Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

236 Murdoch and Iozzo
3. Dilute the crude extract to a total protein content of 2.5 mg/mL or less with column buffer.
Cold osmotic shock extracts may not need diluting. Load the extract onto the column at
[10 × (diameter of column in cm)
2
] mL/h (~0.5 mL/min for an XK16 column).
4. Wash the column with 12 volumes of column buffer (or with an excess overnight).
5. Elute the bound fusion protein in column buffer containing 10 mM maltose, collecting
fractions of one-fifth column size (usually 2–3 mL). Fusion protein will usually start to
elute within the first column volume and should be quite a tight peak; collecting beyond
3 column volumes is usually not necessary.
6. Determine the protein content of fractions with the Bradford assay and pool the fractions
containing protein.
7. Check the protein concentration of the pooled material and run samples on SDS-PAGE
gels to verify the integrity of the protein.
8. Protein can be dialyzed, or concentrated and buffer-exchanged in Centricon concentra-
tors (Amicon) if it is necessary for your application.
3.5. Inclusion Body Solubilization
1. This method is taken from Sambook et al. (12), based on a method of Marston (13) and
uses a combination of 8 M urea and alkaline pH for solubilisation. Unless stated, all pro-
cedures should be carried out on ice or in a cold room.
2. Pellet up to 1 L of an induced culture of cells by centrifugation at 4000 g for 10 min at 4°C.
3. Decant the supernatant and weigh the pellet. Resuspend the pellet in 3 mL of lysis buffer
per gram wet weight.
4. Add 8 µL of 50 mM PMSF per gram of cells (see Note 2) and then 80 µL of 10 mg/mL
lysozyme per gram. Incubate for 20 min with occasional mixing.
5. Stir the lysate with a glass rod and, while stirring, add 4 mg of deoxycholate per gram of
original cell weight. Move the lysate to a 37°C water bath and continue to stir. When the
lysate becomes viscous, add 20 µL of 1-mg/mL DNAse I per gram of cells, mix well, and
incubate at room temperature.
6. Check the lysate periodically for viscosity. After approx 30 min, the mixture should be no
longer viscous.
7. Centrifuge the lysate at 12,000 g for 15 min at 4°C.
8. Resuspend the pellet in 9 vol of inclusion body wash buffer and incubate at room tem-
perature for 5 min (first wash).
9. Centrifuge at 12,000 g for 15 min at 4°C.
10. Remove the supernatant and keep. The pellet can be rewashed several times. At each
wash, save the supernatant and analyze small (10-µL) samples along with a small sample
of the final pellet resuspended in 100 µL of H
2
O by SDS-PAGE. If large amounts of
the fusion protein are washing out of the pellet, the washes can be kept and pooled with
the solubilised and dialyzed material from the pellet (below). The small amount of
Triton X-100 in a diluted sample applied to an amylose column may interfere with MBP
binding, but will have to be determined on an empirical basis.
11. Resuspend the pellet in 100 µL of inclusion body solubilization buffer. Store at room
temperature for 30 min.
12. Add the mixture to 9 vol of alkaline pH buffer. Incubate at room temperature for 30 min.
Check the pH by removing very small samples to pH strips at regular intervals. If the pH
begins to drop, add KOH to maintain it at 10.7.
13. Adjust the pH to 8.0 with HCl and incubate for a further 30 min at room temperature. It is
important during these alterations in the pH that the temperature is not allowed to rise
above room temperature.
Prokaryotic Expression of Proteoglycans 237
14. Centrifuge at 12000 g for 15 min at room temperature. Decant the supernatant and store.
Resuspend the pellet in 100 µL of SDS-PAGE sample buffer. Run 10 µL samples of
supernatant and resuspended pellet on an SDS-PAGE gel to check the amount of solubilized
material.
15. The supernatant should be dialysed into pMAL column buffer before affinity chromatogra-
phy (above). We have found a single-step dialysis in the presence of β-mercaptoethanol to
be satisfactory, but depending on the fusion partner and the downstream application a
stepwise dialysis from 8 M urea or dropwise dilution into an excess of column buffer may
be tried.
4. Notes
1. The glucose in the growth medium represses the expression of E. coli amylase. Expres-
sion of amylase can lead to problems with the affinity purification due to degradation of
the amylose resin.
2. Some of the protocols listed here use PMSF as a serine protease inhibitor. In many cases
this compound can now be replaced with an equimolar concentration of the much safer and
water-soluble AEBSF (Calbiochem).
3. In our hands the pMAL-c2 and -p2 vectors are not as stable as other plasmids such as pBluescript.
In order to generate plasmids containing insert with no obvious deletions or rearrangements of
the vector, it is normally necessary to pick at least 10 colonies at a time for overnight liquid
culture. More than one set of 10 may need to be screened by restriction digestion.
4. The conditions for IPTG induction may need to be optimized for the particular fusion.
Lower or higher IPTG concentrations, induction for shorter or longer times, and induction
at higher cell densities can all be tested. Problems with fusion protein solubility can also be
improved by altering the growth and induction conditions—for example, expressing the
protein at a lower temperature—but often it is easier to optimize for maximum expression
and then solubilize the protein inclusions, especially if the application does not require
native folded protein.
5. Adding additional protease inhibitors at this step may help with protein stability if this is a
problem.
6. Many refolding protocols are available. A good selection to start from can be found in the
pMAL handbook (at http://www.neb.com).
References
1. Dudhia, J., Davidson, C. M., Wells, T. M., Vynios, D. H., Hardingham, T. E., and Bayliss,
M. T. (1996) Age-related changes in the content of the C-terminal region of aggrecan in
human articular cartilage. Biochem. J. 313, 933–940.
2. Zimmerman, D. R., Dours-Zimmerman, M. T., Schubert, M., and Bruckner-Tuderman, L.
(1994) Versican is expressed in the proliferating zone in the epidermis and in association
with the elastic network of the dermis. J. Cell Biol. 124, 817–825.
3. Murdoch, A. D., Liu, B., Schwarting, R., Tuan, R. S., and Iozzo, R. V. (1994) Widespread
expression of perlecan proteoglycan in basement membranes and extracellular matrices of
human tissues as detected by a novel monoclonal antibody against domain III and by in situ
hybridization. J. Histochem. Cytochem. 42, 239–249.
4. Whitelock, J. M., Murdoch, A. D., Iozzo, R. V., and Underwood, P. A. (1996) The degra-
dation of human endothelial cell-derived perlecan and release of bound basic fibroblast
growth factor by stromelysin, collagenase, plasmin, and heparanases. J. Biol. Chem. 271,
10,079–10,086.
238 Murdoch and Iozzo
5. Ujita, M., Shinomura, T., Ito, K., Kitagawa, Y., and Kimata, K. (1994) Expression and
binding activity of the carboxyl-terminal portion of the core protein of PG-M, a large chon-
droitin sulfate proteoglycan. J. Biol. Chem. 269, 27,603–27,609.
6. Brinkmann, T., Weilke, C., and Kleesiek, K. (1997) Recognition of acceptor proteins by
UDP-D-xylose proteoglycan core protein beta-D xylosyltransferase. J. Biol. Chem. 272,
11,171–11,175.
7. Weilke, C., Brinkmann, T., and Kleesiek, K. (1997) Determination of xylosyltransferase
activity in serum with recombinant human bikunin as acceptor. Clin. Chem. 43, 45–51.
8. Williamson, R. A., Natalia, D., Gee, C. K., Murphy, G., Carr, M. D., and Freedman, R.
B. (1996) Chemically and conformationally authentic active domain of human tissue
inhibitor of metalloproteinases-2 refolded from bacterial inclusion bodies. Eur. J.
Biochem. 241, 476–483.
9. Ruppert, R., Hoffman, E., and Sebald, W. (1996) Human bone morphogenetic protein 2
contains a heparin-binding site which modifies its biological activity. Eur. J. Biochem.
237, 295–302.
10. Guan, C., Li, P., Riggs, P. D., and Inouye, H. (1987) Vectors that facilitate the expression
and purification of foreign peptides in Escherichia coli by fusion to maltose-binding pro-
tein. Gene 67, 21–30.
11. Maina, C. V., Riggs, P. D., Grandea, A. G. III, Slatko, B. E., Moran, L. S., Tagliamonte,
J. A., McReynolds, L. A., and Guan, C. (1988) A vector to express and purify foreign
proteins in Escherichia coli by fusion to, and separation from, maltose binding protein.
Gene 74, 365–373.
12. Sambrook, J., Fritsch, E. F., and Maniatis, T., ed (1989) Molecular Cloning: A Laboratory
Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, MA.
13. Marston, F. A. O., Lowe, P. A., Doel, M. T., Schoemaker, J. M., White, S., and Angal, S.
(1984) Purification of calf prochymosin (prorennin) synthesised in Escherichia coli. Bio/
Technology 2, 800–804.

Proteoglycan Gene Targeting 239
239
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
23
Proteoglycan Gene Targeting in Somatic Cells
Giancarlo Ghiselli and Renato V. Iozzo
1. Introduction
1.1. Overview of Gene Targeting in Mammalian Cells
Gene targeting allows the generation of specifically designed mutations in cells by
the mean of homologous recombination between exogenous DNA and endogenous
genomic sequences (1–3). This is possible because a fragment of genomic DNA intro-
duced into a cell can locate and recombine with the chromosomal homologous
sequences (4). Although the mechanisms of exogenous DNA transfer to the nucleus
and of homologous recombination are still poorly understood, important advances have
been made in identifying the requirements for the targeting vector and the desirable
features of the targeted locus that maximize gene targeting (5). Presently, various
gene targeting techniques can be successfully applied to a variety of organisms. In
mammals, gene targeting strategies have been developed mainly for the genetic
manipulation of mouse embryonic stem (ES) cells. Mating of the transgenes leads to
the generation of animals in which the functional significance of a specific gene
silencing can be investigated in different conditions. Cell lines can also be established
from mouse organ biopsies. There are, however, important limitations to the attain-
ability of cell lines of interest from transgenic animals. First, ES gene knockout tech-
niques are currently limited to the mouse. Because there are major interspecies
differences with regard to gene pattern of expression and implication in disease,
extrapolation from the results obtained with mice cells to the pathophysiological
mechanisms in humans is not warranted. This is particularly the case when genes
involved in malignant transformation and development are investigated. Second, the
availability of established cell lines bearing specific mutations makes somatic gene
targeting highly desirable. In this case the implication of the expression of a particular
gene on the cell behavior can be assessed directly against a well-defined genetic
background. Third, the establishment of an organ-specific cell line involves complex
genetic rearrangements leading to immortalization. In other words, cell lines, even if
240 Ghiselli and Iozzo
derived from the same animal cannot be considered isogenic in the sense that the pat-
tern of expression of several genes — as opposed to the single targeted gene—is
affected. In addition, the genetic mutation that has been introduced through homolo-
gous recombination in ES cells may be lethal even at a stage preceding embryonic
development, thus precluding the possibility of establishing a cell line. Finally, the
cost of originating and maintaining a transgenic animal colony is high.
These potential drawbacks are particularly relevant when considering the gene
knockout of proteoglycan core proteins. There is in fact ample evidence that
proteoglycans play a crucial role, both in development as well as in modulating the
phenotypic expression of somatic cells. This is a case where the ES cell knockout
strategy is of dubious value, as the generated transgenic animals will likely display a
phenotype that is the result of a constellation of effects ensuing at different stages of
life. A mechanistic interpretation of the function of a gene at a cellular level is thus
difficult. This is more so in the case of diseases in which an array of genes rather than
a single gene play a role, such as in cancer.
The subject of homologous recombination and gene targeting has been reviewed
extensively in the recent past (5,6). The reader is directed to that literature for a critical
examination of the various strategies devised for achieving successful gene targeting
and knockout. In this chapter, we will discuss those aspects of the targeting vector
design that are pertinent to somatic gene knockout and the methodology for vector
transfer into established cell lines other than ES cells. The design of an effective
screening strategy for the identification of homologous recombinants by genotyping
and functional assays is also discussed. As proteoglycans play a role in cell differen-
tiation and growth (7) their gene knockout can significantly affect cell behavior. Con-
sequently, care should be taken in devising a screening strategy that is not negatively
biased toward the selection of clones with a rate of growth different from that of the
parent cell line, as this would lead to poor recovery of the clones in which homologous
recombination has occurred.
1.2. Targeting Strategies for Gene Knockout
A major limitation of gene targeting is that DNA introduced into the mammalian
genome integrates by random, nonhomologous recombination at a rate 100 to 100,000
times more frequently than by homologous recombination. In order to identify and
recover the small fraction of cells in which a homologous recombination occurs,
several strategies have been developed. Through these strategies, successful homolo-
gous recombination have been reported to occur in at about 1% of stable transfected
ES clones. Two of these strategies, the so-called positive–negative selection (PNS) (8)
and the promotorless strategy (9,10) have been found to be particularly advantageous.
The PNS approach exploits the use of two selectable markers in the targeting vector.
The first marker gene harboring a drug resistance cassette is inserted within the region
of homology of the vector and is driven by an exogenous promoter. This marker serves
to disrupt the gene translation and at the same time to select the cells that have
incorporated the homologous DNA region of the targeting vector within the chromo-
somal DNA. Examples of selectable markers are neo, which confer resistance to G418,
Proteoglycan Gene Targeting 241
an analog of neomycin that is toxic to mammalian cells, and hydro and puro, which
confer resistance to the hygromycin and the puromycin antibiotic, respectively. The
second marker’s gene, also driven by an exogenous promoter, is placed outside the
homology region of the targeting vector and confers sensitivity to a toxic agent (e.g.,
Herpes simplex virus thymidine kinase [HSV-tk] which impart sensitivity to purine
analog such as ganciclovir, FIAU, and acyclovir through phosphorylation of these
agents). The rationale is that, unless a double reciprocal crossover (i.e., a replacement)
is taking place and the HSV-tk gene is excised out of the inserted vector, cells become
sensitive to the purine toxic agent as the negative-selection gene is incorporated into
the chromosomal DNA. The main advantage of the PNS vector strategy is that there is
no requirement for the targeted gene to be expressed, as both markers gene are driven
by their own promoters. This strategy has been adopted extensively in ES cells. The
occurrence of homologous recombination is somatic cell lines is, however, two to
three orders of magnitude lower than in ES cells (5) and targeting strategies have been
developed that allow the identification of homologous recombinant events with
higher efficiency than that allowed by the PNS strategy. The attainment of high screen-
ing efficiency is crucial for somatic gene knockout inasmuch as a double round of
gene targeting is required. The so-called promotorless selectable marker strategy relies
on a single selectable marker that becomes activated if the targeting vector is incorpo-
rated in a chromosomal region that allows the gene to be driven by an endogenous
promoter. The expected transcriptional product is represented by a fusion protein com-
prising the N-terminal region of the targeted protein and the selectable marker. Since
activation by cellular promoters occurs in about 1% of random integration events, the
use of promotorless vectors provides a 100-fold enrichment for homologous recombi-
nation by reducing the background of nonhomologous recombination. By careful
manipulation of the concentration of the selection agent it has been possible to increase
the rate of legitimate recombination to values approaching or even exceeding 10%
(11). A recent survey of the literature (12) of 23 experiments of gene targeting with a
promotorless vector has revealed that gene targeting frequency in human cell lines is
highly variable, ranging from 1/3 for the p21 gene targeted in HCT116 colon carci-
noma cells to 1/940 for the p53 gene in the same cell line, suggesting important locus-
to-locus variability. A significant advantage of the promotorless strategy with
reference to somatic gene knockout is that since the end point of the experiment is
represented by the suppression of a cell phenotypic trait, assays relying on the expres-
sion of the targeted gene product can be considered for screening purposes. This can
greatly facilitate the screening of the stable transfected clones, bypassing the problem
of identifying the modality of gene mutation through genomic characterization of the
targeted locus.
1.3. Design of a Promotorless Targeting Vectors for Somatic Knockout
of Proteoglycan Genes
Although the description of the method is general, this approach can be applied to
the somatic knockout of any given proteoglycan gene. A targeting vector is designed
to recombine with and mutate a specific chromosomal locus. The construction of a
242 Ghiselli and Iozzo
promotorless targeting vector involves the selection of a suitable genomic fragment
harboring an exon into which a positive selection cassette can be inserted in frame.
The positive selection marker serves two functions: (1) to isolate the rare transfected
cells among which there are those that have properly integrated DNA, and (2) to
silence the gene by mutation. The selected genomic fragment must be free of any
functional promoter/enhancer elements and of repeating sequences that might increase
the rate of detectable spurious recombinations. The desirable features of the homolo-
gous sequence to be incorporated in the targeting vector are:
1. The presence of regions coding for the N-terminus of the protein such that the expression
of a proteins with functional activity is rigorously precluded.
2. The absence of repetitive sequences in order to reduce the chances of scattered illegitimate
recombination in the chromosomal DNA (4).
3. The coding region harboring the marker should be located 5 kb or more from the transcrip-
tional starting site. This is to prevent the gene promoter influencing the expression of
selectable marker (5,11).
4. The selectable marker should be preferably inserted within an exon initiating and terminat-
ing in different splicing phase. This to avoid that splicing of the mutated exon might give rise
to a functional gene product (13).
5. The size of the homologous sequence should be between 3 and 6 kb. Efficiency of
homologous recombination is dependent on the length of the homologous region, but
there is little evidence that beyond 6 kb the frequency of homologous recombination
occurs at significantly higher rate (14–16). On the other hand, limitation in the size of the
homologous region facilitates the construction of the vector
6. The rate of recombination is affected by the length of homologous end regions of the
targeting vector (17). A nonmutated region of 500 bp at either end of the homologous
region should provide sufficient linearized DNA area such that hybridization of
exogeneous and chromosomal DNA at the site of crossover might occur efficiently.
7. The targeting vector needs to be linearized such that a single crossover event (insertion
targeting) or rather a double crossover event (replacement targeting) is favored (18). Gene
knockout by insertion occurs at a rate 10 times higher than by replacement (13). In order
to favor insertion targeting, the targeting vector is linearized at a restriction site located
within the region of homology. On the other hand, a replacement vector requires linear-
ization outside the region of homology, within the vector backbone where unique cutting
sites are already mapped. In this sense, the design of a replacement vector is facilitated
compared to that of an insertion vector.
Detailed mapping of the region of homology is required, especially when the use of
an insertion vector is contemplated. In-depth knowledge of the targeting site and its
restriction fragment mapping is also required in order to generate DNA probes that can
provide informative results on the successful integration of the targeting vector by
Southern hybridization screening. Insertion and replacement targeting vectors give
raise to different integration products (6). The genotype screening strategies should
take into account that although some of the crossover events are favored over others,
there is the possibility that a rather wide range of integration products is obtained,
especially when using an insertion vector. This is because the pattern of integration is
largely dependent on the DNA recombination and repair machinery of the host cell

Proteoglycan Gene Targeting 243
line (4). Furthermore, there is clear evidence for a locus-to-locus susceptibility for
integration of foreign DNA that affects both the rate of homologous integration as
well as the pattern of integration (12). Generally, the final recovery product of a
replacement vector is equivalent to the replacement of the homologous chromosomal
sequence with all the components of the vector except for the regions flanking the
homologous sequence, as all the heterologous part of the vector is excised and lost
(17) (see Fig. 1A). The favored final product of an insertion vector on the other hand,
is an increase in length of chromosomal DNA corresponding to the full length of the
targeting vector including the vector backbone (see Fig. 1B).
Fig. 1. Integration pattern of the targeting vector into a genomic locus. A promotorless
targeting vector is constructed by inserting a mutagen-selectable marker (illustrated by the
heavy shaded box) into the coding region of the gene of interest (shown as lightly shaded
boxes) such that a fusion protein terminated by the stop codon of the selectable marker is
generated. The expression of the selectable marker (usually a gene whose product confer-resis-
tance toward a cytotoxic drug) is driven by an endogenous cellular promoter coinciding with
that of the targeted gene when a legitimate recombination takes place. Targeting vectors
linearized at a site external to the region of homology integrate into the chromosomal DNA
preferentially by replacing the endogenous DNA through a double crossover event (A). Gene
disruption by insertion is favored when cells are transfected with a targeting vector that has
been linearized within the region of homology (B). In this case the vector backbone becomes
part of the mutated chromosomal locus. Because a single crossover event is necessary for the
integration of foreign DNA, mutagenesis by insertion occurs at higher frequency than by
replacement.
244 Ghiselli and Iozzo
1.4. Somatic Gene Knockout: Overall Strategy
After a suitable cell line and the targeting strategy have been selected, the somatic
gene targeting involves the following general steps: (1) electroporation of the target-
ing vector into the host cell line, (2) selection of stable transfected cell colonies by
drug selection, (3) rescue and growth of the cell colonies, (4) preparation of frozen
stock and of duplicate plates for identification of the targeted clones by Southern blot
analysis, PCR, or RT-PCR, (5) new round of targeting in the heterozygous clones
utilizing a targeting vector harboring a new selectable marker, and (6) cloning, cell
rescue, and gene analysis. The knockout is then confirmed by genotyping, Northern
blot hybridization, and immunoassay techniques. For the construction of the
promotorless targeting vector in p-Bluescript (Stratagene) or another suitable cloning
vector, the neomycin cassette harboring the Neomycin phosphotransferase gene (neo)
and its polyA tail is generated by PCR from a pSV2-Neo template using primers that
allow for the in-frame ligation of the cassette into the selected coding region of
the targeting vector. Following cloning and linearization of the construct, the targeting
vector is introduced into the selected human cell line by electroporation using
50–100 µg DNA per 10
7
cells. Human colon carcinoma HCT116 cells has been
frequently used for this purpose (12). This cell line offers some key advantages over
other normal or malignant cell lines. First, HCT116 carries a DNA mismatch repair-
deficient gene, thereby enhancing the stability of the inserted foreign DNA (19). Sec-
ond, these cells are sensitive to the cytotoxic effect of G418 over a relative wide range
of concentrations (between 0.4 and up to 10 mg/mL), which is useful when attempting
to improve the rate of recovery of successful transfectants by increasing the drug con-
centration (11). Third, unlike most transformed cell lines, HCT116 has a normal
euploid, which implies that somatic knockout is completed in two rounds of gene
targeting. Within 2 wk after electroporation and selection in G418, the drug-resistant
colonies are ring-cloned and expanded. The targeting of the second allele is pursued
by a second round of transfection with a targeting vector carrying a different drug
resistance gene, usually hygro or puro. After genotyping of the rescued clones by
Southern hybridization, final confirmation of the functional destruction of the gene of
interest is performed by immunoassays or RNA-based techniques (20). The targeting
strategy and the analysis of the integration products by Southern blot hybridization of
the human perlecan gene in HCT116 colon carcinoma cells is illustrated in Fig. 2. For
the targeting of this gene, a knockout replacenment strategy was considered.
2. Materials
1. Low-electroendoosmosis agarose for electrophoresis (from Fisher).
2. TE buffer: 10 mM Tris-HCl, pH 7.4, 1 mM Na
2
EDTA.
3. Absolute ethanol (95%), reagent grade.
4. DNA restriction enzymes and related reaction buffers can be purchased from various vendors.
5. Cell electroporation apparatus (example: Hoefer’s Progenetor II Electroporation unit).
6. DMEM/FCS: Dulbecco’s Minimal Essential Medium with 10% fetal calf serum.
7. DPBS without Ca/Mg: Dulbecco’s phosphate buffer saline without calcium and magnesium.
8. Trypsin solution: 0.05% trypsin, 0.53 mM Na
4
EDTA in Hank’s phosphate buffer without
Ca/Mg.
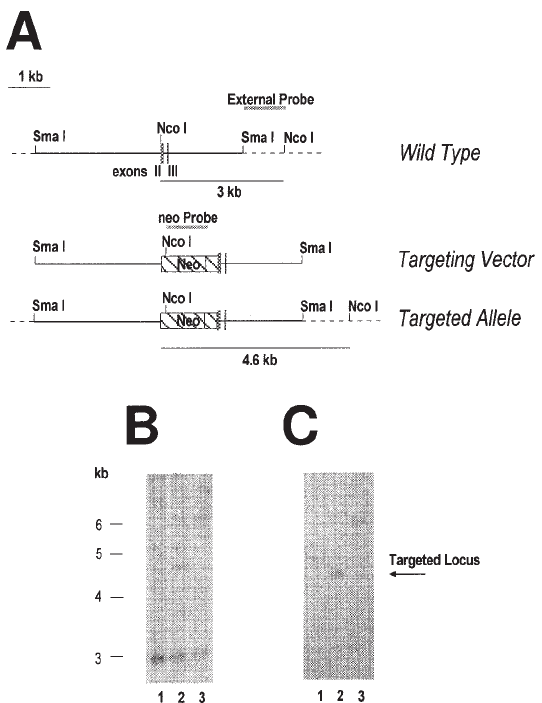
Proteoglycan Gene Targeting 245
Fig. 2. (A) Strategy for targeting of the human perlecan gene in HCT 116 colon carcinoma
cells by replacement. The restriction site map of the wild-type allelic locus is illustrated at the
top. The solid lines represent the targeted DNA, whereas the dotted line correspond to its flank-
ing regions. An in-scale diagram of the targeting vector constructed by in-frame insertion of the
neo selectable marker at the NcoI restriction site of exon II of perlecan is also illustrated. Note
the presence of the NcoI site within the neo insert that facilitates the RFLP analysis of the
recombination products. The genomic DNA of G418-resistant HCT116 cell clones was digested
with NcoI, the products separated by agarose gel electrophoresis, and transferred to a nitrocel-
lulose membrane for hybridization with two
32
P-DNA probes identified by the shadowed bars
in the figure. Panel (B) illustrates the autoradiogram obtained by using the “external” probe
flanking the 3' region of the targeted locus whereas panel (C) illustrates the results with the
same blot using a “neo” probe spanning the neo DNA. Lane 1, unsuccessful targeting; lane 2,
successful targeting identified by the presence of a NcoI restriction fragment of the expected
size (4.6 kb) that hybridizes with both probes; lane 3, evidence for a third integration product
giving a larger-than-expected restriction fragment likely generated by the rearrangement of the
targeting vector and/or of the targeted locus.
