Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

256 Handler and Iozzo
3.4. Luciferase Assay
Following the first stable transfection, neomycin-resistant clones are transiently
transfected with the plasmid pTRE-LUC. This plasmid is similar to the response
plasmid except that the luciferase gene is expressed in place of a specific cDNA under
the control of the TRE and the minimal CMV promoter. By measuring the level of
luciferase activity with a functional assay, it is possible to determine which individual
clones display the highest level of inducibility as well as the lowest background. This
allows the investigator to screen the clones quickly before performing a second stable
transfection.
1. Transiently transfect each neomycin resistant clone with 10 µg of pTRE-LUC plasmid
according to calcium phosphate protocol. We recommend using the CalPhos Maximizer
(Clontech) for maximal transfection efficiency. Although we have suggested introducing
the luciferase vector via calcium phosphate transfection, electroporation can also be uti-
lized (see Note 5).
2. Cells are grown in 35-mm dishes ± doxycycline (2 µg/mL) until 90% confluent.
3. Growth medium is removed from the cells and then rinsed twice with 1× PBS. Then 500 µL
of lysis buffer (25 mM Tris-phosphate, pH 7.8, 2 mM DTT, 2 mM 1,2-diaminocyclohexane-
N,N,N,N-tetracetic acid, 10% glycerol, 1% Triton X-100) are added to each dish and
the cells are scraped off and placed in a sterile Eppendorf tube.
4. Tubes are then spun briefly (~5 s) in a Beckman microcentrifuge at 12,000g to pellet
large debris.
5. Next, 20 µL of room-temperature cell extract is mixed with 100 µL of room-temperature
Luciferase Assay Reagent (Promega).
6. The sample is placed in a luminometer to measure the release of light as the luciferin is
being oxidized (see Note 7).
3.5. Generation of Antisense Inducible Clones
Once a clone has been selected to have low background levels and high inducibility
based on the luciferase assay results, it undergoes a second stable transfection. It
is transfected with two plasmids: a response plasmid containing specific cDNA under
the control of the TRE and minimal CMV promoter, as well as a plasmid expressing
the hygromycin resistance gene. Because the cells are already neomycin resistant, it is
necessary to use a different antibiotic resistance marker to select clones.
1. The most optimal stably transfected clone, as determined by luciferase assay, is cotransfected
with 40 µg of the pTRE construct and 2 µg of the hygromycin plasmid by electroporation
as described previously. Because the clone is already G418 resistant, hygromycin is
needed as a second antibiotic resistance marker.
2. After 48 h, 200 µg/mL hygromycin was added to the culture medium (see Note 4).
3. Then 30–40 hygromycin-resistant clones are isolated by ring cloning and expanded as
described previously.
3.6. Southern Blot Analysis
Although the clones have been selected through antibiotic resistance, it is also
important to show that the regulatory and response plasmids have in fact integrated
into the genome. This can be achieved by PCR with primers that amplify a specific
region of the regulatory and response plasmids or by Southern analysis utilizing these
Antisense Expression 257
plasmids as probes. We have found that Southern analysis is a much more reliable
although labor-intensive method. It is preferable to perform Southern analysis after the
first stable transfection as well before proceeding with the second stable transfection.
1. Extract genomic DNA from a 35-mm dish of each clone to be screened. DNA should be
phenol:chloroformed and ethanol precipitated prior to restriction digestion.
2. Separate endonuclease digestions should be performed with an enzyme(s) that will liber-
ate a distinct region within either the response plasmid and/or the regulatory plasmid.
Digest 10 µg of genomic DNA overnight at 37°C, followed by inactivation with 0.5 µL of
0.5 M EDTA, phenol:chloroform extraction and ethanol precipitation.
3. Genomic digestions are run on a 0.8% agarose gel in TAE buffer at 80 V for 4–5 h. Gel
percentage and duration of electrophoresis will depend on the size of the fragments that are
expected.
4. Plasmid DNA should be digested with the same enzymes as genomic DNA and inserts
purified as described previously.
5. After gel is finished running, it is denatured in 1.5 M NaCl and 0.5 M NaOH for 30 min at
room temperature and then neutralized in 1 M ammonium acetate and 0.5 M NaOH for
30 min at room temperature. The DNA is transferred onto a nylon membrane in 10× SSC
overnight and then UV cross-linked in a Stratalinker.
6. The plasmid probes should be labeled with
32
P dCTP or, if preferred, a nonradioactive
isotope such as digoxigenin, by random priming with Klenow according to the
manufacturer’s protocol.
7. Genomic DNA filters are prehybridized at 65°C for 3 h and then hybridized with the
corresponding DNA probes at 65°C overnight. Membranes are then washed and exposed to
autoradiography film. Development of the film should yield the expected results.
3.7. Northern Blot Analysis
Northern analysis is needed to determine that the tTA or rtTA and the antisense
cDNA are expressed. This will confirm expression of both the regulatory and response
transcripts. Proper controls include extraction of total RNA in both the presence and
absence of doxycycline. Once again, Northern analysis should ideally also be per-
formed after the first stable transfection.
1. Total RNA is extracted from 35-mm confluent dishes of the clones to be screened by
commonly used guanidium thiocyanate protocols (e.g., TRI-Reagent, Sigma) (see Note
8).
2. Then 20 µg of total RNA are run on a 1% agarose/formaldehyde denaturing gel in MOPS
buffer at 70 V for 4–5 h.
3. Total RNA is transferred onto a nylon filter and cross-linked with a UV Stratalinker. Filters
are prehybridized at 42°C for 3 h and hybridized at 42°C overnight with the probes labeled
the same as described for Southern analysis.
4. Membranes are then washed and exposed to autoradiography film. Development of the film
should yield the expected results.
3.8. Western Immunoblotting Analyses
Western analysis is necessary to determine that there is a decrease in specific pro-
tein expression upon addition or removal of doxycycline. Which system is being used
(Tet-On or Tet-Off) will dictate whether expression should be blocked with or without
doxycycline. It is also possible to perform functional assays for the protein as well.
258 Handler and Iozzo
1. Antisense transfected clones are expanded into 35-mm dishes and then incubated for 48 h
in serum-free defined media with or without 2 µg/mL doxycycline.
2. Serial dilutions of the conditioned media from each clone are vacuum blotted onto a nylon
filter (Schleicher and Scheull).
3. The filter is then blocked in 3% milk for 3 h and then incubated overnight at room tempera-
ture with a primary antibody to Protein X.
4. After several washes, the blot is incubated with an appropriate horseradish peroxidase-conju-
gated secondary antibody.
5. Enhanced chemiluminescence is performed according to the manufacturer’s protocol
(Amersham).
6. Autoradiographs are then scanned by laser densitometry and quantitated to determine the
relative protein levels.
4. Notes
1. When working with tissue cultures, sterility is of concern. Although antibiotics are added
to the culture media, be sure to wipe down the hood with 70% ethanol before and after
use, and wear gloves throughout the procedure.
2. Any DNA to be transfected into cells should be of highest quality. That is, after plasmid
preparation, DNA should be phenol:chloroformed and ethanol precipitated under a sterile
tissue culture hood. Also, as a final check, it is a good idea to run an aliquot on an agarose
gel prior to transfection.
3. Be sure to determine the correct capacitance and voltage for the cell line and electroporator.
4. Due to variations in potency between different lots, it may be necessary to titrate the proper
concentrations of G418 and hygromycin for each cell line.
5. It is important to mention that certain cell lines can alter the effectiveness of the tetracycline
controlled gene expression (11). The type of transfection method as well as the amount of
plasmid that is transfected can also alter considerably the background levels and inducibility
of each clone. Therefore, it may be necessary to test several experimental parameters for
each cell line and screen many clones in order to find one that is highly inducible but also
gives very low levels of background expression. Although labor intensive, the results will
be much more trustworthy.
6. When ring cloning, we have found that plastic rings work better than metal rings and require
less vacuum grease to attach to the plate. Also, while picking clones, try to work rapidly,
because the plates can dry out quickly and the cells will die.
7. We have noticed that often there are extremely high levels of background luciferase expres-
sion in many of the selected clones. Therefore, it may be necessary to select more than the
recommended number of clones until one of low background can be isolated.
8. Be certain when working with RNA that gloves are always worn and are changed fre-
quently. RNA is very susceptible to degradation from RNases that are found on most
surfaces, including skin.
References
1. Gossen, M. and Bujard, H. (1992) Tight control of gene expression in mammalian cells by
tetracycline-responsive promoters. Proc. Natl. Acad. Sci. (USA) 89, 5547–5551.
2. Gossen, M., Bonin, A. L., and Bujard, H. (1993) Control of gene activity in higher eukary-
otic cells by prokaryotic regulatory elements. TIBS 18, 471–475.
3. Gossen, M., Freundlieb, S., Bender, G., Muller, G., Hillen, W., and Bujard, H. (1995)
Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769.
Antisense Expression 259
4. Baron, U., Freundlieb, S., Gossen, M., and Bujard, H. (1995) Co-regulation of two gene
activities by tetracycline via a bidirectional promoter. Nucleic Acids Res. 23, 3605–3606.
5. Baron, U., Gossen, M., and Bujard, H. (1997) Tetracycline-controlled transcription in
eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res.
25, 2723–2729.
6. Freundlieb, S., Baron, U., Bonin A.L., Gossen, M., and Bujard, H. (1997) Use of tetracy-
cline-controlled gene expression systems to study mammalian cell cycle. Meth. Enzymol.
283, 159–173.
7. Furth, P. A., Onge, L. S., Boger, H., Gruss, P., Gossen, M., Kistner, A., Bujard, H., and
Henninghausen, L. (1994) Temporal control of gene expression in transgenic mice by a
tetracycline-responsive promoter. Proc. Natl. Acad. Sci. (USA) 91, 9302–9306.
8. Kistner, A., Gossen, M., Zimmermann, F., Jerecic, J., Ullmer, C., Lubbert, H., and Bujard,
H. (1996) Doxycycline-mediated quantitative and tissue-specific control of gene expression
in transgenic mice. Proc. Natl. Acad. Sci. (USA) 93, 10,933–10,945.
9. Baron, U., Schnappinger, D., Helbl, V., Gossen, M., Hillen, W., and Bujard, H. (1999)
Generation of conditional mutants in higher eukaryotes by switching between the expression
of two genes. Proc. Natl. Acad. Sci. (USA) 96, 1013–1018.
10. Bonin, A. L., Gossen, M., and Bujard, H. (1994) Photinus pyralis luciferase: vectors that
contain a modified luc coding sequence allowing convenient transfer into other systems.
Gene 141, 75–77.
11. Gossen, M. and Bujard, H. (1995) Efficacy of tetracycline-controlled gene expression is
influenced by cell type: commentary. BioTechniques 19, 213–216.

Cell-Mediated Transfer of PG Genes 261
261
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
25
Cell-Mediated Transfer of Proteoglycan Genes
Jens W. Fischer, Michael G. Kinsella, David Hasenstab,
Alexander W. Clowes, and Thomas N. Wight
1. Introduction
Replication-defective retroviral systems for transduction of genes into target
eukaryotic cells have grown recently in popularity. In general, the use of a retroviral
packaging system involves the preparation and transfection by standard molecular
biological techniques of the retroviral vector into packaging cell lines for the produc-
tion of replication-defective retrovirus (1,2). Virus-laden medium from packaging cells
is then used to infect target cells. Retroviral transduction of cDNA sequences has dis-
tinct advantages and disadvantages when compared to other gene transfer technolo-
gies. Foremost among the advantages of viral gene delivery approaches are the high
cellular infection rates. This efficiency allows the preparation and rapid selection of
large pools of transduced cells without the added step of cell cloning. This is critical in
the preparation of sufficient numbers of low-passage primary cells for both character-
ization of cellular phenotype and expression of the transgene in vitro, as well as for
experimental use in vivo. Moreover, because pools of transduced cells can be used
rather than clonal cell lines, clonal variability unrelated to transgene expression is not
a major issue in interpretation of experimental results. A major advantage in the use of
the retroviral delivery system is that the transduced gene is rapidly incorporated into
the host genome of dividing cells, thus ensuring stable expression of the gene product,
although typically a single copy of the target gene is inserted. However, because the
insertion of the viral sequences requires mitosis of the target cell, retroviral vectors
cannot be used effectively to transfer genes to nondividing cells. Thus, retroviral trans-
duction is usually performed on cells in culture, and gene transfer in animal models
can be performed by implantation of cells that express the transgene, a process re-
ferred to as cell-mediated gene transfer. One advantage of the cell-mediated gene trans-
fer approach is that long-term expression of the transgene in vivo can be achieved (3).
Moreover, local expression of the gene can be induced in an adult animal without
concern that adaptive processes that compromise function or viability might occur
262 Fischer et al.
during development, such as is possible in transgenic and knockout models. However,
the preimplantation culture of the target cells in vitro may induce a phenotype that
differs from that of endogenous cells. The approach of cell-mediated gene transfer has
been used both to model disease processes and to evaluate therapeutic agents in vivo.
For example, the role of tissue factor in the thrombotic occlusion of restenotic vessels
has been examined by local overexpression of tissue factor by cell-mediated gene
transfer (4). Retrovirally transduced cells that overexpress the human granulocyte
colony stimulating factor (G-CSF) gene have been used to examine the effects of the
systemic delivery of that factor on circulating neutrophil levels (5). Several studies
have used cell-mediated gene transfer to express locally potential therapeutic agents
in vivo. For example, tissue inhibitor of metalloproteinases has been assessed as an
approach to limiting vessel aneurysms in an animal model (6), and the local expres-
sion of decorin induced by cell-mediated gene transfer has been assessed as an agent
to limit the growth of the neointima (7). We include protocols that we have used for
cell-mediated gene transfer to the injured rat carotid artery as examples of this ap-
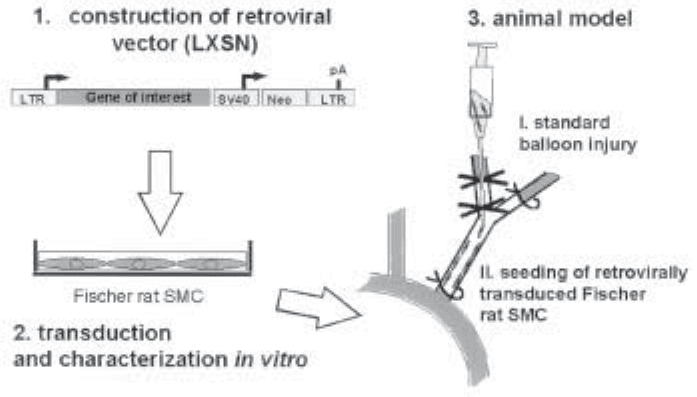
proach (Fig. 1).
2. Materials
2.1. Retroviral Transfection of Target Cells
1. PE501 cells.
2. PA317 cells.
3. NIH 3T3 TK– cells.
4. LXSN vector (~20 µg/transfection).
5. 2.5 M CaCl
2
, pH 7.2.
Fig. 1. Flow diagram of protocols involved in cell-mediated proteoglycan gene transfer. In
step 1 the dark panel indicates the gene of interst.
Cell-Mediated Transfer of PG Genes 263
6. 2× HEPES buffered saline (2× HBS): 50 mM HEPES, 280 mM NaCl, 1.5 mM Na
2
HPO
4
,
pH 7.2.
7. Polybrene (hexadimethrine bromide) stock solution, 4 mg/mL, store at 4°C. Polybrene is
added to the media of cultures to be infected in order to increase infection rates.
8. G-418 (Geneticin) stock solution, 50 mg/mL in PBS. G-418 is a neomycin analog used for
cell selection after transduction of vector.
9. Standard tissue culture supplies.
10. Culture medium: Dulbecco’s Minimal Essential Medium (DMEM) with 10% fetal
bovine serum.
2.2. Internal Seeding of Retrovirally Transfected Rat Smooth Muscle
Cells into the Injured Rat Carotid Artery
The following is an example of how cell mediated gene transfer, using retrovirally
transduced smooth muscle cells (3,8), can be used to overexpress proteoglycans in the
vessel wall and to investigate their effect on neointima formation (7). For this purpose
smooth muscle cell from Fischer 344 rats were retrovirally transduced using the proto-
col described above. Since Fischer 344 rats are an inbred rat strain, Fischer 344 rat
smooth muscle cells can be transferred into any Fischer 344 rat without inducing an
immune response (see Note 1).
1. Fischer 344 rats, male, 250–300 g.
2. Anesthetic: 50 mg/kg ketamine, 5 mg/kg xylazine, 1mg/kg acepromazine.
3. Heparin, 10U/kg.
4. 2F Fogarty balloon embolectomy catheter (Baxter) filled with water, attached to 1-cm
2
syringe filled with lactate ringer’s solution.
5. 1-cm
3
syringe attached to silicone elastomer medical grade tubing (0.012 in. id × 0.025
in. od).
6. Lactated Ringer’s solution.
7. Gauze sponges.
8. Cotton-tipped applicators.
9. 4.0 silk sutures.
10. Scalpel.
11. Ethanol, betadine.
12. Instruments: fine point (watchmaker’s) forceps; blunt-curved forceps, fine curved forceps,
retractor, 3 mosquito clamps, arterial (vanna) scissors, regular scissors.
2.3. Peri-adventitial Seeding on Decellularized Carotids
In some circumstances, it may be advantageous not to have endogenous cells
present in the media and neointima and to repopulate a vessel entirely with exog-
enously seeded cells. Rapid freeze/thaw cycles can be used to decellularize the artery
before seeding the cells in the peri-adventitial region. Transduced smooth muscle
cells can be cultured in 5'-Bromo-2'-deoxyuridine (BrdU)(Roche, 24 h in 0.06 µg/mL),
which will be incorporated in the DNA and serve as a genomic tag for identifying
seeded cells at later times. Smooth muscle cells seeded in the peri-adventitial region
will migrate through the media and form a neointima by 2 wk. This protocol is well
suited for measuring the effects of gene expression on migration in vivo (4,10).
1. As under Subheading 2.2.1.
2. Liquid nitrogen.
264 Fischer et al.
3. 5'-Bromo-2'-deoxyuridine (BrdU).
4. Teflon sheet.
5. Anti-BrdU antibodies.
6. Secondary antibody and standard supplies for immunocytochemistry (ICC).
3. Methods
3.1. Retroviral Transfection of Target Cells
1. Insert the gene of interest into the LXSN vector using standard procedures (see Note 2).
2. Transfection of primary ecotropic (PE501) cells with the retroviral vector (LXSN and L
gene of interest SN) and transduction of secondary amphotropic (PA317) packaging cells
with the replication-defective retrovirus (see Note 3):
Day 1: Plate PE501 cells at 5 × 10
5
cells/60mm dish, using 4 mL of medium.
Day 2: Prepare tube A with 20 µg of vector DNA, and 50 µL of 2.5 M CaCl
2
. Add H
2
O
to make 500 µL. Prepare tube B with 500 µL of 2× HBS solution. Add contents of tube A
dropwise to tube B, mixing by constant moderate aeration of tube B with a Pasteur pipet.
Incubate at ambient temperature for 30 min (a precipitate will form). Add 1 mL of the
mixture to the medium in each plate and incubate cells overnight at 37°C in the incubator.
Day 3: Change medium of transfected PE501 cells. Plate secondary packaging cell
line (PA317) at 5 × 10
5
cells/60-mm dish; include an extra dish with PA317 cells to
monitor the G-418 selection (see Note 4). Note: After transfection, cells, media and asso-
ciated culture materials must be considered Biohazardous!
Day 4: Collect 16-h-conditioned medium from PE501 cells. Centrifuge collected cul-
ture supernatant for 10 min at 4800g (see Note 5). Prepare medium for infection of PA317
cells by mixing fresh medium 1/1 with the conditioned medium of PE501 cells and add
Polybrene from 1000× stock at final concentration of 4 µg/mL. Replace the culture
medium of the target PA317 cells with the freshly prepared viral infection medium and
incubate overnight.
Day 5: Replate infected and control PA317 cells at various dilutions (1/10, 1/100,
1/1000) into 100-mm culture dishes with medium containing 800 µg of G-418/mL for
selection
of virally transduced cells (see Note 4).
3. Selection and cloning of secondary packaging cell line (PA317): Replace selection me-
dium every 2–3 d until all cells in the control dish die (approximately 7–10 d). Pick
clones with the use of cloning rings following standard procedures and pass individual
clones into 60-mm plates. Continue growth in selection media for 3 d more. Freeze clones
back before you determine the virus titer of PA317 clones (step 4).
4. Titer virus produced by PA317 clones. In this step, the relative number of virus produced by
the different clones is determined, in order to select the PA317 clone that produces the
highest titer for transduction of target cell lines.
Day 1: Plate cells from the PA317 clone to be titered at 5 × 10
5
cells/60-mm dish.
Day 2: Replace medium of the PA317 cells with 4 mL of fresh medium and plate one
dish of NIH 3T3 TK– cells at 5 × 10
5
cells/60-mm dish for each clone to be titered. Plate
one additional dish to use as a selection control.
Day 3: Collect 16-h-conditioned medium of PA317 cells, centrifuge for 10 min at
4800g, prepare medium for infection of NIH 3T3 TK– cells by mixing fresh medium with
10 µL of the virus-containing medium of PA317 cells and add Polybrene at a final con-
centration of 4 µg/mL. Replace the medium of the NIH 3T3 TK– cells with the freshly
prepared infection medium. Add fresh medium only to the selection control dish. Incu-
bate overnight at 37°C.
Cell-Mediated Transfer of PG Genes 265
Day 4: Trypsinize 3T3 TK
–
cells and replate three different dilutions (1/20, 1/100,
1/500) of the cells into 6-well plates in medium containing G-418 (600 µg/mL). Grow
the cells for 7–10 d until the cells in the selection control dish have died and the colonies
in the titering plates are big enough to be counted. To count clones, remove media, rinse
with PBS, fix for 2 min with 100% methanol, rinse with distilled water, and stain
cells with 0.1% methylene blue in 50% ethanol for 5 min. After staining, dishes are rinsed
with water until excess stain is removed and clones are visible, then dishes are air-dried,
and the colonies counted by visual inspection. The number of clones and the dilution
factors allow the calculation of the number of virus particles produced by the different
clones. For example, 10 clones at the 1/500 dilution equals 5000 infectious units (cfu) in
10 µL of medium or 5 × 10
5
cfu/mL.
After virus-titering, selected clones are expanded and frozen back under liquid nitrogen.
5. Infection of the target cells (see Note 6).
Day 1: Plate the PA317 clone with the highest titer at 5 × 10
5
cells/60-mm dish
(see Note 7).
Day 2: Change medium of PA317 cells (4 mL), plate target cells at 5 × 10
5
cells/
60-mm dish. Plate a control dish of target cells to monitor the G-418 selection.
Day 3: Collect 16-h-conditioned medium from PA317 cells (see Note 7) and centri-
fuge for 10 min at 4800g. Prepare infection medium for the target cells by mixing
fresh medium 1/1 with the conditioned medium of PA317 cells and adding Polybrene
at 4 µg/mL. Remove medium from the target cells and apply the freshly prepared infec-
tion medium.
Day 4: Replate cultures of transduced target cells into 100-mm dishes and add G-418
at the previously determined concentration for selection.
Days 5–14: Continue selection in G-418, with medium changes every 3 d, until all of
the cells in nontransduced control cultures die. Target cells are now stably transduced and
can be passed and frozen back following standard tissue culture procedures.
6. Characterization of the transduced cells: The characterization procedures typically in-
clude Northern and Western blot analysis to demonstrate mRNA and protein expression
of the transgene. The DNA sequences that encode the gene of interest and the neomycin
phosphotransferase gene are expressed in a single contiguous mRNA. Therefore, the
mRNA for the transgene is ~3.1 kb larger than that of the endogenous gene, allowing the
retrovirally expressed gene product to be easily distinguished from the endogenous
mRNA. Further characterization after the Western blotting depends largely on the gene of
interest. If the target gene is a proteoglycan, it is important to determine the extent to
which glycosaminoglycan chains are added and elongated on the core protein. The appro-
priate procedures are described elsewhere.
3.2. Internal Seeding Of Retrovirally Transfected Rat Smooth Muscle
Cells into the Injured Rat Carotid Artery
1. Preparation of cells for the seeding procedure (see Note 9): After characterization of the
transduced smooth muscle cells in vitro, grow cells nearly to confluence and trypsinize.
Suspend the cells in DMEM/10% FCS to inactivate the trypsin, centrifuge cells at 960g
(1000 rpm) and wash with DMEM without serum, repeat the washing step once, count the
cells, and resuspend at 2.5 × 10
6
cells/mL in serum-free DMEM. The estimated amount of
cell suspension per carotid is 40 µL; to allow for waste, prepare 150 µL per seeded carotid
(see Note 10).
2. Anesthetize and weigh animals (see Note 11).
266 Fischer et al.
3. Shave throat.
4. Restrain rat lying on its back (make sure that the tongue does not obstruct the airway).
5. Disinfect the shaved throat area.
6. Make a midline incision from breast bone to chin bone.
7. Blunt dissect until the carotid artery is visible.
8. Set retractor to open neck cavity.
9. Dissect the common carotid artery from chin bone (distal to the bifurcation) to approx 1 cm
proximal of the bifurcation.
10. Put a double, temporary loop around the common carotid artery as far proximally as possible.
11. Install a single loop around the external carotid just distal of the bifurcation.
12. Ligate the external carotid as distal as possible.
13. Put a double, temporary loop around the internal carotid.
14. Attach hemostats to all ties for better control.
15. Shut off blood flow by tightening the double loop around the proximal part of the common
carotid artery.
16. Make an arteriotomy between the distal tie and the single temporary loop aroung the external
carotid artery (step 11). Use tips of the Vanna scissors at a 45° angle, close and insert the
tips of the scissors into the arteriotomy, and carefully open the scissors to spread the
arteriotomy, then close the scissors again before pulling it out.
17. Hold the arteriotomy open with the cut tip of a 30-g needle and insert tip of the catheter filled
with lactated Ringer’s and flush to remove blood.
18. Insert balloon catheter and perform balloon injury to the carotid artery (Clowes et al.
[1983], Lab Invest 49, 208), afterwards tighten loop to prevent blood flow.
19. Flush again with lactated Ringer’s.
20. Resuspend cells thoroughly (no clumps) and draw about 150 µL into the 1cm
3
syringe,
attach the catheter, remove air, and insert the catheter into the ateriotomy, proximal of
the middle tie at the bifurcation. Tie the catheter in with the middle loop (make sure
that it seals) and instill the cells into the carotid artery while pulling the catheter
back toward the arteriotomy. Be careful not to pull the catheter tip out. Invert the rat for
2 min, then return the rat to its original position for 13 min (cover the wound with
lactated Ringer’s-soaked gauze).
21. To close, remove the catheter, loosen the proximal tie of the common carotid artery and flush
the artery with blood to remove the cells that did not adhere to the denuded vessel wall.
22. Ligate the middle loop around the external carotid artery around the bifurcation.
23. Restore blood flow by loosening the proximal tie around the common carotid artery.
24. Remove the loop on the internal carotid artery, and check blood flow through the internal
and common carotid artery.
25. Cut the ends of the ties; leave the proximal loop loosely around the common carotid
artery as a marker.
26. Close the incision.
27. At the end of the experimental period, seeded carotid arteries can be harvested as fresh
tissue for further examination by methods of molecular biology or protein and proteoglycan
biochemistry, or the animal can be perfusion fixed at physiological pressures for morpho-
logical examination.
3.3. Peri-adventitial Seeding on Decellularized Carotids
1. Cells are prepared as described previously except that 24 h before trypsinization, 0.06 µg/mL
of BrdU are added to the culture medium. This dose should be determined for each cell
