Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.


Methods in Molecular Biology
TM
HUMANA PRESS
Proteoglycan
Protocols
Edited by
Renato V. Iozzo,
MD
HUMANA PRESS
Methods in Molecular Biology
TM
VOLUME 171
Proteoglycan
Protocols
Edited by
Renato V. Iozzo,
MD

Isolation from Cell Cultures and Tissues 3
3
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
1
Isolation of Proteoglycans from Cell Cultures and Tissues
Masaki Yanagishita
1. Introduction
Proteoglycans are a class of glycosylated proteins characterized by the presence of
glycosaminoglycans as a carbohydrate component, which endow them with unique
biological as well as biochemical properties. Therefore, isolation of proteoglycans
from various biological sources such as cell cultures and tissues could be achieved by
ordinary molecular purification procedures utilizing their general molecular proper-
ties and by those taking advantages of the presence of glycosaminoglycan moiety.
This chapter focuses on the latter experimental procedures, which are particularly use-
ful for obtaining total proteoglycan and glycosaminoglycan species from various
biological sources. These protocols could be followed by purification procedures
specific to individual proteoglycan species (i.e., by using antibodies to core proteins,
or binding proteins to specific sequences of glycosaminoglycans) to select specific
molecules.
Two major classes of well-established purification procedures aiming at the pres-
ence of glycosaminoglycans in the molecule have been used extensively. They
include (1) density gradient ultracentrifugation, and (2) anion-exchange chroma-
tography. The former procedure makes use of the fact that the glycosaminoglycan
moiety of proteoglycans has high specific gravity, so, proteoglycans with a large
number of glycosaminoglycans have high molecular densities (often as high as
those of nucleic acids). Therefore, such a procedure is particularly suited for the
purification of proteoglycans with many glycosaminoglycan chains (e.g., aggrecan,
the major proteoglycan in cartilage tissues). On the other hand, the procedure is not
suitable for isolating proteoglycans with only few glycosaminoglycans and high
protein contents (e.g., “small, leucine-rich proteoglycans” and cell surface heparan
sulfate proteoglycans). Purification procedures belonging to the latter class take
advantage of high negative charges contributed by sulfate groups and carboxyl
groups universally present in glycosaminoglycans. Thus, they can be widely used
4 Yanagishita
for isolating any species of proteoglycans and glycosaminoglycans, and are the main
subject of following discussion.
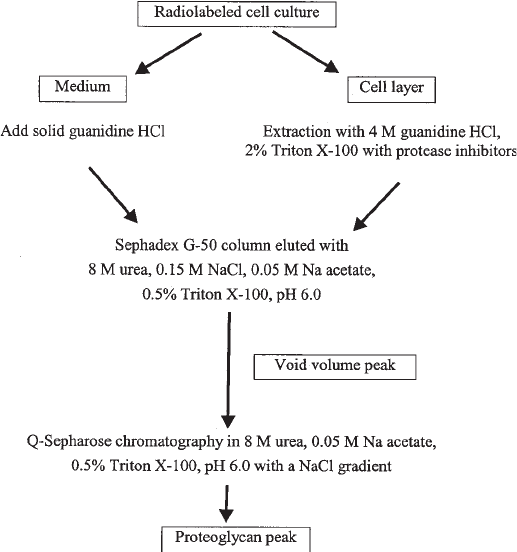
This chapter outlines a proteoglycan-isolating protocol from a metabolically radiola-
beled cell culture (see Fig. 1) as an example case, which addresses technical problems
often encountered when working with small quantities of proteoglycans. Other reviews
on similar subjects can be consulted for more detailed discussions (1–3).
2. Materials
2.1. Extraction
1. Buffer: 4 M guanidine HCl, 0.05 M Na acetate, pH 6.0, containing 2% (w/v) Triton X-100
and protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 10 mM N-ethylmaleimide,
10 mM disodium ethylenediaminetetraacetic acid (4).
2. Stock solutions (100 × concentrated) of phenylmethylsulfonyl fluoride and N-ethylmaleimide
are prepared in ethanol and added to the guanidine HCl buffer just prior to use (they are
unstable in aqueous solutions).
2.2. Solvent Exchange
1. Sephadex G-50, fine (Amersham Pharmacia Biotech). Preswelling of Sephadex can be
done in hot water (off the heater), which achieves sterilization, degassing, and shortening of
swelling time. Extreme caution should be exercised when adding Sephadex powder to
Fig. 1. A flow diagram of proteoglycan isolation from a radiolabeled cell culture.
Isolation from Cell Cultures and Tissues 5
boiling water, to avoid flushing. A convenient concentration of gel (50% slurry) can be
made by mixing 5 g of Sephadex G-50 with 100 mL of water. Bacteriostatic agents (e.g.,
0.02% Na azide) should be added for long-term storage.
2. 10 mL plastic disposable pipet (Falcon).
3. Glass wool, #3950 (Corning).
4. Buffer: 8 M urea, 0.20 M NaCl, 0.05 M Na acetate, 0.5% Triton X-100, pH 6.0.
2.3. Anion-Exchange Chromatography
1. Q-Sepharose, fast flow (Amersham Pharmacia Biotech). Q-Sepharose has to be
preequilibrated with the low salt buffer as described below.
2. Low salt buffer: 8 M urea, 0.20 M NaCl, 0.05 M Na acetate, 0.5% Triton X-100, pH 6.0.
3. High salt buffer: 8 M urea, 1.5 M NaCl, 0.05 M Na acetate, 0.5% Triton X-100, pH 6.0.
4. Gradient former (a simple configuration can be made with two beakers).
5. Peristaltic pump.
2.4. Gel Filtration Chromatography
1. Superose 6, HR 10/30 (Amersham Pharmacia Biotech).
2. Buffer: 4 M guanidine HCl, 0.05 M Na acetate, 0.5% (w/v) Triton X-100, pH 6.0.
3. Methods
General experimental procedures introducing radioactive precursors into
proteoglycans using cell cultures and tissue cultures are reviewed elsewhere (1).
3.1. Extraction
1. After removing media from the cell layer, approximately 2 mL of extraction buffer (per
35-mm-diameter cell culture dish) is added to a culture plate (see Note 1).
2. Proteoglycans are extracted within 2–3 h of constant shaking at 4°C.
3. Passing the extract through a 1-mL pipet tip up and down approximately 10 times reduces
the viscosity of the solution caused by DNA.
4. Extraction of proteoglycans from tissues: As used originally in the extraction of
proteoglycan from cartilage tissue (3), 4 M guanidine generally provides excellent solu-
bilization of proteoglycans.
5. When tissues are to be extracted, ordinarily approximately 10 times volume of 4 M guanidine
HCl buffer successfully solubilizes proteoglycans from finely minced tissues in 12 h at 4°C.
6. Additional consideration should be made when extraction of cell-associated proteoglycans
is attempted; i.e., inclusion of sufficient amounts of detergent may be necessary (such as 2%
Triton X-100) for the solubilization of proteoglycans (5).
7. Secreted proteoglycans in cell culture media are generally already soluble, but, in order to
minimize interactions between highly charged proteoglycans and other molecules, direct
addition of solid guanidine HCl (0.53 g of solid guanidine HCl per milliliter of media makes
4 M guanidine HCl solution) is frequently used.
3.2. Solvent Exchange
1. In order to prepare the extracted proteoglycans in 4 M guanidine HCl for the anion-
exchange chromatography procedure in the next step, guanidine HCl has to be replaced
with a solvent compatible with the procedure.
2. A preferred solvent is a urea buffer, since it disrupts molecular interactions by interfering
with the formation of hydrogen bonds.
3. A convenient buffer-exchange procedure can be done by gel filtration (such as Sephadex
G-50 chromatography) using a small disposable pipet. This process is also very conve-
6 Yanagishita
nient to remove unincorporated radioactive precursors, when radiolabeled cell cultures
were extracted with a 4 M guanidine HCl solvent.
4. Preparation of Sephadex G-50 column: Pour preswollen Sephadex G-50 (50% slurry) into
a 10-mL plastic disposable column (which has been cut at the top with a file and plugged
with glass wool at the bottom) to make 8 mL bed volume.
5. Remove excess water and equilibrate the column with 8 M urea buffer (a total of 9 mL is
sufficient to equilibrate the column).
6. Carefully prepare a flat gel surface with a glass Pasteur pipet and remove excess urea buffer.
Apply a 2-mL sample and discard the eluent.
7. After the entire sample is in the column, carefully overlay 3 mL of buffer and collect
eluent until the entire buffer is in the column (3-mL fraction collected). This fraction
contains proteoglycans and other macromolecules in 8 M urea buffer, while leaving
small molecules in the original sample (guanidine HCl, isotope precursors, etc.) behind
in the column.
8. At this point, the column can be safely disposed of as a radioactive waste.
3.3. Anion-Exchange Chromatography
3.3.1. Preparation of Q-Sepharose Column (
see
Note 2)
and Sample Application
1. 2 mL of preequilibrated Q-Sepharose (1 mL of Q-Sepharose can bind up to 3–5 mg of
proteoglycans) is packed into a small column (10-mL plastic pipet cut by a file and plugged
with glass wool at the bottom).
2. Alternatively, 2 mL of Q-Sepharose is mixed with the sample in 8 M urea buffer and
gently shaken for 1 h, then packed into the column; this latter method gives uniform
binding of proteoglycans to Q-Sepharose, resulting in a better flow property, especially
when a large quantity of materials is used.
3. After sample application, the column is washed with 10 mL of the low-salt buffer.
4. Then the column is connected to a gradient former and eluted with a total of approximately
40 mL of buffer with a flow rate of 10–15 mL/h.
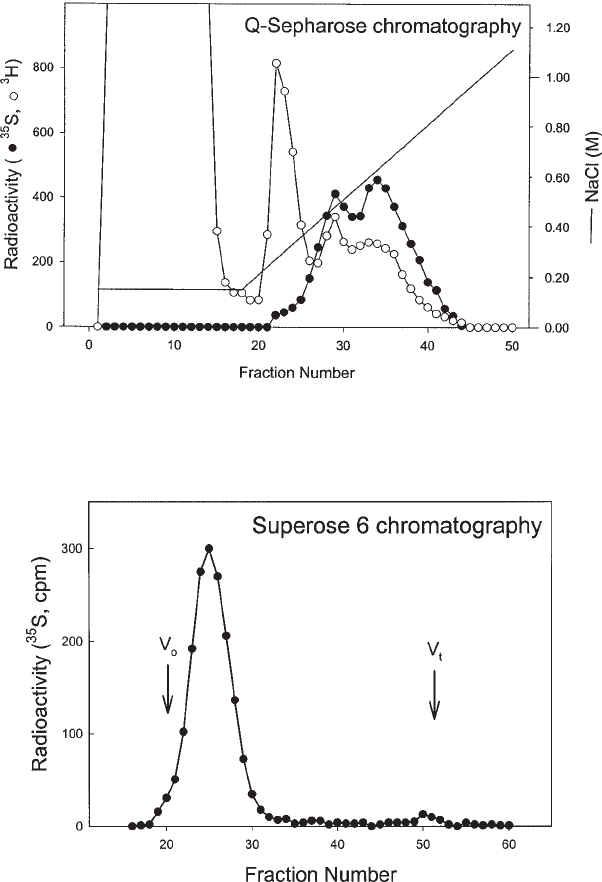
5. Every 1- to 2-mL fraction is collected and monitored for NaCl concentration by conductivity
measurement (see Fig. 2). Eluent fractions are monitored for proteoglycans (by radioac-
tivity detection or colorimetric procedures; a convenient and sensitive colorimetric pro-
cedure using Safranin O is described in ref. 6).
6. Typically, heparan sulfate proteoglycans are eluted in a peak at approximately 0.5 M
NaCl and chondroitin sulfate proteoglycans at 0.65 M NaCl.
3.4. Gel Filtration Chromatography
1. Proteoglycans with differing molecular weights purified by Q-Sepharose chromatogra-
phy are further separated by gel filtration chromatography. Superose 6 (or equivalent gel)
is suitable for resolving small proteoglycans (“small, leucine-rich proteoglycans”, cell
surface heparan sulfate proteoglycans, etc.) from large proteoglycans (e.g., aggrecan,
versican, and perlecan) and partially degraded proteoglycans.
2. Superose 6 is equilibrated in 4 M guanidine HCl buffer containing 0.5% Triton X-100
(see note 2), and up to 0.5 mL of sample can be loaded to the column.
3. The column is eluted at a flow rate of 0.4 mL/min and each 1-min fraction is collected
(see Fig. 3).
4. Eluent fractions are analyzed for proteoglycan content (by radioactivity detection or colo-
rimetric procedures).
Isolation from Cell Cultures and Tissues 7
4. Notes
1. Extraction of cell-associated proteoglycans from cultured cell requires the use of deter-
gent (5). Usefulness of 4 M guanidine HCl in the presence of 2% Triton X-100 has been
rigorously demonstrated.
2. One of the major technical problems associated with anion-exchange chromatography
of proteoglycans, especially when purifying molecules present in small quantities (e.g.,
Fig. 2. Q-Sepharose anion-exchange chromatography. A sample from a cell culture that was
metabolically radiolabeled with [
3
H]leucine and [
35
S]sulfate was analyzed. A large peak contain-
ing
3
H-labeled proteins was efficiently separated from
35
S-labeled proteoglycan peaks eluting
later in high-salt fractions.
Fig. 3. Superose 6 chromatography of
35
S-labeled cell surface heparan sulfate proteoglycan.
8 Yanagishita
isolation of proteoglycans from cell cultures), is poor recovery of materials. This
can be, in most cases, overcome by the use of detergents (either nonionic or zwitte-
rionic). Routinely, the use 0.5% (w/v) Triton X-100 (or NP-40) dramatically
improves recovery of proteoglycans (even glycosaminoglycans) from ion-exchange
columns. Most nonionic detergents (such as Triton X-100, NP-40) possess strong
absorbance in the ultraviolet (UV) range, thus making UV tracing for protein detec-
tion difficult. If this causes problems in analyses, non-UV-absorbing, nonionic
detergents such as Genapol X-100
TM
(Calbiochem) can be used. Also, when the
removal of detergents is required in later experimental steps, the use of those with
high critical micellar concentrations (such as CHAPS
TM
, Calbiochem) in place of
Triton X-100 is beneficial.
References
1. Hascall, V. C., Calabro, A., Midura, R. J., and Yanagishita, M. (1994) Isolation and
characterization of proteoglycans. Meth. Enzymol. 230, 390–417.
2. Yanagishita, M., Midura, R. J., and Hascall, V. C. (1987) Proteoglycans: Isolation and
purification from tissue cultures. Meth. Enzymol. 138, 279–289.
3. Hascall, V. C. and Kimura, J. H. (1982) Proteoglycans: Isolation and characterization.
Meth. Enzymol. 82, 769–800.
4. Oike, Y., Kimata, K., Shinomura, T., Nakazawa, K. and Suzuki, S. (1980) Structural analy-
sis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases
(chondroitinases) and endo-beta-D-galactosidase (keratanase). Biochem. J. 191, 193–207.
5. Yanagishita, M. and Hascall, V. C. (1984) Proteoglycans synthesized by rat ovarian
granulose cells in culture; isolation, fractionation, and characterization of proteoglycans
associated with the cell layer. J. Biol. Chem. 259, 10,260–10,269.
6. Lammi, M. and Tammi, M. (1988) Densitometric assay of nanogram quantities of
proteoglycans precipitated on nitrocellulose membrane with Safranin O. Anal. Biochem.
168, 352–357.

Isolation of Proteoglycans from Tendon 9
9
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
2
Isolation of Proteoglycans from Tendon
Kathryn G. Vogel and Julie A. Peters
1. Introduction
Proteoglycans make up less than 1% of the dry weight of a dense connective tissue
such as tendon (1). Most of this proteoglycan is a small molecule called decorin.
Because decorin has only one glycosaminoglycan chain, it cannot be separated from
other proteins by the CsCl density-gradient centrifugation method that was originally
used to purify aggrecan from cartilage.
The basic approach described in this chapter is to extract proteoglycans from the
tissue with 4 M guanidine, a solution that will denature collagen and disrupt most kinds
of noncovalent molecular interactions. The cross-linked collagen of adult tendon remains
insoluble during this extraction, allowing the proteoglycans and other soluble proteins to
be separated from the bulk of the tissue by centrifugation. Proteoglycans are then sepa-
rated from other extracted proteins by ion-exchange chromatography, taking advantage
of the anionic nature of the glycosaminoglycan chain.
This method has been used to quantitate and isolate proteoglycans from the tensile
(proximal) and compressed (distal) regions of bovine deep flexor tendon. These
mechanically distinct regions of flexor tendon are characterized by differences in
proteoglycan amount and type (2). The method is equally applicable to isolation of
proteoglycans from human tendon or from other dense connective tissues (3). Once
isolated, the large proteoglycans can be separated from smaller ones by sieve chroma-
tography. These isolated proteoglycans and their unique core proteins and glycosami-
noglycan chains are of sufficient purity to then be examined by specific analytical
techniques or used in functional assays.
2. Materials
2.1. Quantitation of Glycosaminoglycans
1. Single-edge razor blades.
2. Heated water bath.
10 Vogel and Peters
3. Microcentrifuge.
4. Lyophilizer.
5. Papain digestion solution: 0.05 M sodium acetate, 5 mM L-cysteine HCl, 10 mM EDTA,
pH 5.5. Add 50 µg/mL papain just before starting digestion.
6. DEAE cellulose (Whatman, DE52).
7. Disposable columns (Bio-Rad Poly Prep, 0.8 × 4 cm).
8. Column elution solutions: 0.02 M HCl, 1 M HCl.
9. Uronic acid detection reagent (orcinol reagent): 2 g of orcinol (5-methylresorcinol;
3,5-dihydroxytoluene monohydrate) in 50 mL of 1.5% FeCl
3
, 850 mL of conc. HCl, and
150 mL of water (4). Orcinol is sensitive to light and is irritating to eyes, the respiratory
system, and skin. Dissolve orcinol in FeCl
3
solution, then add HCl and water. Use caution
and work in a fume hood when adding water to concentrated HCl.
10. Uronic acid standards: stock solution of 100 µg/mL of glucuronolactone diluted to stan-
dards of 5 µg/mL, 10 µg/mL, 20 µg/mL, and 30 µg/mL.
11. Pyrex test tubes.
12. Heat block.
13. Spectrophotometer.
2.2. Isolation of Proteoglycans
1. Single-edge razor blades.
2. Tissue-grinding device (we have used a food mill made by Arthur Thomas, Incorporated,
Philadelphia, PA).
3. Lyophilizer.
4. 16-mL centrifuge tubes with sealing screw cap (Nalge Nunc).
5. 4 M guanidine buffer: 4 M guanidine, 0.05 M sodium acetate, pH 6.0. Add protease inhibitors
just before starting extraction: 5 mM benzamindine, 5 mM phenylmethylsulfonyl fluoride,
and 1 mM N-ethylmaleimide.
6. Nutator (Clay-Adams) or other rocking device.
7. Centrifuge (Beckman J2, JA-17 rotor).
8. 7 M urea buffer: 7 M urea, 0.05 M sodium acetate, pH 6.0.
9. 7 M urea buffer plus protease inhibitors: shortly before use add 5 mM benzamindine, 5 mM
phenylmethylsulfonyl fluoride, 1 mM N-ethylmaleimide.
10. Dialysis tubing (MWCO: 12,000–14,000).
11. DEAE cellulose (Whatman, DE52).
12. Disposable columns, Bio-Rad Poly-Prep, 0.8 × 4 cm.
13. Column elution solutions: 7 M urea buffer + 0.1 M NaCl; 7 M urea buffer + 0.2 M NaCl;
7 M urea buffer + 0.8 M NaCl.
2.3. SDS Polyacrylamide Gel Electrophoresis of Proteoglycans
1. 2-mL screw-capped centrifuge tubes.
2. 95% ethanol.
3. Microcentrifuge.
4. Enzyme digestion buffer: 0.1 M Tris base, brought to pH 8.0 with acetic acid.
5. Chondroitinase ABC (Seikagaku, 5 U/vial) resuspended in 0.5 mL of 0.1 M Tris-HCl,
pH 8.0 mL (10 U/mL).
6. Gel sample buffer: for 100-mL, combine 50 mL stacking gel buffer, 5 g sodium dodecyl
sulfate, 20 g glycerol, 4 mg bromophenol blue, and water to volume.
7. Electrophoresis apparatus and power source.
Isolation of Proteoglycans from Tendon 11
8. Polyacrylamide: 30% acrylamide, 0.8% bis-acrylamide.
9. Separating gel buffer: 0.75 M Tris, 4 mM EDTA, pH 8.8.
10. Stacking gel buffer: 0.25 M Tris, 4 mM EDTA, pH 6.8.
11. 0.5% ammonium persulfate.
12. TEMED.
13. Electrode buffer stock: 0.1 M Tris, 0.768 M glycine, 8 mM EDTA, pH 8.7. After adjust-
ing pH (if necessary), add 0.4% SDS. The upper buffer is diluted 1/4; the lower buffer is
diluted 1/8.
14. Gel fixative: 50% methanol, 7% acetic acid, 43% dH
2
O.
15. Coomassie blue staining solution: 0.1% Coomassie Brilliant Blue R-250, 40% methanol,
10% acetic acid, 50% dH
2
O.
16. Gel destain: 25% methanol, 7% acetic acid, 68% dH
2
O.
17. Alcian blue staining solution: 0.5% Alcian blue (8GX, Sigma Chemical Co), 7% acetic
acid, 93% dH
2
O.
18. Alcian blue destain solution: 7% acetic acid, 93% dH
2
O.
3. Methods
3.1. Quantitation of Glycosaminoglycans
1. Dissect tendon tissue free of muscle and fat, cut into chunks about 3 mm on a side, weigh,
lyophilize, weigh again (see Note 1 and bovine tendon water content, Table 1).
2. Add papain digestion solution to tissue at 50 mg dry tissue/1 mL enzyme in 2-mL screw cap
tubes. Incubate at 65°C until tissue pieces have disappeared (6–18 h, see Note 2).
3. Centrifuge and remove the supernatant. Dilute supernatant with equal volume of dH
2
O to
assure low salt concentration.
4. Pour columns containing 0.3 mL DE-52 in water (see Note 3).
5. Apply 1 mL of diluted sample to DEAE cellulose in column; collect the flow-through
(see Note 4).
6. Rinse column with 20 mL of 0.02 M HCl to remove loosely adherent molecules. Elute the
glycosaminoglycans into a clean tube with 2 mL of 1 M HCl.
Table 1
Water Content of Adult Flexor Tendons
Tissue 1 2 3
Tens. 65.6 61.5 62.7 _
65.2 62.1 63.4 X = 63.7 + 1.7 %
66.6 62.1 63.7
Comp. 70.2 70.6 73.1 _
70.8 67.3 71.1 X = 71.0 + 1.7 %
70.9 70.9 73.7
Samples of fresh tendon from animals between 1 and 2 years of age were cut into small pieces with
a razor blade, weighed, lyophilized, and weighed again to determine the percent of tendon wet weight
that was water. Triplicate samples of about 130 mg each were assessed. Tens., tissue from the tensile/
proximal region of tendon; Comp., tissue from the compressed/distal region of tendon. Samples are
from the front (1) and rear (2) leg of one animal and the rear (3) leg of another. Note that the water
content is lower in the tensile tendon.
