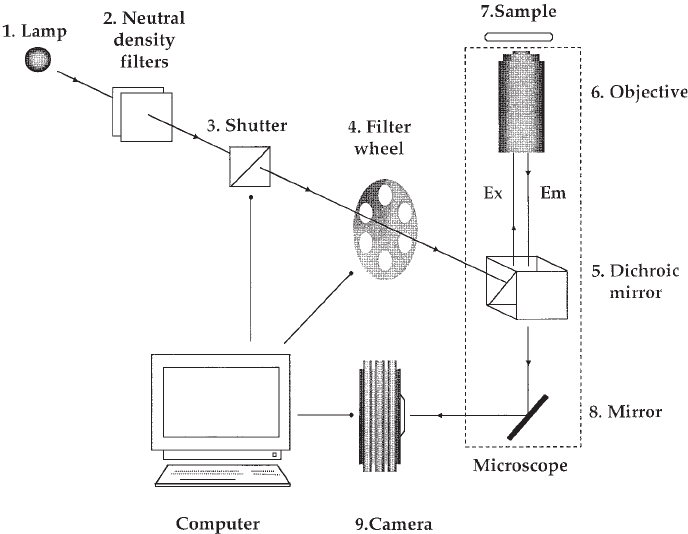
Central to an imaging system (Fig. 3) is the epifluorescence microscope that houses
appropriate optics to allow delivery of the exciting light and isolation of the emitted
fluorescence. Briefly, cells grown on a glass cover slip are loaded with a fluorescent
indicator and placed into a holder on the stage of the microscope. Excitation light of
the appropriate wavelength for the indicator is selected (usually with a filter) and
directed to the cells via the objective lens through which the cells are imaged. Part of
the resulting emitted fluorescence is collected by the objective and discriminated from
the excitation light by a dichroic mirror. The light is then focused onto a camera, digi-
Fig. 3. Schematic representation of a typical imaging system. Light from an arc lamp
(1) is attenuated by a neutral density filter (2) and passed through a shutter (3) to a filter
wheel (4) that selects the appropriate excitation wavelength. The excitation light (Ex) then
enters the microscope and is reflected by a dichroic mirror (5) up into the objective (6) to
the sample (7) loaded with the fluorescent dye. The emitted fluorescence (Em) is then
collected by the objective and directed back to the dichroic mirror, where it is transmitted
to a second mirror (8) that reflects the light to the camera (9). The shutter, filter wheel, and
camera are computer-controlled.

440 Patel and Iozzo
tized, and stored to a computer. By periodically illuminating the cells through the use
of a computer-controlled shutter, a series of images is collected. Regions of interest,
corresponding to individual cells (or subcellular regions) are selected by the user, and
data are extracted from the image at each time point. Fluorescence intensities/ratios
can then be calibrated to [Ca
2+
] for individual cells in the entire field. A brief description
of the principle components of an imaging system is given in Table 1.
Many imaging systems are now available commercially. Normally, an inverted as
opposed to an upright microscope configuration is adopted, since access to the sample (for
perfusion or microinjection) is unhindered. The choice of camera is one of the most impor-
tant considerations in low-light-level studies. Cameras can be broadly classified according
to their output, which may in the form of a standard video signal (e.g., silicon-intensified
target cameras) that is later digitized or direct digital readout (e.g., cooled charged-coupled-
device cameras). The latter provide better signal-to-noise ratios but are inherently slower.
High-sensitivity cameras allow illumination intensity to be reduced without compromis-
ing signal, thus reducing photobleaching of the dyett. Imaging formats such as 1024 by
1024 pixels are typical. The range of digitisation (8–16 bit) determines the dynamic range
(256–65536 intensity levels per pixel) (see ref. 16 for further details).
1.4 Calibration of Fluorescence Data
Once fluorescence data are acquired, it is relatively straightforward to convert them
to [Ca
2+
]. Data should first be corrected for background fluorescence that is, cell-
independent (“instrument”) noise. For intensiometric indicators, fluorescence inten-
sity (F) is related to [Ca
2+
] by the following equation:
(F – F
min
)·K
d
(F
max
– F)
where F
min
and F
max
are the fluorescence intensities of the Ca
2+
-free and Ca
2+
-satu-
rated dye, respectively, and K
d
is the dissociation constant of the dye for Ca
2+
. With
these indicators, an in situ calibration is necessary since measured fluorescence is
dependent on dye concentration (see Subheading 3.4.1.). This involves exposing cells
to a Ca
2+
ionophore such as ionomycin in order to equilibrate Ca
2+
across the plasma
membrane, and then setting the extracellular [Ca
2+
] to zero and saturating levels to
derive F
min
and F
max
, respectively. The parameters are thus determined under similar
dye loading levels as those during experimentation.
For ratiometric indicators, the fluorescence ratio, R (Ca
2+
-bound fluorescence/Ca
2+
-
free fluorescence) is related to [Ca
2+
] by the following equation:
(R –R
min
)K
d
· S
f2
(R
max
–R)S
b2
where R
min
and R
max
are the fluorescence ratios of the Ca
2+
-free and Ca
2+
-saturated
dye, respectively, K
d
is the dissociation constant of the dye for Ca
2+
, and S
f2
/S
b2
is the
ratio of fluorescence values for the Ca
2+
-free and Ca
2+
saturated dye at the wavelength
used to monitor the Ca
2+
-free indicator. For fura-2, R is the fluorescence of the dye at
340 nm excitation/fluorescence of the dye at 380 nm excitation and S
f2
/S
b2
is
determined at 380 nm. At 37°C, the K
d
of fura-2 is 224 nM.
[Ca
2+
] =
[Ca
2+
] =
·
Principles of Ca
2+
Imaging 441
R
min
and R
max
(from which S
f2
/S
b2
is calculated) can be determined in situ with iono-
phore as with intensiometric indicators. However, as ratios are independent of dye con-
centration, calibration parameters can also be obtained (for the particular instrument
configuration employed) in vitro that is, in the absence of cells (see Fig. 4A; Subhead-
ing 3.3.2.). This is achieved using an intracellular-like solution supplemented with the
free (de-esterified) form of the dye in the presence of BAPTA and excess Ca
2+
to deter-
mine the fluorescence of the Ca
2+
-free and Ca
2+
-bound dye, respectively. Unlike in situ
calibrations, which need to be performed at the end of each experimental run, in vitro
calibrations need only be performed once on the day of experimentation. Furthermore, it
is often difficult to completely equilibrate Ca
2+
across the plasma membrane in in situ
calibrations. However, a major assumption in in vitro calibrations is that the conditions
mimic the environment of the dye within the cell, which may not necessarily be the case.
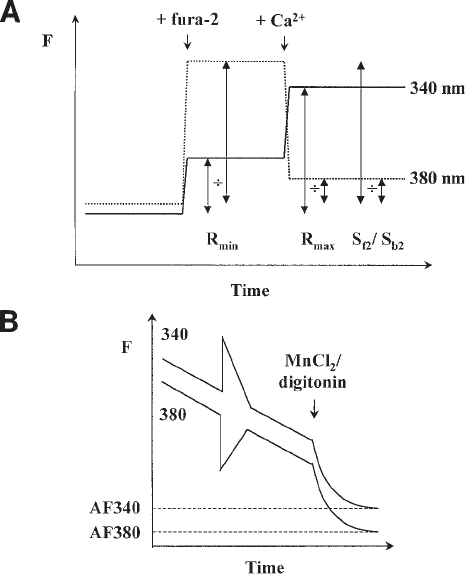
Fig. 4. In vitro calibration of ratiometric fluorescence data. (A) A cytosol-like calibration
medium (initially Ca
2+
-free) is used to determine calibration parameters (see Table 2). For
fura-2, fluorescence of the medium at 340-nm (solid line) and 380-nm (dotted line) excitation is
acquired first in the absence of dye to determine background fluorescence. Fura-2 free acid
(1 µM) and then CaCl
2
(500 µM) are added to determine R
min
, R
max
, and S
f2
/S
b2
as shown. These
parameters are used to convert acquired ratio values to [Ca
2+
] using Eq. (2). (B) Autofluorescence
(AF) is measured with either MnCl
2
or digitonin (see text) and subtracted from all experimental
data prior to calculation of the fluorescence ratio.

442 Patel and Iozzo
442
Table 1
Components of a Single-Cell Imaging System
Component Function Notes
Arc Lamp Provide illumination Xenon arc lamps are preferred over mercury lamps, because they provide a
more even illumination over the range of wavelengths required for excitation
commonly used Ca
2+
indicators.
Neutral-density Filter Attenuate light source Attenuates light evenly at all wavelengths.
Computer-controlled Provide illumination only Prevents premature burning out of filters shutter during acquisition and
shutter unnecessary photobleaching.
Excitation selector Provide appropriate wavelength of Normally achieved with a barrier filter. For dual-excitation studies (as with
light for optimal excitation of the fura-2), it is necessary to have a device for the rapid changing of the excitation
fluorophore wavelength. This can be achieved with a computer-controlled rotating filter
wheel, which houses the appropriate excitation filters (18,19). Alternatively,
a monochromator system can be employed, which is more flexible in that the
number of available wavelengths is essentially unlimited.
Dichroic mirror Reflect excitation light to, Designed to reflect a specific range of wavelengths while allowing light of
and isolate emitted fluorescence other wavelengths to pass through. Chosen such that the cutoff for passing
from sample light is greater than the wavelength of exciting light. Excitation light is
therefore reflected up to the sample, whereas the resulting emitted
light (of longer wavelength) passes through. For fura-2, a dichroic mirror
with a cutoff of 400 nm is appropriate to separate excitation light (340/380 nm)
and fluorescence emission (peak 510 nm).
Objective To deliver excitation light 16 × or 20 × magnification is sufficient to accurately resolve most single
and collect emitted fluorescence cells. Higher magnification (40 × /63 ×) is required for subcellular recording.
Must efficiently transmit light at wavelengths to be used. Fura-2, for example
requires quartz or Fluor objectives in order to pass light of 340 nm.
Lenses with high numerical apertures (NA), an index of light-collecting
capacity, are preferred.
Sample chamber Chamber that fits onto In an inverted microscope configuration, the cover slip itself forms the base
microscope stage and houses the of the chamber. It is usually thermostatted to physiological temperatures.
cover slip containing adherent cells

Principles of Ca
2+
Imaging 443
443
Emission selector Select appropriate emission A filter designed to pass a broad range of wavelengths (long-pass) is
wavelengths normally used, in order to maximize the fluorescence signal, but sufficiently
removed from the cutoff wavelength of the dichroic mirror in order to filter
out stray excitation light. For Fura-2, a 420- to 600-nm filter can be used
with a 400-nm cutoff dichroic mirror.
For dual-emission dyes such as indo-1, a filter system similar in design to
that used to select alternate excitation wavelengths can be fitted to the exit port
of the microscope.
Camera Capture emitted fluorescence Silicon-intensified target (SIT) cameras or cooled charged-coupled-device (CCD)
cameras are commonly used.
444 Patel and Iozzo
Before calculation of fluorescence ratios in dye-loaded cells, it is important to sub-
tract any fluorescence not emanating from Ca
2+
-sensitive dye from the measured sig-
nal. Such autofluorescence can derive from many sources, including flavoproteins and
reduced adenine nucleotides. Autofluorescence is determined by first eliminating the
fluorescence due to the dye and then subtracting the residual fluorescence from the
data. For fura-2, this can be achieved by introducing Mn
2+
into the cell (using iono-
phore), which quenches dye fluorescence (see Fig. 4B, Subheading 3.4.1.). Note that
with intensiometric indicators, autofluorescence is cancelled out in both the numera-
tor and denominator of Eq. (1), and thus does not need to be determined separately.
In some cells, AM loading can result in accumulation of the dye into noncytosolic
compartments such as the endoplasmic reticulum. Since Ca
2+
levels in the endoplas-
mic reticulum are much higher than in the cytosol, compartmentalized dye will, under
normal conditions, remain saturated, thereby contributing to background fluorescence.
In this case, the Mn
2+
quench method for determining autofluorescence is unsuitable,
because ionomycin will equilibrate Mn
2+
into most cellular compartments. An alterna-
tive method to determine autofluorescence in cells where there is significant compart-
mentalization of dye (or when using dyes, such as fluo-3, that are not completely
quenched by Mn
2+
) is to permeabilize selectively using, the plasma membrane with
detergents such as digitonin (see Fig. 4B, Subheading 3.4.2.). This method effects
release of just the cytosolic dye from the cell. More problematic is compartmentaliza-
tion of dye into organelles such as mitochondria, where indicators used for measuring
cytosolic Ca
2+
will also report mitochondrial Ca
2+
changes. In such cases “mixed”
signals will result, since cytosolic and mitochondrial Ca
2+
changes may occur asyn-
chronously (see Note 2).
2. Materials
1. Loading of the cells with Ca
2+
indicator and data acquisition are performed in an extracel-
lular-like, imaging medium (see Table 2 for composition), which should be prepared
fresh on the day of experimentation. Alternatively, the medium can be prepared in bulk,
sterile filtered, aliquoted, and stored at 4°C.
2. Autofluorescence and calibration media (see Table 2) are made in bulk and stored at 4°C.
3. AM esters of Ca
2+
indicators (1 mM) should be reconstituted in dimethylsulfoxide
(DMSO), aliquoted into single-use vials, and stored at –20°C.
4. Free acids of Ca
2+
indicators (1 mM) should be reconstituted in H
2
O, aliquoted into single-
use vials, and stored at –20°C.
5. Ionomycin (2 mM) should be prepared in DMSO, aliquoted into single-use vials, and
stored at –20°C.
6. Digitonin (10 mg/mL) and MnCl
2
(1 M) stock solutions should be prepared in H
2
O and
stored at room temperature.
3. Methods
3.1. Dye Loading
1. Cells should be plated onto glass (no. 1 thickness) cover slips and cultured until 70–80%
confluent in the appropriate tissue culture medium.
2. Remove tissue culture medium and rinse cover slips twice with 2–3 mL of prewarmed
imaging medium.

Principles of Ca
2+
Imaging 445
3. Incubate cover slip with fresh imaging medium supplemented with the appropriate con-
centration of Ca
2+
indicator and incubate with gentle agitation (see Note 3).
4. Remove medium and rinse cover slips twice with 2–3 mL of imaging medium.
5. Transfer cover slip to incubation chamber.
6. Leave for sufficient time (e.g., 10 min) to effect complete de-esterification of the dye.
3.2. Data Acquisition
1. Experiments can be performed either in a static chamber or by continual perfusion of the
cells. The former approach is recommended, because much smaller quantities of (usually
precious) materials are required. Indeed, experiments can be performed in volumes as small
as 0.3 mL.
2. Capture images for at least 60 s prior to stimulation of the cells in order to obtain an
accurate measure of the basal [Ca
2+
] and to characterize any possible spontaneous changes
in [Ca
2+
] (see Note 4).
3. For static chambers additions can be made by complete removal (by pipet) of the medium
and addition of fresh medium supplemented with the test agent. Alternatively, a small
aliquot of the medium can be removed, mixed with the appropriate volume of a concen-
trated stock solution of the test agent, and the entire volume added back. This method is
less prone to addition artefacts than complete exchange of the medium.
3.3. Calibration of Fluorescence Data
3.3.1. In Situ Calibration
1. This method can be used to calibrate fluorescence data from both intensiometric and
ratiometric indicators. For ratiometric indicators, autofluorescence (see below) must be
subtracted prior to calculation of the ratio.
2. At the end of the experimental run, rinse cells into imaging medium (without added Ca
2+
)
supplemented with 2 mM BAPTA and add 2 µM ionomycin to equilibrate Ca
2+
across the
cell membrane.
3. Reinitiate data acquisition and monitor until fluorescence intensity reaches a stable pla-
teau to obtain F
min
(for intensiometric indicators) or R
min
(for ratiometric indicators).
Table 2
Solutions for Single Cell Ca
2+
Imaging
Imaging medium Autofluorescence medium Calibration medium
NaCl 121 mM 10 mM 10 mM
KCl 4.7 mM 120 mM 120 mM
MgCl
2
1.2 mM 2 mM 2 mM
CaCl
2
2 mM 150–300 µM —
BAPTA — 500 µM 500 µM
KH
2
PO
4
1.2 mM ——
NaHCO
3
5 mM ——
Glucose 10 mM ——
BSA 0.25% — —
HEPES 20 mM 20 mM 20 mM
pH 7.4 @ 37°C pH 7.2 @ 37°C pH 7.2 @ 37°C
446 Patel and Iozzo
4. Add 10 mM CaCl
2
to saturate the dye with Ca
2+
.
5. Reinitiate data acquisition and monitor until fluorescence intensity reaches a stable pla-
teau to obtain F
max
(for intensiometric indicators) or R
max
(for ratiometric indicators).
6. Use eq. (1) (for intensiometric) or eq. (2) (for ratiometric indicators) to calculate [Ca
2+
].
3.3.2. In Vitro Calibration
1. This method is suitable for ratiometric indicators for a given instrument configuration.
2. Place 2 mL of calibration buffer (see Table 2) in the incubation chamber and acquire
5–10 images. This is the background fluorescence, which should be subtracted from sub-
sequent data (below) at both wavelengths before calculation of ratios.
3. Add 1 µM of the free acid form of the indicator and acquire 5–10 images. Calibration
medium is initially Ca
2+
-free, thus the fluorescence ratio gives R
min
.
4. Add 500 µM CaCl
2
to saturate the dye and acquire 5–10 images. This fluorescence ratio
is R
max
.
5. Use eq. (2) to convert the acquired experimental ratio values (corrected for
autofluorescence) to [Ca
2+
] at each time point.
3.4. Determination of Autofluorescence
3.4.1. Mn
2+
Quench
1. At the end of the experimental run, remove medium and rinse twice with 2 mL of imag-
ing buffer.
2. Reinitiate data acquisition and add MnCl
2
(2–4 mM) and ionomycin (2 µM).
3. Monitor until fluorescence intensity falls to a stable plateau.
4. Subtract final image from all acquired images.
3.4.2. Digitonin Permeabilization
1. At the end of the experimental run, remove medium and rinse cells twice with 2 mL of
autofluorescence buffer.
2. Reinitiate data acquisition and add digitonin (20–50 µg/mL).
3. Monitor until fluorescence intensity falls to a stable plateau.
4. Subtract final image from all acquired images.
4. Notes
1. Photobleaching and dye extrusion can be distinguished by briefly interrupting data acqui-
sition and comparing the fluorescence intensity before and after resuming acquisition. If
the fluorescent intensities are comparable, then the loss of signal is due to photobleaching,
in which case illumination intensity should be reduced by decreasing exposure time and/
or increasing attenuation of the excitation source. Dye extrusion, however, should not be
affected by stopping acquisition, thus the fluorescence signal should continue to fall.
Loss of dye can be slowed by reducing the working temperature and/or including organic
anion-transport inhibitors such as bromosulphophthalein (100 µM) in the imaging medium
to inhibit active extrusion pathways (also see Note 2).
2. Compartmentalized dye can be recognized by a punctate cellular dye distribution and/
or a dim nucleus and can be reduced by reducing the loading temperature and/or
loading time. Alternatively, the free acid (de-esterified) form of the dye can be intro-
duced directly into the cytosol by micropipet or dialysis through a patch pipet. This
method, although more disruptive, has the added advantage of allowing delivery of
dyes conjugated to inert dextrans, thereby preventing dye extrusion (see ref. 17 for
further discussion).
Principles of Ca
2+
Imaging 447
3. The conditions for dye loading are highly dependent on cell type and should be determined
empirically. Cells are typically loaded for up to 1 hour with 1–10 µM of the AM ester. Dye
loading can be increased by premixing the dye with dispersants such as Pluronic F-127 (0.02%)
prior to dilution into imaging medium (to increase dye solubilization) and/or by decreasing
cell density. Care should also be taken not to overload cells with dye, since at high concentra-
tions, the indicator may buffer Ca
2+
increases, resulting in sluggish or blunted responses.
4. As a rule, minimize the time that cells are exposed to the excitation light to prevent
photobleaching (see Table 1). This is achieved by adjusting shutter exposure time and
attenuation (neutral-density filter). Also, the delay between capturing successive images
(acquisition delay) will determine total exposure time. A common problem encountered
during acquisition is uneven fluorescence throughout the imaging field. This is likely to be
due to misalignment of the excitation source. Also, excitation bulbs have a finite life span.
Bulbs should be replaced if high frequency noise becomes a problem during experimentation.
Acknowledgements
We would like to thank Dr. David McQuillan for providing decorin, and G. C.
Churchill and A. Galione for helpful discussions. This work was supported by a
Wellcome Prize Travelling Research Fellowship.
References
1. Berridge, M. J., Bootman, M. D., and Lipp, P. (1998) Calcium—a life and death signal.
Nature 395, 645–647.
2. Berridge, M. J. (1993) Inositol trisphosphate and calcium signalling. Nature 361, 315–325.
3. Rhee, S. G. and Bae, Y. S. (1997) Regulation of Phosphoinositide-specific Phospholiapse
C Isoenzymes. J. Biol. Chem. 272, 15045–15048.
4. Joseph, S. K. (1996) The inositol triphosphate receptor family. Cell Signal. 8, 1–7.
5. Patel, S., Joseph, S. K., and Thomas, A. P. (1999) Molecular properties of inositol
1,4,5-trisphosphate receptors. Cell Calcium. 25, 247–264.
6. Sjaastad, M. D. and Nelson, W. J. (1997) Integrin-mediated calcium signaling and regula-
tion of cell adhesion by intracellular calcium. BioEssays 19, 47–55.
7. Iozzo, R. V. (1999) The biology of the small leucine-rich proteoglycans. J. Biol. Chem.
274, 18843–18846.
8. Patel, S., Santra, M., McQuillan, D. J., Iozzo, R. V., and Thomas, A. P. (1998) Decorin
activates the epidermal growth factor receptor and elevates cytosolic Ca
2+
in A431 carci-
noma cells. J. Biol. Chem. 273, 3121–3124.
9. Vogel, W., Gish, G. D., Alves, F., and Pawson, T. (1997) The Discoidin Domain Receptor
Tyrosine Kinases Are Activated by Collagen. Mol. Cell 1, 13–23.
10. Shrivastava, A., Radziejewski, C., Campbell, E., Kovac, L., McGlynn, M., Ryan, T. E.,
Davis, S., Goldfarb, M. P., Glass, D. J., Lemke, G., and Yancopoulos, G. D. (1997) An
Orphan Receptor Tyrosine Kinase Family Whose Members Serve as Nonintegrin Col-
lagen Receptors. Mol. Cell 1, 25–34.
11. Schlessinger, J. (1997) Direct Binding and Activation of Receptor Tyrosine Kinases by
Collagen. Cell 91, 869–872.
12. Thomas, A. P., Bird, G. St. J., Hajnóczky, G., Robb-Gaspers, L. D., and Putney, J. W.,Jr.
(1996) Spatial and temporal aspects of cellular calcium signaling. FASEB J. 10, 1505–1517.
13. Haughland, R. P. (1996) Handbook of Fluorescent Indicators. Molecular Probes, Eugene, OR.
14. Grynkiewicz, G., Poenie, M., and Tsien, R. Y. (1985) A new generation of Ca
2+
indicators
with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450.
