Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

Substrate Gel and Inverse Substrate Gel Technique 395
10. Rinse the gel twice with distilled water.
11. Establish the molecular sizes of hyaluronidase inhibitors by comparison with protein
molecular-weight standards.
4. Notes
1. This protocol is designed for two Bio-Rad minigels (8 × 10 cm.). For other sizes or thick-
nesses, volumes of stacking and separating gels, and operating current, must be adjusted
accordingly.
2. Not all Coomassie blue preparations work equally well. We have found the product of
BDH Chemicals, (Poole, UK) optimal. There are others that do not work at all.
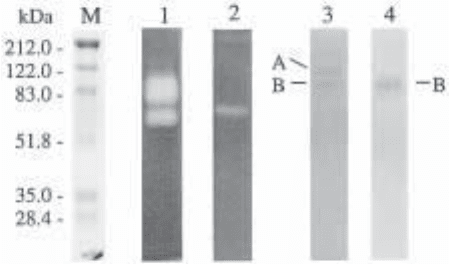
3. Analysis of bovine testicular hyaluronidase on an HA-substrate gel demonstrates two forms
of the enzyme (see Fig.1, lane 1) corresponding to the soluble and membrane-bound forms
of the enzyme (8,9). Analysis of human serum on the HA-substrate gel performed at pH 3.7
reveals a band at 57 kDa, which is Hyal-1 (see Fig. 1, lane 2). On an inverse HA-substrate
gel performed at pH 7.4, two bands, at 120 and 83 kDa, are identified in mouse serum (see
Fig. 1, lane 3). The data can be compared using the pattern obtained from a conventional
HA-free gel (see Fig. 1, lane 4). The 83-kDa band persists, demonstrating that this is an
artifact, corresponding to an endogenous plasma glycoprotein. The 120-kDa band corre-
sponds to one of the hyaluronidase inhibitors in mouse serum, an inhibitor of neutral-active
PH-20 enzyme.
4. Alcian blue is commonly used for staining HA (17–21). Acidification of the Alcian blue
is recommended. This enhances staining and prevents precipitation of dye. For optimal
Fig. 1 Detection of hyaluronidase and hyaluronidase inhibitors using HA-substrate gel
and inverse HA-substrate gel procedures. M), molecular-weight markers. Lanes 1 and 2:
HA-substrate gel for the detection of hyaluronidase activities. Examination of bovine testicular
hyaluronidase, PH-20 (lane 1), and human plasma hyaluronidase, Hyal-1 (lane 2), were per-
formed at pH 7.4 and pH 3.7, respectively. Lanes 3 and 4: Inverse HA-substrate gel for the
examination of hyaluronidase inhibitor in mouse serum. The HA-containing gel, to which mouse
serum had been applied, was digested with 0.5 rTRU/mL of bovine testicular hyaluronidase at
pH 7.4 (lane 3). The position of the hyaluronidase inhibitors corresponds to a band in which the
HA remained undigested (lane 3, A). A false positive corresponds to a band of endogenous
plasma glycoprotein and possible HA-binding protein. This band could be identified by running
a corresponding gel that does not contain HA (lane 4, B). Lane 1, 0.5 rTRU bovine testicular
hyaluronidase; lane 2, 0.5 µL human plasma; lanes 3 and 4; 4 µL human plasma.
396 Mio et al.
results, staining should be performed for 16 h in a solution of 0.5% Alcian blue in 3%
acetic acid. When sequential staining with Alcian blue and Coomassie blue is performed,
staining for 1 h with Alcian blue is sufficient. For the inverse substrate gel procedure, a
single staining step with Alcian blue is recommended.
5. The hyaluronidase digestion step in the inverse HA-substrate gel procedure is obviously
critical but can be difficult, as the gel is very fragile at this stage. Enzyme activity can also
vary with minor changes of pH and temperature. Optimization of the concentration of
hyaluronidase in the gel digestion step may be required with each experiment. We rou-
tinely utilize three different levels of hyaluronidase with each experiment (0.25, 0.50, and
2.00 rTRU/mL) to avoid over- and underdigestion.
6. High levels of a protein can prevent dye penetration into the gels (9,10) and can gener-
ate false positive bands. Albumin introduces such an artifact when plasma and serum
samples are examined. Albumin is also a HA-binding protein (22–24), which may
explain its persistence in these HA-containing gels. Pronase treatment of the gels elimi-
nates such false positives. Proteins present in lesser amounts will appear as blue bands
following Coomassie blue staining. These should disappear if a pronase digestion step
is interposed.
Acknowledgements
This work was supported by Lion Corporation, Kanagawa, Japan, to K. M., and by
National Institutes of Health (USA) Grant 1P50 DE/CA11912, to R. S.
References
1. Kreil, G. (1995) Hyaluronidases-a group of neglected enzymes. Protein Sci. 4, 1666–1669.
2. Csóka, T. B., Frost, G. I., and Stern, R. (1997) Hyaluronidases in tissue invasion. Invasion
Metastasis 17, 297–311.
3. Frost, G. I., Csóka, T. B., Wong, T., and Stern, R. (1997) Purification, cloning, and expres-
sion of human plasma hyaluronidase. Biochem. Biophys. Res. Commun. 236, 10–15.
4. Csóka T.B., Frost G.I., Wong T., and Stern R. (1997) Purification and microsequencing of
hyaluronidase isozymes from human urine. FEBS Lett. 417, 307–310.
5. Csóka, T. B., Frost, G. I., Heng, H. H. Q., Scherer, S. W., Mohapatra G., and Stern R.
(1998) The hyaluronidase gene HYAL1 maps to chromosome 3p21.2-3p21.3 in human
and 9F1-F2 in mouse, a conserved candidate tumor suppressor locus. Genomics 48, 63–70.
6. Csóka, A. B., Scherer, S. W., and Stern, R. (1999) Expression analysis of six paralogous
human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics 60,
356–361.
7. Gmachl, M., Sagan, S., Ketter, S., and Kreil, G. (1993) The human sperm protein PH-20 has
hyaluronidase activity. FEBS Lett. 336, 545–548.
8. Cherr, G. N., Meyers, S. A., Yudin, A. I., VandeVoort, C. A., Myles, D. G., Primakoff, P.,
and Overstreet, J. W. (1996) The PH-20 protein in Cynomolgus macaque spermatozoa:
identification of two different forms exhibiting hyaluronidase activity. Develop. Biol. 175,
142–153.
9. Meyer, M. F., Kreil, G., and Aschbauer, H. (1997) The soluble hyaluronidase from bull
testes is a fragment of the membrane-bound PH-20 enzyme. FEBS Lett. 413, 385–388.
10. Haas, E. (1946) On the mechanism of invasion. I. Antinvasin I, An enzyme in plasma,
J. Biol. Chem. 163, 63–88.
11. Dorfman, A., Ott, M. L., and Whitney, R. (1948) The hyaluronidase inhibitor of human
blood. J. Biol. Chem., 223, 621–629.
Substrate Gel and Inverse Substrate Gel Technique 397
12. Moore, D. H. and Harris, T. N. (1949) Occurrence of hyaluronidase inhibitors in fractions
of electrophoretically separated serum. J. Biol. Chem. 179, 377–381.
13. Guntenhoener, M. W., Pogrel, M. A., and Stern, R. (1992) A substrate-gel assay for
hyaluronidase activity. Matrix 12, 388–396.
14. Miura, R. O., Yamagata, S., Miura, Y., Harada, T., and Yamagata, T. (1995) Analysis of
glycosaminoglycan-degrading enzymes by substrate gel electrophoresis (zymography).
Anal Biochem. 225, 333–340.
15. Mio, K., Carette, O., Maibach, H. I., and Stern, R. (2000) A serum inhibitor of hyalu-
ronidase. J. Biol. Chem., July 24.
16. Laemmli, UK. (1970) Cleavage of structural proteins during the assembly of the head of
bacteriophage T4. Nature 227, 680–685.
17. Wardi, A. H. and Michos, G. A. (1972) Alcian blue staining of glycoproteins in acrylamide
disc electrophoresis. Anal. Biochem. 49, 607–609.
18. Cowman, M. K., Slahetka, M. F., Hittner, D. M., Kim, J., Forino, M., and Gadelrab, G.
(1984) Polyacrylamide-gel electrophoresis and Alcian blue staining of sulphated
glycosaminoglycan oligosaccharides. Biochem. J. 221, 707–716.
19. Turner, R. E. and Cowman, M. K. (1985) Cationic dye binding by hyaluronate fragments:
dependence on hyaluronate chain length. Arch. Biochem. Biophys. 237, 253–260.
20. Wall, R. S. and Gyi, T. J. (1988) Alcian blue staining of proteoglycans in polyacrylamide
gels using the “critical electrolyte concentration” approach. Anal. Biochem. 175, 298–299.
21. Ghiggeri, G. M., Candiano, G., Ginevri, F., Mutti, A., Bergamaschi, E., Alinovi, R., and
Righetti, P. G. (1988) Hydrophobic interaction of Alcian blue with soluble and erythrocyte
membrane proteins. J. Chromatogr. 452, 347–357.
22. Johnston, J. P. (1955) The sedimentation behavior of mixtures of hyaluronic acid and
albumin in the ultracentrifuge. Biochem. J. 59, 620–627.
23. Davies, M., Nichol, L. W., and Ogston, A. G. (1963) Frictional effects in the migration of
mixtures of hyaluronic acid and serum albumin. Biochim. Biophys. Acta 75, 436–438.
24. Gramling, E., Niedermeier, W., Holley, H. L., and Pigman, W. (1963) Some factors
affecting the interaction of hyaluronic acid with bovine plasma albumin. Biochim. Biophys.
Acta 69, 552–558.

Affinity Coelectrophoresis 401
401
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
40
Affinity Coelectrophoresis
of Proteoglycan–Protein Complexes
James D. San Antonio and Arthur D. Lander
1. Introduction
Affinity coelectrophoresis (ACE) was developed as a tool to measure the strengths
of interaction between proteoglycans (PGs) or glycosaminoglycans (GAGs) and pro-
teins, and to assess the specificity of the interaction (i.e., to detect and fractionate GAG
or PG sample constituents that differentially bind to protein) (1). In ACE, trace con-
centrations of radiolabeled GAG or PG are subjected to electrophoresis through agar-
ose lanes containing protein at various concentrations. The electrophoretic pattern of
the radiolabeled GAG or PG is then visualized by autoradiography, or using a
phosphorimager, and the apparent dissociation constant (K
d
) is calculated as the protein
concentration at which the GAG or PG is half-shifted from being fully mobile at very
low protein concentrations (or between protein-containing lanes) to being maximally
retarded at saturating protein concentrations (see Figs. 1–3).
ACE holds many advantages over other means of studying GAG or PG–protein inter-
actions since it: (1) uses only trace quantities of the interacting molecules (typically a
microgram or less of GAGs, and a milligram or less of protein); (2) studies behaviors of
native proteins and of radiolabeled PGs or GAGs that can be essentially unmodified
(e.g., through metabolic radiolabeling) or minimally modified (e.g., by radioiodination);
(3) can measure strengths of binding even for relatively weak interactions characteristic
of GAG or PG-protein interactions (e.g., 100 nM or weaker K
d
); (4) can detect protein-
binding heterogeneity in a GAG or PG population and can even be used to isolate the
differentially binding subpopulations for further analysis (see Fig. 4); and (5) is a low
cost, simple, and rapid method, and is amenable to quantitative analysis.
The theory of ACE has been described in detail elsewhere (1,2) and will not be
repeated here. Rather, this chapter will serve as a description of ACE methods, and
will also include a protocol for the preparation of radioiodinated heparin samples,
which are often useful in various ACE applications. However, before
attempting ACE, there are several important questions which need to be addressed:
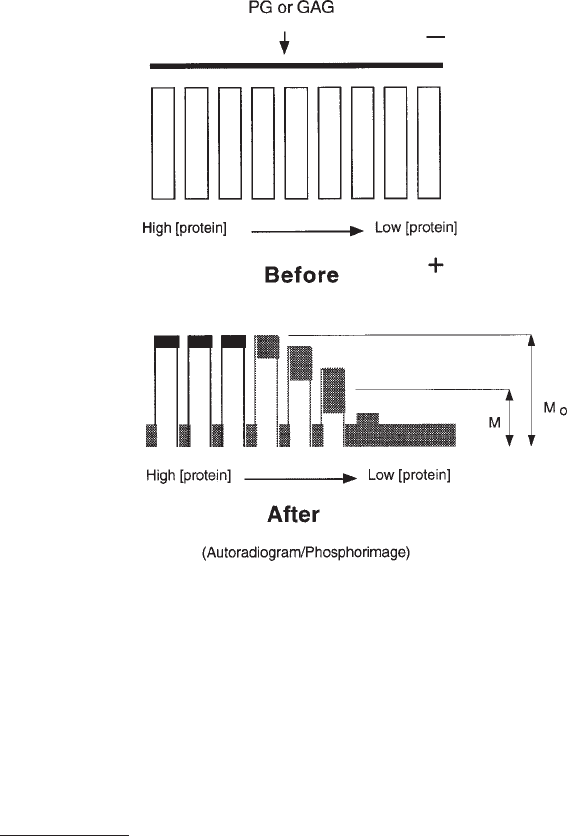
402 San Antonio and Lander
Fig. 1. Analytical ACE schematic. Top panel: ACE gels poured using a casting stand and
Teflon combs and strips as shown in Fig. 5 are used to create nine parallel rectangular wells,
which are filled with protein–agarose mixtures, each at a different protein concentration. Radiola-
beled GAG or PG is loaded into the slot above the protein-containing wells (shown as a dark line),
and after electrophoresis of the GAG or PG through the protein-containing lanes, its migration as
a function of protein concentration is visualized by autoradiography or phosphorimaging (shown
here as a pattern of peaks and valleys). The degree of GAG/PG retardation at the various protein
concentrations is used to calculate the apparent K
d
of GAG– or PG–protein binding (see text for
details). Artwork by Shawn M. Sweeney.
Fig. 2. (opposite page) ACE analysis can reveal the affinity of interactions between PGs or
GAGs and various proteins. For these experiments, syndecan-1 was electrophoresed through
types I–VI collagens in ACE gels. (A) Images of PG migration patterns were obtained using a
phosphorimager. The electrophoretograms indicate that some collagens bind strongly to
syndecan-1 (e.g., type V), and others bind weakly (e.g., type II). Protein concentrations in nM
are shown beneath gels. (B) Calculation of affinities of syndecan-1 for various human col-
lagens. From each electrophoretogram in panel (A), retardation coefficients (R) for syndecan-1
were determined (see text) and are plotted against protein concentration. Smooth curves rep-
resent nonlinear least-squares fits to the equation R = R
∞
(1 + (K
d
/[protein])
2
). Data are
adapted from (5).
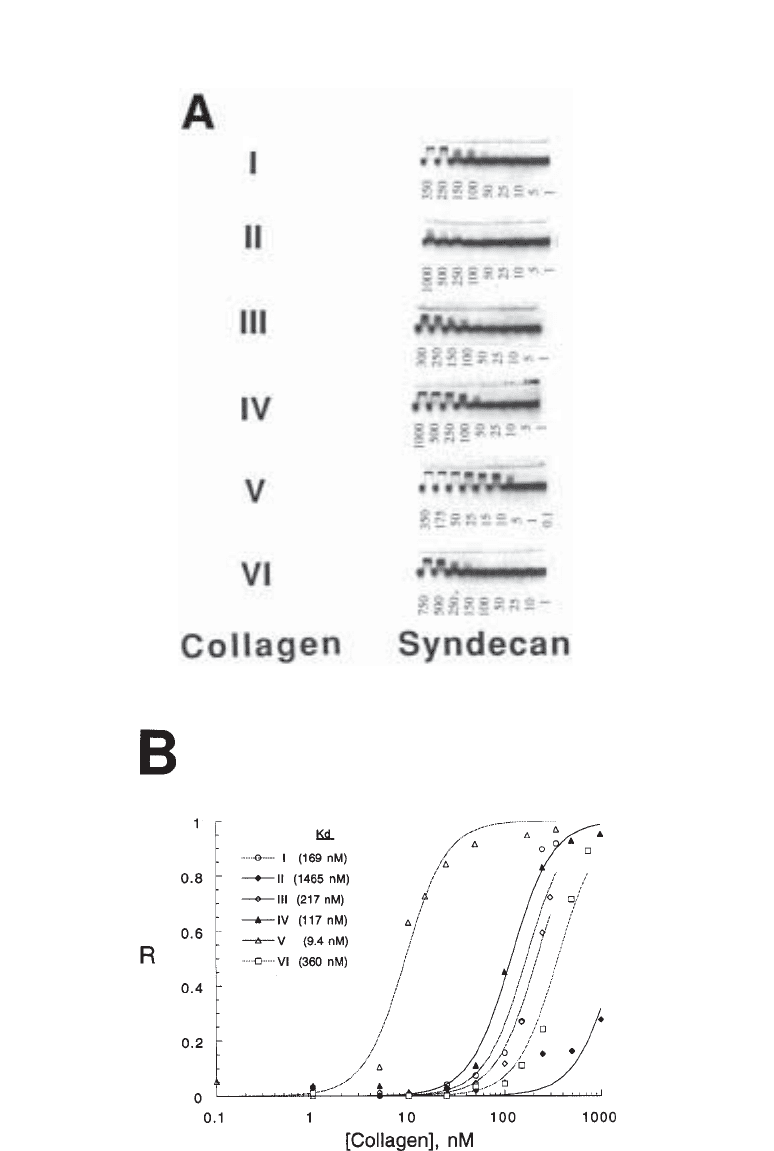
Affinity Coelectrophoresis 403

404 San Antonio and Lander
1.1. Do I Have Enough Protein?
There is no way of knowing a priori how much of a protein sample one needs for an
ACE gel, since it depends on a yet to be determined value, i.e., the affinity the protein will
exhibit for GAGs or PGs. However, some general idea about the amounts of protein
required can be derived from the following example. For type I collagen, a protein of
M
r
⯝ 300,000 Da that exhibits a heparin-binding K
d
in the range of 100–200 nM, one
needs 150 µg of protein per ACE gel (using the ACE gel dimensions specified under
Subheading 2.). This amount allows for the creation of nine protein–agarose samples of
250-µL each, at concentrations of 1000, 500, 250, 100, 50, 25, 10, 5, and 1 nM. Since ACE
gels should be repeated at least three times to derive a reasonable estimate of the K
d
, then
one would need a minimum of 450 µg of type I collagen for three experiments. Other
proteins such as growth factors are much smaller than collagens, and often exhibit much
higher affinities for GAGs and PGs, and thus can require considerably less protein for
three experiments, i.e., on average < 50 µg total, or in some cases even much less—e.g., for
basic fibroblast growth factor, < 1 µg is required.
1.2. Will the Protein Remain Native?
The native state of the protein, its solubility, and propensity to aggregate as a function
of its concentration or solvent are key considerations, and must be determined for each
protein used in ACE. For example, in the case of laminin, which tends to aggregate in
solution, ethylendiamminetetraacetic acid (EDTA) can be supplemented to the protein
samples and the ACE buffers to inhibit aggregation (3). In the case of the collagens,
Fig. 3. ACE analysis can reveal selectivity in PG– or GAG–protein interactions. Example of
ACE analysis of the interactions between a basic peptide and
35
S-sulfate metabolically labeled
PGs/GAGs secreted by endothelial cells in vitro. ACE gel image was obtained using a
phosphorimager. At least two populations of PG/GAG, seen as two bands of radiolabeled mate-
rial migrating with different mobilities at low protein concentrations (< 50 nM), indicates het-
erogeneity in size and/or charge density within the PG/GAG mixture. Potential heterogeneity
in PG/GAG–peptide interactions is also obvious at a peptide concentration of 250 nM, in which
a fractionation of the PG species through the peptide-containing lane is evident as a broad
smear throughout the lane, and as a sharp band that migrates approximately halfway down the
lane. Thus, components of the PG/GAG sample are binding strongly to the peptide (i.e., are
retained closer to the top of the peptide-containing lane), and others are binding more weakly to
the peptide (i.e., are not significantly retained and migrate further within the peptide-contain-
ing lane). In such cases preparative ACE can be used to recover differentially binding PG/GAG
populations for further characterization. Data are adapted from (11).
Affinity Coelectrophoresis 405
which undergo fibrillogenesis within about 20 min after being brought from an acidic to
a neutral solution, such fibrils are insoluble and are impossible to subject to a serial
dilution, as is required in ACE. Thus, to avoid this problem one must bring collagen
solutions from the acid soluble to the neutralized state, and then mixed into agarose and
pipetted into ACE gels before fibrillogenesis occurs (4,5).
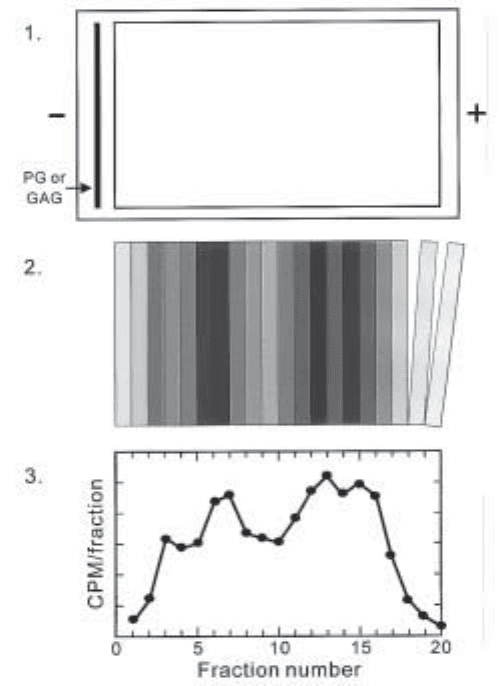
Fig. 4. Preparative ACE schematic. Top panel: A preparative ACE gel is poured using a
casting stand as shown in Fig. 5, except instead of using protein well-forming Teflon combs, a
single Plexiglas block is used to create one large rectangular well to be filled with a single
protein–agarose mixture. Radiolabeled GAG or PG is loaded into the slot above the protein-
containing wells (shown as a dark line to the left in the gel schematic), and electrophoresed through
the protein-containing zone. Middle panel: The agarose gel surrounding the protein-containing
zone is trimmed away, and the remaining protein-agarose block is sectioned into 2-mm-thick
segments. The amount of radiolabeled GAG or PG in each segment is then determined. Bottom
panel: Actual plot of CPM/fraction of heparin octasaccharide mixture electrophoresed through
1000 nM type I collagen, showing the partial resolution of four differentially binding populations.
(From San Antonio and Lander, unpublished data). Artwork by Drew Likens.
406 San Antonio and Lander
1.3. Are the GAGs or PGs of Interest Suitable?
One of the requirements of ACE is that the GAG or PG concentration is much less than
the Kd of GAG– or PG–protein binding (1). Thus, radiolabeled GAGs and PGs are used at
trace concentrations in ACE gels, i.e., generally far less than 1 µg of GAG or PG/gel will
suffice. However, the GAG/PG must be radiolabeled or labeled otherwise, and present in
great enough quantities to be detecteD (generally for radiolabeled samples at least 10,000
cpm/gel). Therefore, the GAGs or PGs must be metabolically radiolabeled with
35
S-sulfate
or
14
C-D-glucosamine in culture and subsequently purified, or purified in their native forms
but derivatized with, for example, Bolton-Hunter reagent (6), fluoresceinamine (1), or
tyramine (3), followed by radioiodination. Here we have presented a method for the
tyramine endlabeling of heparin for use in ACE. Another factor that must be considered in
the analysis of data from ACE gels is the potential multivalency of GAGs or PGs in terms
of their interactions with proteins; this issue is addressed elsewhere (1).
2. Materials
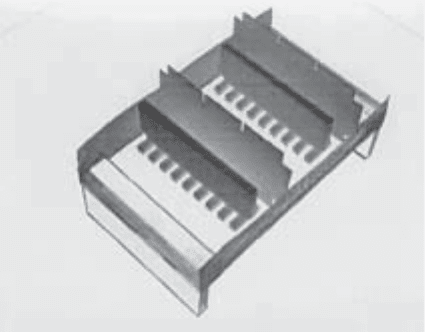
1. ACE casting apparatus (see Fig. 5): casting stage(s), protein well-forming comb(s),
PG/GAG lane-forming comb(s), and tape (autoclave or equivalent). Combs are precision
tooled from Teflon blocks (for protein well-forming combs) or sheets (for PG/GAG
lane-forming combs). The ACE casting apparatus we most commonly use includes a cast-
ing stage made of Plexiglas with a gel platform of 100 long × 75 wide × 6 mm deep;
protein well-forming combs consisting of nine parallel rectangular blocks spaced 3 mm
apart, each 15 long × 4 × 4 mm; and PG/GAG lane-forming combs cut from a 25 × 75 mm
Fig. 5. Oblique view of apparatus for pouring two ACE gels, each with protein-containing
lanes 15 mm in length. Plexiglas casting stand contains a clear piece of gel bond (not visible in this
photograph), on which are placed two Teflon combs that are each used to create 9 agarose–
protein-containing lanes, and two Teflon strips that are each used to create a GAG/PG loading
slot. The stand is bordered on two sides by masking tape, which retains the agarose and Teflon
strips in place. After filling the stand with agarose, upon solidification the combs and strips are
removed, forming two ACE gel templates to be run as described in the text and shown
diagramatically in Fig. 1.
Affinity Coelectrophoresis 407
rectangle of 1-mm-thick Teflon. Rectangles of 4.5 × 10 mm are removed from two
corners to produce a comb with one 66-mm edge, which is stood on its short edge and
held upright in the casting apparatus by pressing the tape used to seal the apparatus against
the overhanging tabs of the comb.
2. At least 1.2 L of running buffer (RB). To make 2 L, add 22.53 g of sodium 3-(N-mor-
pholino)-2hydroxypropanesulfaonate (MOPSO) to 1.8 L of distilled water with
stirring. Add 20.51 g of sodium acetate, anhydrous, or 34.02 g of sodium acetate, trihydrate.
Use 5 M NaOH to bring the pH to 7.0. Bring to 2 L with distilled water. Store at 4°C, will
last no longer than several months. This buffer can also be prepared as a 5× concentrated
stock and diluted before use.
3. GelBond, 85 × 100 mm sheets (FMC, #53734).
4. 1.053% agarose: 1 g of low-melting-point (LMP) (Sea Plaque Agarose; FMC) in 95 mL
of RB.
5. 2.22% agarose: 1 g of LMP agarose (Sea Plaque Agarose; FMC) in 45 mL of RB.
6. 10% CHAPS in distilled water.
7. Leveling device.
8. ACE gel running box (we use the Hoefer Super-Sub apparatus).
9. Low voltage electrophoresis power supply, capable of delivering 75 V.
10. Waterbath set to 37°C.
11. Boiling-water bath or microwave oven.
12. Circulating water chiller (unnecessary if cold-water tap is available at lab bench where the
gel will be run).
13. Small space heater (1500 W).
3. Methods
3.1. Analytical ACE
This protocol is used to estimate the K
d
of GAG or PG binding to a protein, or to
visualize heterogeneity in binding between a PG or GAG mixture and a protein.
1. Place the casting stand on a level benchtop. On a piece of GelBond, determine which is the
bonding side by placing a drop of distilled water on one of the sides. If the drop beads up,
it is the nonbonding side and is placed down on the casting apparatus; if the bead spreads
out, it is the bonding side and is placed face up. Using scissors, cut the GelBond to fit the
casting apparatus, then use 1–2 drops of distilled water to hold the nonbonding side in place.
Press excess water out from between the GelBond and the casting stage and, using
Kimwipes, make sure that the sides of the stage are dry.
2. Use autoclave tape to seal the edges of the casting stage, making sure to leave about 1 in
(2.5 cm) excess on both ends so it can be folded against itself to make a tab to facilitate its
removal later. Place the sample-forming comb(s) in the casting stand using the tape to
hold it in place, then place the protein-lane forming comb by centering it and leaving a
space of about 2 mm between it and the sample comb. Using the 15-mm-long protein
well-forming combs and the gel casting stand specified here, either one or two ACE gels
can be poured per stand.
3. When agarose is made fresh, to promote its rapid dissolution allow at least 20 min for it
to soak in room temperature RB before the mixture is boiled. Place a glass bottle
containing the 1.053% agarose into the water bath, making sure it cannot tip and that
the cap is very loose, so that upon heating it will not explode. The bottle should be left
in the boiling-water bath or microwaved until the agarose has come to a boil and is
