Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

Proteoglycans and Glycosoaminoglycans from Drosophila 45
4. Ultrafree–MC DEAE microcentrifugal filtration units with DEAE-derivatized membranes
(Millipore).
5. 0.3 M sodium phosphate buffer (pH 6.0).
6. Loading buffer: 50 mM sodium phosphate buffer (pH 6.0) containing 0.15 M NaCl.
7. Elution buffer: loading buffer containing 1.0 M NaCl.
8. Heparin lyase mixture: 200 mIU/mL each of heparin lyase I (heparinase, E.C. 4.2.2.7),
heparin lyase II (heparitinase II), heparin lyase III (heparitinase I, E.C. 4.2.2.8) (all from
Seikagaku America).
9. Digestion buffer: 0.1 M sodium acetate buffer (pH 7.0) containing 10 mM calcium acetate.
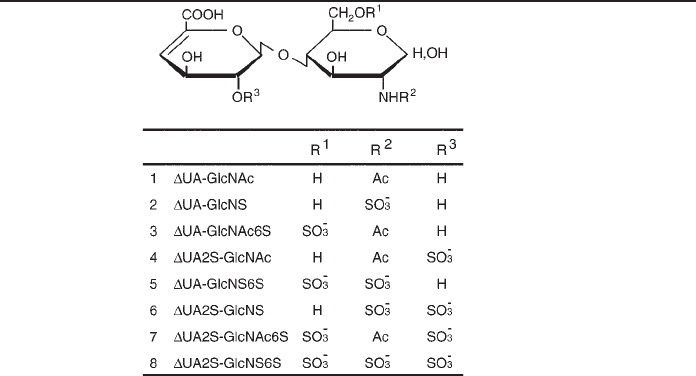
10. Unsaturated disaccharide standards from heparin/heparan sulfate (Seikagaku America;
see Table 2).
11. A Speed-Vac centrifugal evaporator (Savant).
2.6. HPLC Analysis
1. 1.2 mM tetra-n-butylammonium hydrogen sulfate in 8.5 % acetonitrile.
2. 0.2 M NaCl in 1.2 mM tetra-n-butylammonium hydrogen sulfate and 8.5% acetonitrile.
3. 0.5% 2-cyanoacetamide.
4. 1.0% NaOH.
5. HPLC system shown in Fig, 2. For a detailed description of equiptment used by the
authors, see Note 7.
3. Methods
3.1. Purification of Proteoglycans from Drosophila
3.1.1. Carry out all steps on ice or at 4
°
C.
1. Homogenize 40 third-instar larvae thoroughly in 3 mL of lysis buffer on ice, using 25–30
strokes with a Dounce homogenizer with the B pestle. One can also use a motorized pestle
Table 2
Disaccharide Standards from Heparin/Heparan Sulfate
46 W. D. Staatz et al.
in a glass homogenizer. Pieces of adult fly cuticle and larval mouth parts will remain as
black fragments in the homogenate, but the soft tissues should be completely dissociated.
(see Note 1).
2. Centrifuge the lysate to clarify and remove excess lipid. To remove the major debris,
centrifuge the lysate at 12,000g for 5 min at 4°C. Then recentrifuged supernatant at
30,000g for 30 min so that any lipid is removed from the surface of the clarified superna-
tant. (see Note 8).
3. Place 1 mL of DEAE Sephaeose suspension in a 15-mL conical centrifge tube and let the
resin settle. Remove the overlying buffer and resuspend the resin in 10 mL of high-salt
buffer. Equilibrate the resin with occasional mixing for 30 min, then let the resin settle and
remove the buffer. Wash the resin twice with 10 mL column buffer by resuspension and
settling.
4. The clarified supernatant is mixed with 0.3 mL of DEAE Sepharose (Amersham Pharmacia)
and rocked at 4°C for 1 h (see Note 8).
5. The Sepharose resin is then allowed to settle and the overlying liquid is replaced with 1 mL
of column buffer. Save the removed liquid (and all further fractions) on ice for analysis at
the end of the column run.
6. The resin suspension is rocked for 5 min at 4°C, then allowed to settle and the overlying
buffer is removed and saved as above. The resin is then resuspended in 1 mL of column
buffer and packed into a 0.5 × 3 cm column. If the resin still contains dark flakes from the
tanning reaction, repeat the washing step until the flakes are no longer visible to the naked
eye. Then pack the column.
7. The column is washed successively with the following buffers. Collect fractions equivalent
to 1 column volume:
a. 3 column volumes of column buffer.
b. 3 column volumes of low-salt buffer.
c. 3 column volumes of urea wash buffer (see Note 2).
d. 3 column volumes of pH 3.5 wash buffer. At this pH the carboxy side chains of Asp and
Glu residues are protonated, thus releasing them from the column. Sulfates on GAG
chains, however, remain negatively charged and bound to the column.
e. 4–5 column volumes of pH 8.0 wash buffer to return the pH of the column to 8.0. Check
the pH of the 10 µL effluent with 1 µL of 0.1% phenol red. If it is still yellow, continue
washing until the effluent is red-orange.
8. To elute the sulfated PGs from the column, add 1 column volume of high-salt buffer and let
it drain to the surface of the resin. Close the column, add 1 column volume of buffer, and let
it equilibrate for 20–30 min. Then reopen the column and collect the eluate. Close the
column, and repeat the elution step with 1 column volume of high-salt buffer. Then wash
with the remaining high-salt buffer without closing the column (see Note 9).
9. Store fractions on ice, and assay 10 µL of each fraction for protein content using the BCA
or other suitable protein assay.
10. Samples can then be dialyzed, precipitated, and assayed by PAGE or other means. (see Fig. 2).
3.1.2. Alternative Stepped NaCl Gradient Elution
1. Follow the standard procedure through step 7b.
2. Wash the column successively with 2-mL aliquots of column buffer containing 0.25, 0.5,
0.75, 1.0, and 1.5 M NaCl.
3. Elute the most tightly bound material from the column with 3 mL of buffer with
2.0 M NaCl.
Proteoglycans and Glycosoaminoglycans from Drosophila 47
4. Dialyze and analyze fractions as for the standard protocol.
3.2. DEAE Isolation of Sulfated Proteoglycans
from Drosophila S-2 cells
3.2.1. Transfection of Drosophila S-2 Cells
1. Add 2 µg each of expression vector and selection plasmid DNA to be transfected and 10
µg CellFECTIN reagent to 0.5 mL of serum-free medium, vortex briefly, and incubate at
room temperature for 20–40 min. (see Note 6).
2. Resuspend 2 × 10
6
S-2 cells in mid-log phase in 0.5 mL of serum-free medium. (see Note 5).
3. Gently mix the DNA/CellFECTIN solution with the cell suspension and plate in a 35 mm-
diameter culture dish or in a well of a 6-well culture plate and incubate at 23°C for 4–6 h.
4. Add 1 mL of fresh growth medium to the cells and return to the incubator overnight.
5. In the morning, add 1 mL of fresh growth medium to the cells and return to the incubator for
7–8 h.
6. Gently resuspend the cell pellets by centrifugation at 300g for 5 min. Then wash the cells
once by resuspending and repelleting in serum-free medium to remove excess CellFECTIN
reagent and DNA.
7. Replate the cells in 3 mL of fresh growth medium. If stable cell lines are to be created, add
the selection medium at this time. Use 2 × 10
-7
M methotrexate for cells co-transfected
with the selection plasmid pH8 C0.
8. Transient transfectants can be grown for 12–48 h before use. Stable transfectants are grown
for 1 wk. Aliquots are then frozen in liquid nitrogen.
3.2.2. Harvesting Cells and Fractionation PGs
1. Heat-shock 3 × 10
9
log-phase S-2 cells transfected with the desired gene cloned into F449 or
another suitable vector (see Note 6): 30 min at 37°C, 5 min on ice, 2–4 h recovery at 23°C.
2. Resuspend nonattached cells and put in a centrifuge tube on ice.
3. Remove attached cells by scraping in 2 × 5 mL of ice-cold TBS, pH 7.4, containing
protease inhibitors, and add to centrifuge tube.
Carry out the following work at 4°C.
4. Spin cells for 5 min at 3000g, remove the supernatant, and wash the cells twice with 10
mL of TBS.
5. Homogenize the cells in 10 mL of homogenization buffer, on ice, 3 min, with a motorized
Teflon pestle in a glass homogenizer.
6. Centrifuge homogenate at 12,000g.
7. Place 6 mL of DEAE Sephaeose suspension in a 50-mL conical centrifge tube and let the resin
settle. Remove the overlying buffer and resuspend the resin in 40 mL of high-salt buffer.
Equilibrate the resin with occasional mixing for 30 min, then let the resin settle and remove the
buffer. Wash the resin twice with 40 mL of column buffer by resuspension and settling.
8. Apply supernatant to a 2-mL DEAE Sepharose column and elute with the protocol
described for whole animal lysates (see Subheading 3.1.).
9. Dialyze aliquots of each fraction and analyze by PAGE/Western blotting for the presence
of the desired core proteins.
10. Store the remaining fractions at –20°C.
3.3. Extraction of Glycosaminoglycans from Drosophila
1. To extract GAGs from Drosophila, lyophilize 100 adult flies, 100 larvae or 250 µL of
dechorionated embryos, or wash with distilled water and then lyophilize.
48 W. D. Staatz et al.
48

Proteoglycans and Glycosoaminoglycans from Drosophila 49
2. Homogenize samples (up to 20 mg of lyophilized samples) in 1.0 mL of acetone cooled
on ice. Extract for 30 min on ice, then centrifuge 15 min at 2500g at 4°C. Wash the
precipitate 3 times with 1.0 mL of ice-cold acetone and dry under vacuum.
3. Extract samples in 1.0 mL of GAG extraction solution for 16 h at room temperature with
constant stirring.
4. Neutralize samples with 200 µL of 1.0 M sodium acetate and 300 µL of 1.0 M HCl and
centrifuge 10 min at 2500g, 4°C. Remove particulate matter from the supernatant with a
300-µm-pore disposable filter column.
5. Add 200 µL of 1.0 M HCl to the filtrate and remove insoluble materials by centrifugation
for 10 min at 2500g, 4°C, then add 7 mL of ethanol to the supernatant and allow the
GAGs to precipitate for 2 h at 0°C.
6. Centrifuge 10 min at 2500g at 4°C and remove supernatant. Wash the precipitates once
with 1 mL of 80% ethanol and once with 1 mL of ethanol. These washes should be done
at 4° C. Dry the precipitate under vacuum. At this stage the samples can be stored at –20°C
until needed.
7. For use in determining the chondroitin sulfate and heparan sulfate disaccharide ratios
(following protocols), dissolve the crude GAG pellets in 250 µL of distilled water.
3.4. Microdetermination of Chondroitin Sulfate in Drosophila
1. Dilute 20 µL of crude GAG solution to 100 µL with distilled water.
2. Add 10 µL of digestion buffer, 5 µL of chondroitinase ABC solution, and 5 µL of
chondroitinase ACII solution to 20 µL of the diluted crude GAG solution in a 500-µL
polypropylene microcentrifuge tube, then incubate at 37°C for 3 h.
3. Spin down the tube in an Eppendorff centrifuge for 30 s, then heat the tube for 2 min at
100°C and spin down again.
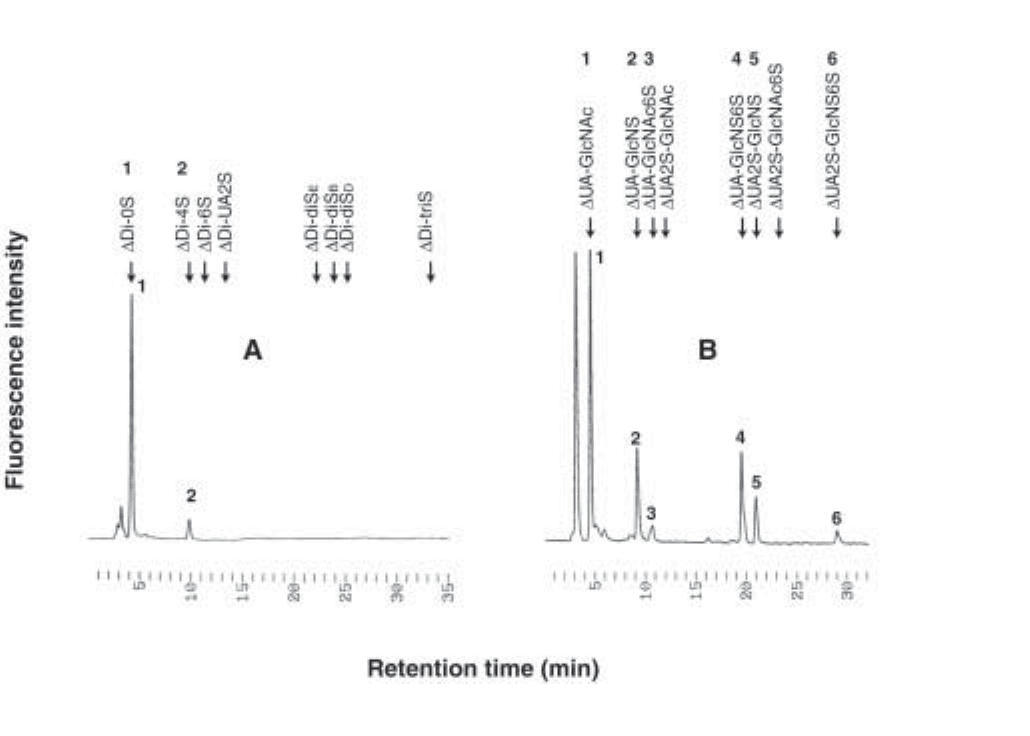
4. Submit 8 µL of the digest to HPLC analysis using the method under Subheading 3.6. Use
2 ppm of each unsaturated disaccharide mixture as standard. A typical chromatogram is
shown in Fig. 3A.
3.5. Microdetermination of Heparan Sulfate in Drosophila
1. Add 400 µL of elution buffer to the Ultrafree–MC DEAE insert and spin at 5000g for
1 min.
2. Empty the microcentrifuge tube. Then add 400 µL of loading buffer to the insert and spin
at 5000g for 1 min. Transfer the insert to a new microcentrifuge tube.
3. Add 50 µL of 0.3 M sodium phosphate buffer (pH 6.0) to 230 µL of crude GAG solution.
4. Filter the mixture through a Ultrafree MC (Durapore®, 0.45 µm).
5. Add the filtrate to the Ultrafree–MC DEAE insert and spin at 5000g for 1 min. Pass the
sample over the membrane twice.
6. Transfer the insert to a new microcentrifuge tube. Add 400 µL of loading buffer to the
insert and spin at 5000g for 1 min.
7. Transfer the insert to a new microcentrifuge tube. Add 100 µL of elution buffer to the insert
and spin at 5000g for 1 min. Repeat 3 times. This fraction is the eluate.
Fig. 3. HPLC profiles of CS and HS disaccharides. Typical chromatograms of unsaturated
disaccharides from chondroitin sulfate (A) and heparan sulfate (B) from adult Drosophila. The
positions at which disaccharide standards migrate are indicated by the arrows. The numbers above
the standards correspond to the numbers of their respective elution peaks. It is interesting to
note that the only sulfated chondroitin disaccharide detected in Drosophila is ∆Di-4S.
50 W. D. Staatz et al.
8. Add the eluate to a Ultrafree MC (Biomax–5) and spin until the retentate is 30 µL.
9. Add 50 µL of distilled water to the retentate and spin. Repeat four times.
10. Remove the retentate to a new tube with a pipette, and rinse the membrane four times with
20 µL of distilled water. Add the washes to the retentate.
11. Dry the sample in a centrifugal evaporator, then dissolve it in 12 µL of distilled water.
12. Add 5 µL of digestion buffer and 5 µL of heparin lyase mixture to 5 µL of the partial purified
heparan sulfate from step 11 in a 500-µL polypropylene microcentrifuge tube, then incu-
bate at 37°C for 16 h.
13. Centrifuge for 30 s at 12,000g, then heat the tube for 2 min at 100°C and recentrifuge.
14. Dry the digest in a centrifugal evaporator.
15. Add 10 µL of water, then inject 8 µL of the sample into the HPLC for analysis using the
method under Subheading 3.6. Use 5 ppm of each unsaturated disaccharide mixture as
standard. A typical chromatogram is shown in Fig. 3B.
3.6. HPLC Analysis
A flow diagram of the HPLC system is shown in Fig. 2. For a detailed description
of the HPLC system used in this method, (see Note 9).
1. Before injecting the sample, heat the column to 55°C and equilibrate with 1.2 mM tetra–
n–butylammonium hydrogen sulfate and 2 mM NaCl in 8.5% acetonitrile. Warm the
postcolumn reaction coil to 125°C.
2. Inject the disaccharides and elute at a flow rate of 1.1 mL/min with an NaCl gradient
consisting of the following segments:
a. 0–10 min : 1–4% 0.2 M sodium chloride.
b. 10–11 min: 4–15% 0.2 M sodium chloride.
c. 11–20 min: 15–25% 0.2 M sodium chloride.
d. 20–22 min: 25–53% 0.2 M sodium chloride.
e. 22–29 min: 53% 0.2 M sodium chloride.
All gradient components contain 1.2 mM tetra–n–butylammonium hydrogen sulfate and 8.5%
acetonitrile. The column is then reequilibrated with 1% 0.2 M sodium chloride for 20 min.
3. To detect the eluted disaccharides, aqueous 0.5% (w/v) 2–cyanoacetamide solution and 1%
(w/v) sodium hydroxide are added to the column effluent at the flow rate of 0.35 mL/min.
The mixture is then passed through the reaction coil at 125°C, and following cooling, the
effluent is monitored fluorometrically at 410 nm with an excitation wavelength of 346 nm.
4. Notes
1. Drosophila larvae and pupae can have high levels of protease activity. It is therefore
imperative that all work be done in the cold and in the presence of a complete cocktail of
protease inhibitors. A mixture of protease inhibitors that inhibit serine-, cysteine-, and
metalloproteases must be included in the lysis buffer and the first 2–3 column wash buff-
ers. The remaining buffers should contain at least 2 mM PMSF and 10 mM EDTA. The
inclusion of these inhibitors will help prevent the degradation of the core proteins of the
proteoglycans being isolated. Several manufacturers supply suitable protease mixtures in
liquid or tablet form.
2. To help solubilize proteoglycan, 6 M urea may be added to the homogenization buffer. In
that case it should be included in all buffers except the pH 8.0 wash buffer and the high
salt buffer. Alternately, 6 M urea may be used as a separate wash step (urea buffer) to help
dissociate protein aggregates from the column. Urea should always be included in the pH
3.5 buffer. It should be noted that urea in solution readily oxidizes to uric acid. Thus, all
Proteoglycans and Glycosoaminoglycans from Drosophila 51
urea-containing solutions should be prepared from the highest-quality urea available and
just prior to use. Including urea in the homogenization buffer may increase the yield of
proteoglycans, but it denatures the core protein and must be removed if the samples are to
be subjected to digestion by GAG lyases.
3. DEAE Sepharose Fast Flow has a maximum binding capacity of 0.11–0.16 mmol/mL
resin, which is equivalent to 3–4 mg of a 90-kDa protein or proteoglycan. Thus, a 0.3 mL
column has a theoretical capacity for PGs from several hundred third-instar larvae.
4. Calcium phosphate has also been used extensively to transfect S-2 cells, and electroporation
has been used with Drosophila KC167 cells. These methods are well reviewed by Cherbas
et al. (6).
5. Drosophila cells can be maintained in a number of media. Most commonly used for S-2
cells are M3 (7), D-22 (8), and Schneider’s medium (9), all of which are commercially
available (Sigma, Life Technologies) and must be supplemented with 5–12.5% fetal bovine
serum. Purchase fetal bovine serum that has been tested for use with tissue culture cells,
because not all lots of serum support insect cell growth. Hyclone’s HQ-CCM3 is a serum-
free medium that works well with S-2 cells and, lacking the need for serum supplementa-
tion, is less expensive than the other media. Cherbas et al. (6) report that HQ-CCM3 is also
preferable to M3 for methotrexate and G418 selection.
6. The most commonly used expression vectors used in Drosophila cells put the gene of
interest under the control of promoters for HSP 70, metallothionein, or ecdysone (reviewed
by Cherbas et al. [6]). Since S-2 cells take up several hundred to several thousand copies
of the transfected DNA, it is not necessary to have the resistance gene used to generate
stable cell lines on the same plasmid as the gene of interest. In all but a few excep-
tional cases, co-transfecting a selection plasmid which carries a selectable resistance
gene with the gene of interest in a separate expression vector provides stable cell lines
expressing the desired gene product. Methods using selection plasmids carrying resis-
tance to methotrexate, G418, and hygromycin can be found in Ashburner (10).
7. The chromatographic equipment used by the authors includes an L-7000 gradient pump and
D-7500 chromato–integrator (Hitachi Instruments) and a model 7125 sample injector with
a 20-µL loop (Rheodyne). Samples were separated on a 4.6 mm × 150 mm Senshu Pak
DOCOSIL A column (Senshu Scientific, Tokyo, Japan).The postcolumn reaction system
consists of a double-plunger pump, AA–100–S (Eldex Laboratories), a CH–30 column
heater, a FH–40 dry reaction bath, and a TC–55 thermocontroller (Brinkmann Instruments).
Samples are detected with a RF–10AXL fluorescence spectrophotometer (SHIMADZU
SCIENTIFIC).
8. The wound-healing response of Drosophila and other insects involves a rapid tanning
reaction that converts components in the hemolymph into dark, cuticle-like material.
When Drosophila are homogenized, this reaction can produce a fine suspension of par-
ticles that are not always removed by centrifugation at modest speeds. These particles can
plug columns and disrupt biochemical fractionation. Two rounds of centrifugation, fol-
lowed by loading and washing the ion-exchange resin in batches, allows the particles to
be washed away before the resin is packed into a column.
9. By washing 1 column volume of elution buffer through the column, then closing the column
and letting the resin equilibrate for 20–30 min with 1 column volume of fresh buffer, most
of the bound ligand will be released into the second elution buffer fraction.
10. The most practical quantitative approach to the analysis of the unsaturated disaccharides
derived enzymatically from GAGs is detection of their ultarviolet absorption at 232 nm.
However, detection systems are not sensitive enough for the microdetermination of biologi-
52 W. D. Staatz et al.
cal samples. To improve the detection limits and specificity, pre- and postcolumn detec-
tion techniques have been investigated. The postcolumn method using 2-cyanoacetamide
(11) was specifically developed to detect optimally unsaturated disaccharides from small
amounts of Drosophila GAGs.
References
1. Herndon, M. E. and Lander, A. D. (1990). Diverse set of developmentally regulated
proteoglycans is expressed in the rat central nervous system. Neuron 4, 949–961.
2. Toyoda, H., Kinoshita-Toyoda, A., and Selleck, S. B. (2000). Structural analysis of
glycosoaminoglycans in Drosophila and C. elegans and demonstration that tout-velu, a
Drosophila gene related to EXT tumor suppressors, affects heparan sulfate in vivo. J.
Biol. Chem. 275, 2269–2275.
3. Struhl, G. (1985). Near-reciprocal phenotypes caused by inactivation or indiscriminate
expression of the Drosophila segmentation gene ftz. Nature 318, 677–80.
4. Rebay, I., Fleming, R. J., Fehon, R. G., Cherbas, L., Cherbas, P., and Artavanis-Tsakonas,
S. (1991). Specific EGF repeats of Notch mediate interactions with Delta and Serrate:
implications for Notch as a multifunctional receptor. Cell 67, 687–699.
5. Bunch, T. A. and Goldstein, L. S. (1989). The conditional inhibition of gene expression in
cultured Drosophila cells by antisense RNA. Nucleic Acids Res. 17(23), 9761–9782.
6. Cherbas, L., Moss, R., and Cherbas, P. (1994). Transformation techniques for Drosophila
cell lines. Meth. Cell Biol 44, 161–179.
7. Shields, G. and Sang, J. H. (1977). Improved medium for the culture of Drosophila
embryonic cells. Drosophila Information Service 52, 161.
8. Echalier, G. and Ohanessian, A. (1970). In vitro culture of Drosophila melanogaster
embryonic cells. In Vitro 6, 162–172.
9. Schneider, I. (1964). Differentiation of larval Drosophila eye-antennal discs in vitro. J.
Exp. Zool. 156, 91–103.
10. Ashburner, M. (1989). Drosophila: A Laboratory Manual. Cold Spring Harbor Press, Cold
Spring Harbor, NY.
11. Toyoda, H., Yamamoto, H., Ogino, N., Tioda, T.,and Imanari, T. (1999). Rapid and sensi-
tive analysis of disaccharide composition in heparin and heparan sulfate by reversed-phase
ion pair chromatography on 2 µm porous silica gel column. J. Chromatogr. A 830, 197–201.

Cartilage and Smooth Muscle Cell Proteoglycans 53
53
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
7
Cartilage and Smooth Muscle Cell Proteoglycans
Detected by Affinity Blotting Using Biotinylated Hyaluronan
James Melrose
1. Introduction
Chondrocytes and smooth muscle cells synthesise the large CS-rich proteoglycans
aggrecan (1) and versican (2) respectively. Both proteoglycans are capable of interact-
ing with hyaluronan to form molecular aggregates that have important tissue specific
functional roles to play. Aggrecan is a major matrix component of cartilage, the
aggrecan aggregates are physically entrapped within the collagenous extracellular
matrix of this tissue, and it is the collective interplay between this collagenous net-
work and the aggrecan aggregates that equips this tissue with its unique viscoelastic
and hydrodynamic properties and the ability to provide an almost frictionless weight-
bearing surface to articulating joints (3). Smooth muscle cell versican, in comparison,
is a quantitatively minor component of blood vessels but nevertheless it may influ-
ence the physicochemical properties of the vessel wall (4). Although the exact func-
tional role of versican within blood vessels has yet to be fully elucidated, it is known
to accumulate in intimal lesions during atherosclerosis and is implicated in the
entrapment of low-density lipoprotein in the arterial wall during atherogenesis. Such
interactions are likely to influence both the viscoelasticity and permeability of the
vessel wall.
The interaction of aggrecan and versican with hyaluronan, which forms the basis of
the assembly of massive macromolecular proteoglycan arrays within connective tis-
sues, particularly in cartilage, is mediated via an amino-terminal globular domain that
extends from the core protein, the so-called G1 domain or hyaluronan-binding region
(HABR) (3,6). This interaction is further stabilized via a ternary complex formed with
a small glycoprotein link protein that displays an affinity both for hyaluronan and for
the G1 domain (7). A further amino-terminal globular domain in aggrecan, termed the
G2 domain, has also been identified. This domain is absent in versican, and despite
54 Melrose
considerable sequence homology with the G1 domain, it does not bind hyaluronan
and its exact functional role has yet to be defined. Since both the HABR and the link
protein display high affinities for hyaluronan, these hyaluronan-binding proteins
(HABPs) have been utilized as probes for the detection and quantitation of
hyaluronan in biological fluids by enzyme-linked immunosorbent assays (ELISAs)
(8–10), for the localization of hyaluronan in tissue sections by immunohistological
techniques (11–17) or for demonstration of hyaluronan species on Western blots
(18) (see also Chapter 46).
The recent development of biotinylated hyaluronan (19) and its application as an
affinity probe (20–22) now provides a means of monitoring not only electrophoreti-
cally resolved intact aggrecan and versican monomer, but also proteolytically
derived fragments of these proteoglycans containing functional G1 domains that are
likely to be generated in certain disease states. The technique employed, affinity
blotting, uses similar methodology to Western blotting but does not utilize specific
antibodies. Affinity blotting is so named not only to differentiate it from the related
Western technique but also to emphasise the functional nature of the interaction
between the G1 domain and a nonsubstituted stretch of the biotinylated hyaluronan
affinity probe that forms the basis of the technique. This chapter provides a simpli-
fied protocol for the preparation of G1-containing aggrecan fragments and also
demonstrates the utility of the affinity blotting technique for the visualization of
G1-containing proteoglycan species resolved by sodium dodecyl sulfate polyacry-
lamide gel electrophoresis (SDS PAGE) or composite agarose polyacrylamide gel
electrophoresis (CAPAGE).
2. Materials
2.1. Equipment and Chemicals
1. Electrophoresis system for SDS PAGE (Novex Xcell II mini electrophoresis system).
2. Blotting system for transfer of SDS PAGE gels (Novex Xcell II blot module).
3. Novex model 3540 programmable power supply.
4. Prepoured 4–20% polyacrylamide gradient Tris-glycine gels (Novex).
5. SilverXpress silver staining kit (Novex).
6. Electroelution cell (ISCO little blue tank)
(23).
7. Vertical electrophoresis system for CAPAGE (Hoefer SE 600).
8. Blotting system for semidry transfer of CAPAGE gels (Bio-Rad).
9. Platform rocker or orbital shaker.
10. Agarose gel-bond support film (FMC Bioproducts, Rockland, ME, USA).
11. Acrylamide: bis-acrylamide 40% w/v stabilized liquid concentrate (19/1, C = 5%)(Bio-Rad).
12. Agarose, low-electroendosmosis grade (Bio-Rad) .
13. Low-molecular-weight hyaluronan, 170 kDa (Fidia, Abano Terme, Italy) (see Note 1).
14. Avidin conjugated to alkaline phosphatase, 750 U/mg protein (Sigma).
15. EDC (1-ethyl-3-[3-dimethylamino)-propyl]-carbodi-imide) (Sigma).
16. NHS-LC-biotin (sulfosuccinimidyl-6-(biotinamido)-hexanoate) (Pierce).
17. Nitrocellulose (0.22 µm) (Schliecher and Schuell or Novex).
18. Chromaphor® green dye (Promega).
19. Prestained and protein molecular-weight standards for SDS PAGE (Novex).
