Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

408 San Antonio and Lander
completely melted. Check that the agarose is completely mixed by swirling the bottle to
see that the liquid appears homogeneous.
4. To a polypropylene tube, add 1.0 mL of 10% CHAPS and bring to volume with 20 mL
with 1.053% boiling hot agarose, place the lid on tightly and gently invert a few times;
take care not to create bubbles. Pour the agarose quickly into the casting apparatus,
making sure that the agarose flows evenly between the combs. If any of the combs
shifted in position during the pouring, readjust them into proper position while the aga-
rose is still hot. Allow the agarose to solidify completely before removing the tape
(usually at least 30 min). If any agarose leaks out of the casting stand, or there are
significant numbers of bubbles remaining in the agarose after it has solidified, the gel
must be discarded.
5. While the gel is cooling, perform a serial dilution of your test protein, into nine concen-
trations including several that should exceed the K
d
, several that are close to it, and
several that should be considerably lower (see Note 1). Number nine polypropylene tubes
and add the correct amount of diluent, usually RB, to each. Usually, each sample is
diluted in RB to twice its desired final concentration, since later they will be mixed 1/1
with 2.0% agarose before being loaded into ACE gels (see step 8). An exception is for
collagen samples, which are dissolved in 0.5 N acetic acid at eight times their final
concentration, and are quickly mixed 1/1 with 0.5 N NaOH, and then 1/1 with 2× RB,
before being mixed 1/1 with 2.0% agarose.
6. After the gel has solidified, remove the well-forming combs first, by slowly and gently
sliding the comb backwards until it separates from the agarose at the front end, then
sliding it forward until it separates from the agarose at the rear end. Then, while holding
the casting stand firmly against the bench surface, tilt the comb and gently pull it away
from the agarose. Be careful not to rip the gel or deform the lanes. Small rips can be
repaired by reinserting the comb and pipetting hot 1.0% agarose (prepared as before) into
the gel. Should small clumps of agarose remain in sample lanes, these can be removed
using a pipet tip affixed to a vacuum line. The PG/GAG lane-forming comb is next
removed by holding the comb in place while slowly peeling the tape away from each of
its sides, then the comb is lifted straight up, out of the agarose. If rips occur in the agarose
between the sample-forming slot and the gel edge, these can be repaired by reinserting
the comb and pipetting new 1.0% agarose (prepared as before) onto the damaged regions
of the gel. If the PG/GAG lane-forming comb has been removed before the gel is fully
solidified, the sample well may collapse upon itself and the gel must be discarded.
Remove the gel (which should be firmly attached to the gel bond) from the casting
stand. Using a waterproof marker, mark on the gel bond the position of the PG/GAG
loading lane, and any necessary notes about the samples to be added to the protein-
containing wells.
7. To a polypropylene tube, add 10% of the total volume needed of 10% CHAPS and bring
to volume with boiling-hot 2% agarose, place the lid on tightly and gently invert a few
times to mix. Using the ACE gel dimensions specified under Subheading 2., each ACE
gel will require 1.125 mL of CHAPS/2% agarose, although extra volume should be
prepared, as some is lost on the side of the sample tube and on the outside of pipet tips.
Place the tube in the 37°C bath to equilibrate for later use (see Note 2).
8. To load the test proteins into the gel wells, make sure the gel is level and located near the
37°C water bath. From the 2% agarose tube, withdraw an amount equal to the total vol-
ume within each of the nine sample tubes, which would be 125 µL. Add this volume to
the ninth sample tube (which contains the most dilute protein sample) and mix it by
Affinity Coelectrophoresis 409
agitating the pipet tip back and forth while rapidly drawing the solution in and out of the
tip 10 times, taking care not to generate bubbles in the mixture. Then add the mixture
(now totaling 250 µL) to lane 9, which is the rightmost of the nine lanes of the gel, taking
care to overfill the lane slightly. Any excess agarose that spills over into the zone between
sample lanes will quickly solidify and will not interfere with the running of the gel,
whereas underfilling the protein lanes results in anomalous GAG/PG migration through
the gel. Move on to the remaining samples, filling the lanes in the following order: 7, 5, 3,
1, 8, 6, 4, and 2. The key to success during this step is to mix the samples thoroughly but
to make sure to work quickly enough that the agarose–protein mixture does not gel in the
tube. If need be, the mixing can be done while the sample tube remains partially sub-
merged in the water bath.
9. Prepare the PG or GAG sample by mixing the labeled material in sufficient quantity and
activity that it can be detected (usually at least 10,000–20,000 cpm of
35
S-radiolabeled
material is sufficient for a good 3-d exposure in a phosphorimager cassette); tracking
dye(s) (we use 0.05% each of bromophenol blue and xylene cyanol); sucrose so that the
sample will sink through the RB during gel loading (5% w/v), and enough RB to bring the
volume to approximately 100 µL/gel (see Note 3). Once the labeled sample is mixed, it
should be vortexed and any insolubles pelleted at 13,000g for 2 min. When adding the
sample to the gel, be careful not to disturb any pellet that may be at the bottom of the tube.
10. The electrophoresis apparatus is next prepared. Make sure the apparatus is resting prop-
erly on a level stir plate. Attach the cooling hoses to a water source, either cold tap water
or a circulating water chiller set to 10°C, and turn on the water. Fill the apparatus with
cold running buffer to roughly 3 mm above the platform, then place the ACE gels with the
PG/GAG lane end of the gel oriented closest to the cathode, and use glass microscope
slides to hold them in place. Turn on the stir plate to purge air trapped in the buffer
recirculator, then turn the stir plate off. Add more buffer if needed, to be sure that the gels
are covered by about 3 mm of buffer. Using a pipetter with a 200-µL gel-loading pipet tip,
load the labeled sample into the lane, drawing it across the gel while discharging the
sample, so that it fills evenly. Take care not to nick the gel with the pipet tip during
sample loading—this leads to an uneven sample front and/or rapid loss of the sample into
the running buffer during electrophoresis (see Note 4).
11. Attach the cover to the gel box, plug the leads into the power supply, and turn it on to
deliver 60–80 V. After the tracking dye has entered the gel (in approximately 5 min), turn
on the stir plate to an intermediate setting but take care that the resulting agitation of the
buffer does not cause the gels to float away from the platform.
12. Run the gel for the appropriate time. For example, for gels containing protein-containing
lanes 15-mm-long, heparin samples should generally run for about 1.0 h, by this time the
heparin should have migrated most if not all of the way through the lane. PGs and other
GAGs may migrate at different rates depending on their relative sizes, charges, etc. The
position of the tracking dyes can be used as a guide to assess when the gels are finished—
for example, the dyes that we use (see step 9) typically move at approximately one-third
the rate of heparin in the electrophoretic field. After electrophoresis, turn off the power
supply and remove the gel box cover. Remove the gels and place them on an elevated
surface that will not impede the flow of warm air around them. We use 8-cm-high plastic
test tube racks placed about 30 cm in front of a warm air source supplied by a small
personal space heater, such as the Holmes (HFH 195, 1500 W). Allow the gels to dry at
the high heat setting for at least 8.0 h; they are dry when the agarose has flattened to a thin
clear sheet that is not sticky to the touch.

410 San Antonio and Lander
3.2. Data Analysis
ACE gel electrophoretograms can be visualized by autoradiography or phos-
phorimaging, and the approximate K
d
of GAG– or PG–protein binding can often be
estimated by visual inspection. For example, from the phosphorimages of ACE gels
shown in Fig. 2A, it can be seen that the affinity of syndecan type I collagen binding can
be estimated as 50 nM < K
d
< 250 nM, since within these concentration ranges it is
evident that the PG is half-shifted from being fully retarded at high protein concentra-
tions to being fully mobile at very low protein concentrations. However, such gels can
also be analyzed quantitatively, by first measuring GAG or PG mobility using a
Phosphorimager by scanning protein-containing lanes and determining relative radioac-
tivity per 88-µm pixel along the length of each lane (see Fig. 2B) (3). GAG or PG mobil-
ity is taken as the pixel position that divides the curves representing the distribution of
GAG or PG into halves of equal areas. The retardation coefficient R is next calculated
for each lane as the GAG or PG migration position in that lane divided by its mobility in
a protein-free lane (R = (M
o
–M)/M
o
, where M
o
is the mobility of free GAG or PG, and M
is its mobility through protein; see Fig. 1). Under appropriate experimental conditions as
described previously, R is proportional to the fractional saturation of GAG or PG by
protein, so that values of the equilibrium binding constant may be determined from the
relationship between R and protein concentration (1). Data are analyzed graphically, by
curve-fitting to the equation R = R
∞
/(1 + K
d
/[protein]
n
; where R
∞
represents the value of
R at full saturation (i.e., at an arbitrarily high protein concentration), and n is a coeffi-
cient that reflects cooperativity of binding (1). Nonlinear least-squares fits are calculated
using a graphics program, e.g., the Kaleidagraph program (Synergy Software, Reading,
PA). The above analysis provides apparent K
d
values for GAG– or PG–protein interac-
tions, but does not indicate whether the sample of GAG or PG exhibits heterogeneity in
protein binding. Such heterogeneity is usually apparent by visual inspection of the ACE
gel electrophoretogram, as evidenced by a broad smearing of the GAG or PG migration
front throughout the length of protein-containing lanes, or by the presence of multiple
bands of the sample at concentrations near the binding K
d
(see Fig. 3). When binding
heterogeneity is evident, subpopulations of GAGs or PGs that bind strongly or weakly to
protein can be isolated using preparative ACE, and subjected to further analysis.
3.3. Preparative ACE
This method is used to fractionate a heterogeneous population of GAGs or PGs that
bind differentially to a protein. In this technique, radiolabeled GAGs or PGs are
subjected to electrophoresis through agarose containing a single concentration of
protein (1,3) as shown schematically in Fig 4. After individual species from the GAG
or PG mixture are isolated, their affinity for protein can be analyzed by standard ACE
methods, or they can be subjected to other analyses.
1. A 1% low-melting agarose/CHAPs solution in ACE electrophoresis buffer is prepared as
detailed previously and is poured hot onto a piece of GelBond fitted within a Plexiglas gel
casting tray where a 4 × 7 cm Plexiglas block and a Teflon PG/GAG lane-forming comb
are positioned. After the agarose solidifies, removal of the block and strip leaves a 4 × 7
cm well with a 66 × 1 mm slot 2 mm away from and parallel to one of the short edges of
the 4 × 7 cm well (see Fig. 4, top).

Affinity Coelectrophoresis 411
2. A protein is prepared at a concentration that will achieve maximal separation of GAG or
PG species of interest as determined by analytical ACE (e.g., for the protein in Fig. 3, a
concentration of 250 nM may be suitable). The sample is then mixed with agarose as
described in step 8 of the analytical ACE methods to a final agarose concentration of
1.0%, and is loaded into the 4 × 7 cm well and allowed to solidify.
3. Gels are submerged under electrophoresis buffer, radiolabeled GAG or PG is loaded into
the 66 × 1 mm slot, and electrophoresis is carried out, all as detailed in steps 9–12 of the
analytical ACE methods.
4. After electrophoresis, gels are removed and are not dried, but rather are affixed to a surface
marked with a millimeter grid, with the electrophoretic origin at the top. For a surface we use
a sheet of 1-mm lined graph paper under a clear plastic sheet, taped onto a flat Styrofoam block.
5. Regions of the gel to the left and right of the 4 × 7 cm block as well as the 3 mm of the block
itself that are immediately adjacent to the left and right edges are cut away and discarded.
6. The resultant 3.4 cm × 7 cm gel is sectioned into 2-mm segments perpendicular to the direc-
tion of electrophoresis, using a piece of surgical suture thread drawn tightly between the hands.
7. Each agarose segment is lifted with a flat-headed metal spatula and placed in a tube, and the
amount of radiolabeled GAG or PG in each segment is measured with a gamma counter, or by
liquid scintillation counting of a melted gel aliquot. To melt gel segments, tubes are placed in
a
>70°C water bath. They may be pooled or divided into aliquots while they are liquid, and
then stored frozen. Alternatively, they may be melted at 70°C and then brought to 6 M in urea
by addition of solid urea (urea blocks gelation of the agarose). In this case they will remain
liquid for days, even at 4°C. Such samples may then be mixed with running dyes and loaded
directly into analytical ACE gels (see Note 5), or subjected to other analyses.
3.4. Iodination and Molecular-Weight Fractionation of Heparin
Heparin is often used as a model compound in studies of GAG– or PG–protein
interactions because it potentially contains a large number of different protein-interac-
tive domains, is structurally homologous to heparan sulfates that are common to many
cell surface and extracellular matrix PGs, and is inexpensive and readily available
from commercial sources. Commercial heparin is polydisperse in M
r
, often averaging
about 15 kDa. However, in protein-binding studies the use of low-molecular-weight
heparin is advantageous, since it minimizes factors that complicate binding analysis,
such as multivalency (1). Thus, here we have included methods we use to tyramine-
derivatize, radioiodinate, and M
r
-fractionate commercial heparin to be used in ACE
analysis. We have found that derivatization of heparin with tyramine is more suitable
than with fluoresceinamine, as the latter may artifactually enhance heparin-binding
affinity for protein (3).
3.5. Tyramine Labeling of Heparin
1. Dissolve 9 mg of heparin in 750 µl of 5% (w/v) solution of tyramine in formamide, and
place in a sealed tube. We use porcine intestinal mucosa heparin (grade 1A; Sigma) in our
experiments.
2. Heat to 80°C for 1 h and cool to room temperature (the solution may turn yellow).
3. Add 1 mg of Na cyanoborohydride, seal, and incubate overnight at room temperature.
4. Dilute with 9 volumes of distilled water.
5. Dialyze against distilled water using 1-kDa Mr cutoff dialysis tubing.
6. Lyophilize the sample.
412 San Antonio and Lander
7. Resuspend in a small volume of water and measure heparin concentration [e.g., using the
Dische assay (7)].
8. Measure tyramine content by OD
278
. As heparin absorbs to a small extent at 278 nm, tyramine
concentrations must be corrected by subtracting this background. Tyramine content may be
estimated from corrected OD values by the formula: tyramine (mg/mL) = 0.0824 × OD
278
.
3.6. Iodination of Tyramine-Heparin
1. Dissolve 400 µg of Iodogen/mL (Pierce) of dichloromethane. Add 50 µL to the bottom of
5-mL glass test tubes; a glass Pasteur pipet connected to a rubber hose affixed to a
nitrogen tank can be used to direct a slow stream of nitrogen gas over the sample in the
bottom of the rotating tube. Tyramine-coated glass tubes can be covered with Parafilm
and stored under vacuum at room temperature for years.
2. Dilute 2.5 µg of tyramine-labeled heparin in 50 µL of 0.25 M Tris-HCl, pH 7.5.
3. Rinse an Iodogen-coated tube gently with 500 µL of 0.25 M Tris-HCl, pH 7.5 to wash off
any unbound Iodogen. Visually inspect tube to ensure that the Iodogen coat remains intact.
4. React 50 µL of heparin solution and 5 mCi of
125
I at room temperature for 6 min with
intermittent agitation, then add an additional 50 µL of buffer for 6 min more with agita-
tion. The addition of extra buffer helps prevent the Iodogen from increasing the pH, which
inhibits iodination.
3.7. Desalting of
125
I-Heparin on G-25
After iodination of the heparin sample, it must be desalted over a G-25 column
(prepared in a 2.0-mL disposable tissue culture pipet) to remove unbound
125
I.
1. Equilibrate and elute the column with 0.5 × RB.
2. Draw off buffer from column bed.
3. Load the
125
I-heparin sample onto the column and position a test tube rack with numbered
Eppendorf tubes underneath.
4. Collect the column eluate in fractions of 2 drops/tube.
5. Make sure to replenish RB as the column runs.
6. Monitor the passage of
125
I-heparin through the column using a Geiger counter.
7. As fractions are collected, monitor their relative radioactivity using a Geiger counter at a
fixed distance from tubes.
8. Record activities and plot elution profile to determine where bound (the smaller of the two
peaks, which elutes first) and free (the larger of the two peaks, which elutes second) isotope
are eluting. Generally, 10–15 fractions are collected.
9. Pool the fractions containing the bound isotope.
10. Discard the column and the fractions containing the unincorporated isotope.
3.8. Molecular–Weight Fractionation of
125
I-Heparin on G-100
Pooled fractions containing
125
I-heparin from the desalting column are pre-
pared for G–100 chromatography and M
r
-fractionated as follows; our typical
column dimesions are 300 × 10 mm. A typical elution profile of
125
I-heparin
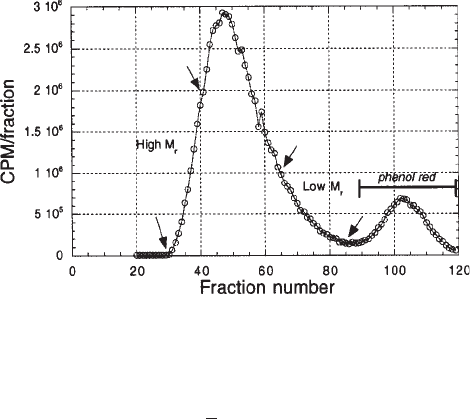
from a G-100 column is shown in Fig. 6.
1. Prepare the sample for G-100 chromatography by mixing
125
I-heparin (generally < 0.5
mL) to 5% sucrose (w/v) plus 10 µL of a saturated phenol red solution. Bring to 2.0 mL
with running buffer, clarify at 13,000g for 2 min, and load on column.
2. Collect fractions of about 8–10 drops (i.e., from 0.5–1.0 mL).
Affinity Coelectrophoresis 413
3. Remove and count several microliters of each fraction using a gamma counter.
4. Plot column profile indicating elution position of phenol red, which should largely overlap
with free iodine peak. See Fig. 6 for an example of a typical profile.
5. The first 12% of radioactive material to elute is the high-molecular-weight fraction.
6. The following 76% to elute is the medium-molecular-weight fraction.
7. The remaining 12% to elute is the low-molecular-weight fraction of M
r
≈ 6 kDa (8–10).
3.8.1. Storage of
125
I-Heparin Samples
1. Pool fractions within each category and cryoprotect with bovine serum albumin (BSA) to
0.1 mg/mL (a 2.0-mg/mL stock of BSA can be used).
2. The low-M
r
fraction, which is the fraction most commonly used in binding experiments,
should be divided into 50- to 100-µL aliquots.
3. The remaining fractions should be divided into samples of about 1.0 mL.
4. Store samples at –80°C. These can be used for approximately 3–6 mo.
4. Notes
1. The accuracy of the ACE technique relies on knowing the exact concentration of the test
protein.
2. If the 2% agarose mixture is not equilibrated to 37°C when it is mixed with test proteins
(see step 8 under Subheading 3.1.), the proteins may become denatured.
3. Tubes containing 5× stock mixtures of dyes/sucrose should be premixed, filtered, and
stored frozen.
4. Loading of the GAG/PG sample is the most technically difficult step of this procedure;
thus, one should practice loading mock samples into ACE gels before working with
radioactive GAG or PG samples.
5. Since urea does not migrate in the electrophoretic field, its removal from samples before
electrophoresis is not required; due to the density of urea, it is not necessary to add sucrose.
Fig. 6. Elution profile of
125
I-tyramine-heparin from G-100 column. The larger of the two
peaks, eluting first, represents the radiolabeled heparin sample; the smaller peak, eluting sec-
ond, is unincorporated
125
I-iodine. The last 12% (marked with arrows) of sample to elute repre-
sents the low-M
r
heparin chains of about < 6 kDa.
414 San Antonio and Lander
References
1. Lee, M. K., and Lander, A. D. (1991). Analysis of affinity and structural selectivity in the
binding of proteins to glycosaminoglycans: development of a sensitive electrophoretic
approach. Proc. Natl. Acad. Sci. (USA) 88, 2768–2772.
2. Lim, W. A., Sauer, R. T., and Lander, A. D. (1991). Analysis of DNA-protein interactions
by affinity coelectrophoresis. Meth. Enzymol. 208, 196–210.
3. San Antonio, J. D., Slover, J. Lawler, J., Karnovsky, M. J., and Lander, A. D. (1993).
Specificity in the interactions of extracellular matrix proteins with subpopulations of the
glycosaminoglycan heparin. Biochemistry 32, 4746–4755.
4. San Antonio, J. D., Lander, A. D., Wright, T. C., and Karnovsky, M. J. (1992). Heparin
inhibits the attachment and growth of Balb c/3T3 fibroblasts on collagen substrata. J. Cell.
Physiol. 150, 8–16.
5. San Antonio, J. D., Karnovsky, M. J., Gay, S., Sanderson, R. D., and Lander, A. D. (1994).
Interactions of syndecan-1 and heparin with human collagens. Glycobiology 4, 327–332.
6. LeBaron, R. G., Hook, A., Esko, J. D., Gay, S. and Hook, M. (1989). Binding of heparan
sulfate to type V collagen. J. Biol. Chem. 264, 7950–7956.
7. Dische, Z. (1947). A new specific color reaction of hexuronic acids. J. Biol. Chem. 167,
189–192.
8. Yamada, K. M., Kennedy, D. W., Kimata, K., and Pratt, R. M. (1980). Characterization of
fibronectin interactions with glycosaminoglycans and identification of active proteolytic
fragments. J. Biol. Chem. 255, 6055–6063.
9. Laurent, T. C., Tengblad, A., Thunberg, L., Hook, M., and Lindhal, U. (1978). The molecu-
lar-weight dependence of the anti-coagulant activity of heparin. Biochem. J. 175, 691–701.
10. Jordon, R., Beeler, D., and Rosenberg, R. D. (1979). Fractionation of low molecular weight
heparin species and their interactions with antithrombin. J. Biol. Chem. 254, 2902–2913.
11. Verrecchio, A., Germann, M. W., Schick, B. P., Kung, B., Twardowski, T., and San Anto-
nio, J. D. (2000). Design of peptides with high affinities for heparin and endothelial cell
proteoglycans J. Biol. Chem. 275, 7701–7707.

Growth Factor Binding 415
415
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
41
Binding Constant Measurements for Inhibitors
of Growth Factor Binding to Heparan Sulfate Proteoglycans
Kimberly E. Forsten and Matthew A. Nugent
1. Introduction
A large number of proteins (>100) have been demonstrated to bind to heparan sul-
fate proteoglycans, and in many instances these interactions have important biological
consequences (1). The best-studied example is the fibroblast growth factor (FGF) fam-
ily of proteins. The FGFs regulate a wide range of cellular functions, including prolif-
eration, differentiation, and migration. The best-characterized FGF family member,
basic fibroblast growth factor (bFGF) or FGF-2, has been shown to require interaction
with heparan sulfate on cell surfaces in order to induce maximal activity (2,3). How-
ever, interaction of bFGF with heparan sulfate within the extracellular matrix can limit
bFGF diffusion and access to cell surfaces (4,5). Thus the interaction of bFGF with
heparan sulfate has been targeted as a site for regulation of both endogenous and exog-
enously administered bFGF. For example, bFGF and many other heparin-binding pro-
teins have been demonstrated to play pivotal roles in the growth of the new blood
vessels (angiogenesis), a process that is essential for both efficient wound healing and
the development of malignant tumors. Indeed, inhibition of endogenous bFGF activity
has been suggested as a possible treatment to prevent tumor growth and metastasis,
whereas enhancement of angiogenesis by pharmacological bFGF has been proposed
to stimulate repair of damaged tissue (i.e., ischemic heart muscle) (6). In both
instances, effective treatments might make use of small compounds, which inhibit
bFGF binding to heparan sulfate proteoglycans. These compounds might have appli-
cations as inhibitors of bFGF binding and activity at cell surfaces, or in turn they
might enhance the transport of added bFGF through connective tissue and allow cell
stimulation distant from the administration site. Compounds that bind either to the
growth factor or to heparan sulfate could block bFGF binding to heparan sulfate, yet
might have very different effects biologically. To screen various potential inhibitors
of growth factor/heparan sulfate binding effectively, a simple, rapid, and semi-quanti-
tative assay is required.
416 Forsten and Nugent
In this chapter we report a simple assay to determine equilibrium binding con-
stants for inhibitors of bFGF/heparan sulfate proteoglycan binding that is based on
the semiquantitative retention of sulfated proteoglycans on cationic nylon filters
(7). Using a modification of previously published conditions, samples containing
proteoglycan and growth factor are subject to filtration through cationic nylon.
While the large negatively charged proteoglycans are retained on the filter, only a
small fraction of growth factor is retained unless bound to the proteoglycan (see
Fig. 1). Thus, using standard preparations of bFGF and heparan sulfate proteoglycan
(isolated from bovine aortic endothelial cell conditioned media [mostly perlecan]),
baseline measurements were made to determine the bFGF/heparan sulfate
proteoglycan binding affinity. A range of concentrations of potential inhibitors
are then included in the reaction, and decreased bFGF retention on the cationic
membrane is measured. These data are then analyzed by fitting to a generalized
equation for binding using a commercially available software package (Mathematica,
version 3.0, Wolfram Research) to determine the binding constant for each inhibi-
tor (binding to either bFGF or heparan sulfate). This assay has been established
with bFGF and has been shown to be effective with inhibitors that bind either
bFGF or heparan sulfate proteoglycans. Minor modifications of the base assay
should allow it to be transferable for the analysis of a large number of heparin-
binding proteins.
2. Materials
1. Human recombinant
125
I-bFGF is prepared by a modification of the Bolton-Hunter proce-
dure (8) and frozen in single-use aliquots to avoid freeze–thaw cycles.
2. Proteoglycans (E-HS) are purified from bovine aortic endothelial cell conditioned
medium as described previously (4) (see Note 1). Briefly, confluent bovine
endothelial cells (isolated from freah calf aorta or purchased from Coriell Cell
Repositories) are established in Dulbecco’s Modified Eagle’s Medium (DMEM) with
10% calf serum. The medium is then replaced by DMEM without any additives and
the cells incubated for 1 h at 37°C as a wash, followed by a 24 h incubation in fresh
DMEM. Conditioned medium is collected and centrifuged at 3000g for 30 min at 4°C
to remove cell debris. The conditioned media is equilibrated with urea (1 M final) for
at least 30 min prior to being applied to an anion-exchange column (Q-sepharose,
Pharmacia-LKB) in Tris buffer (50 mM Tris-HCl, pH 8.0, 0.15 M NaCl, 1 M urea).
The column is washed extensively with 50 mM Tris-HCl, pH 8.0, 0.3 M NaCl, 1 M
urea, and the proteoglycan eluted in the same buffer containing 1.5 M NaCl.
Proteoglycan fractions are identified using the dimethylmethylene blue (DMMB) dye
binding assay (9) and dialyzed extensively in 50 mM Tris-HCl, pH 8.0, 0.15 M NaCl.
Proteoglycan concentration is based on the mass of glycosaminoglycan using the
DMMB assay (9) with a bovine kidney heparan sulfate standard (Sigma).
3. Incubation buffer: 50 mM Tris-HCl, pH 8.0, 0.15 M NaCl, 2 mg/mL bovine serum albu-
min. BSA, biotech grade (Fisher), worked well.
4. Cationic membrane (Zeta-Probe membrane) and Dot-blot apparatus (Bio-Dot
Microfiltration Apparatus) (Bio-Rad)
5. Test compound, we have used glycosaminoglycans (Sigma), protamine sulfate (TCI
America), and sucrose octasulfate.
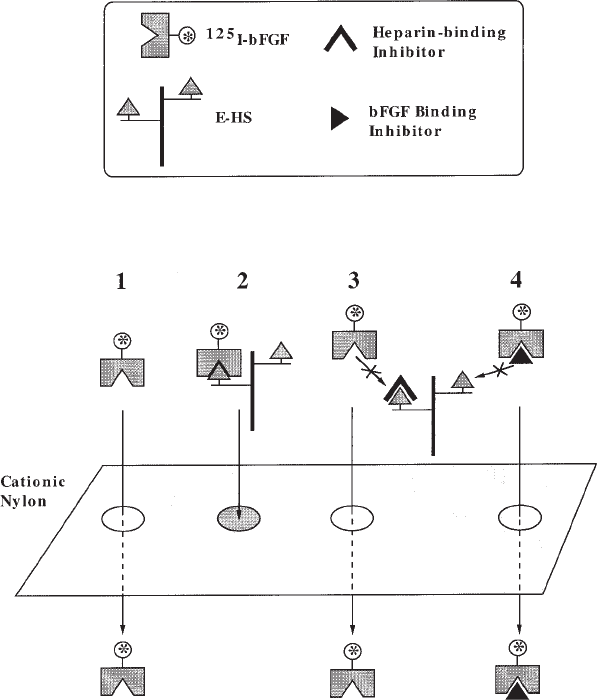
Growth Factor Binding 417
Fig. 1. Schematic of cationic nylon filter binding assay for growth factor/heparan sulfate
proteoglycan interactions. Filtration of various mixtures of growth factor, heparan sulfate
proteoglycan (E-HS), and compounds that block either the heparin-binding domain on the growth
factor or the protein-binding domain on the heparan sulfate chains, through cationic nylon filters
can be used to measure the various binding affinities quantitatively. (1) Cationic nylon filters do
not specifically retain small proteins such as FGF-2. (2) FGF-2 bound to heparan sulfate
proteoglycans will be retained on the filter, providing a direct measure of FGF-2/heparan sulfate
proteoglycan binding. (3) Compounds that bind to E-HS and block the growth factor-binding
domain can reduce FGF-2 retention on the filter in relation to the binding affinity of the inhibitor
compound for E-HS. (4) Compounds that bind to FGF-2 and block its interaction with E-HS can
reduced FGF-2 retention in relation to the binding affinity of the inhibitor compound for FGF-2.
As an example we have provided a description of how this assay can be used to determine the
FGF-2–binding affinity for E-HS and sucrose octasulfate, and the binding affinity of E-HS for
protamine sulfate.
