Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

34 Whitelock
3. Saku, T. and Furthmayr, H. (1989). Characterization of the major heparan sulfate
proteoglycan secreted by bovine aortic endothelial cells in culture. J. Biol. Chem. 264,
3514–3523.
4. Heremans, A., Cassiman, J.-J., Van Den Berghe, H., and David, G. (1988). Heparan sul-
fate proteoglycan from the extracellular matrix of human lung fibroblasts. J. Biol. Chem.
263, 4731–4739.
5. Weis, J. R., Sun, B., and Rodgers, G. M. (1991). Improved method of human umbilical
arterial endothelial cell culture. Thromb. Res. 61, 171–173.
6. Maciag, T., Cerundolo, J., Ilsley, S., Kelley, P. R., and Forand, R. (1979). An endothelial
cell growth factor from bovine hypothalamus: identification and partial characterization.
Proc. Natl. Acad. Sci. (USA) 76(11), 5674–5678.
7. Whitelock, J. M., Murdoch, A. D., Iozzo, R. V., and Underwood, P. A. (1996). The
degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast
growth factor by stromelysin, collagenase, plasmin, and heparanases. J. Biol. Chem.
271(17), 10,079–10,086.
8. Melrose, J. and Ghosh, P. (1993). Determination of the average molecular size of gly-
cosaminoglycans by fast protein liquid chromatography. J. Chromatogr. 637, 91–95.
9. McDevitt, C. A. and Muir, H. (1971). Gel electrophoresis of proteoglycans and gly-
cosaminoglycans on large pore composite polyacrylamide-agarose gels. Anal. Biochem.
44, 612–622.

Nervous Tissue Proteoglycans 35
35
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
5
Isolation and Characterization of Nervous Tissue
Proteoglycans
Yu Yamaguchi
1. Introduction
Nervous tissues contain a variety of proteoglycans (1–3). In tissues, proteoglycans
are present predominantly in extracellular matrices and on cell surfaces. Proteoglycans
in extracellular matrices are generally extracted in soluble fractions by physiological
buffers without detergents, whereas cell surface proteoglycans that are anchored to the
plasma membrane either by transmembrane domains or glycosylphosphatidylinositol
(GPI) linkages are fractionated into membrane fractions. Some proteoglycans in extra-
cellular matrices requires chaotropic agents such as urea and guanidine for solubiliza-
tion (4). It has been shown that a total of 20 µg of proteoglycans (as protein) can be
isolated from 1 g (wet weight) of embryonic rat brain tissues, in which 8 µg are from the
soluble fraction and 12 µg are from the membrane fraction (1). In the case of adult rat
brain, a total of 35 µg of proteoglycans are isolated, 20 µg in the soluble fraction and
15 µg in the membrane fraction. In general, the soluble fraction contains predominantly
chondroitin sulfate proteoglycans (CSPGs), whereas heparan sulfate proteoglycans
(HSPG) are enriched in the membrane fraction.
Herndon and Lander (1) reported that ~25 distinct proteoglycan core proteins can
be identified in embryonic and adult rat brain. Among these bands for putative
proteoglycan core proteins, 16 were identified as chondroitin sulfate proteoglycans
and 9 were heparan sulfate proteoglycans. Not all of these bands represent distinct
proteoglycan core proteins, since some represent proteolytic fragments. Thus far, more
than 20 proteoglycans that are molecularly defined have been shown to be expressed
in embryonic and adult brain. It should be noted that there is a great deal of difference
in the amount of these proteoglycans in the brain at the protein level, and not all of
these proteoglycans have been isolated from the brain in biochemical quantities.
Among CSPGs, brevican, versican, and phosphacan are abundant in adult brain,
whereas neurocan is abundant in embryonic brain (5,6): All these CSPGs have been
isolated in biochemical quantities from the brain. Less is known about the relative
36 Yamaguchi
abundance of individual HSPGs, but at least glypican (glypican-1) and cerebroglycan
(glypican-2) have been isolated for the purpose of N-terminal sequencing (7).
The procedure described here was originally reported by Herndon and Lander (1)
and modified by us for large scale purification (5,8,9). This procedure provides a
straightforward way to isolate fractions that are highly enriched for various
proteoglycans, by taking advantage of negative charges of glycosaminoglycan chains.
Purification of individual proteoglycans in native forms, however, requires methods
specific for each proteoglycan, such as affinity chromatography and immunoaffinity
chromatography. For instance, neurocan and phosphacan were purified using
immunoaffinity chromatography on monoclonal antibodies (10). Brevican can be iso-
lated by using affinity chromatography on a column of tenascin-R fragment (11), while
versican can be isolated by affinity chromatography on hyaluronan (6). Although it is
possible to purify individual proteoglycans by the combination of conventional chro-
matographic procedures, such approaches are usually not so easy. This is because
proteoglycans behave similarly in most biochemical separation methods due to the
large, negatively charged glycosaminoglycan chains shared by all these molecules.
Purification of individual core proteins is less challenging. For this purpose, after
digesting glycosaminoglycan chains with chondroitinase, heparitinase, or both, mix-
tures of core proteins can be fractionated by HPLC (9; see also Fig. 1, lane 3), prepara-
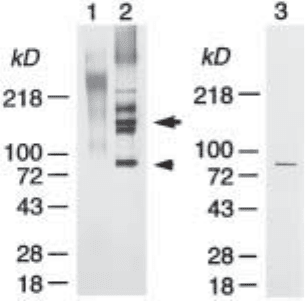
Fig. 1. SDS-PAGE analysis of the total proteoglycan fraction isolated from adult rat brain
(modified from ref. 9). The total proteoglycan fraction was isolated from soluble extracts of
adult rat brain by the methods described here. (Lanes 1, 2) The total proteoglycan fraction was
digested with (lane 2) or without (lane 1) protease-free chondroitinase ABC (Seikagaku
America) and analyzed on 8–16% gradient gel (Novex) under nonreducing condition. Proteins
were visualized by silver staining. Arrow and arrowhead indicate the 145-kDa (full-
length) and 80-kDa (N-terminally truncated) forms of brevican core protein, respectively.
Peptide sequencing also identified the ~180-kDa band above the 145-kDa brevican core pro-
tein is phosphacan and the ~125-kDa band just below the 145-kDa brevican core protein is
neurocan. (Lane 3) The 80-kDa brevican core protein was purified by reverse-phase HPLC on
a Vydac C4 column.
Nervous Tissue Proteoglycans 37
tive PAGE, or other protein separation methods. However, since some core proteins
have similar molecular weights (e.g., all glypican core proteins migrate around ~60
kDa), such core proteins are more difficult to purify.
2. Materials
1. Brain tissues. This procedure has been used for rat, mouse, bovine, and human brain tis-
sues. For large scale purification of proteoglycan-enriched fractions as described below, we
usually start with 100 g of brain tissue. For most applications, we use brain tissues frozen on
Dry Ice and stored at –80ºC. It is preferable to strip blood vessels and meninges from brains,
if contamination of proteoglycans derived from these tissues is to be minimized.
2. Sorvall GSA rotor or equivalent.
3. 250-mL centrifuge bottles for GSA rotor.
4. Sorvall T865 ultracentrifuge rotor or equivalent.
5. 30 mL ultracentrifuge tubes (e.g., Sorvall Ultrabottles, polycarbonate).
6. Polytron homogenizer.
7. Gradient former.
8. DEAE-Sepharose Fast Flow.
9. Buffers: Buffer A: 4 mM Hepes, pH 7.5, 0.3 M sucrose, 0.15 M NaCl, and protease
inhibitors*(see item 17 below).
10. Buffer B: 50 mM Tris-HCl, pH 8.0, 0.15 M NaCl, 1% CHAPS, and protease inhibitors
(see Note 1).
11. Buffer C: 50 mM Tris-HCl, pH 8.0, 0.15 M NaCl, and 0.5% CHAPS.
12. Buffer D: 50 mM Tris-HCl, pH 8.0, 0.25 M NaCl, and 0.1% CHAPS.
13. Buffer E: 50 mM Tris-HCl, pH 8.0, 0.25 M NaCl, 0.1% CHAPS, and 6 M urea.
14. Buffer F: 50 mM sodium formate, pH 3.5, 0.2 M NaCl, 0.1% CHAPS, and 6 M urea.
15. Buffer G (preelution buffer): 100 mM Tris-HCl, pH 8.0, 0.2 M NaCl, and 0.5% CHAPS.
16. Buffer H (elution buffer): 50 mM Tris-HCl, pH 8.0, 1 M NaCl, and 0.5% CHAPS.
17. 1 mM EDTA, 1 µg/ml pepstatin A, 0.5 µg/ml leupeptin, 0.4 mM PMSF (final concentra-
tions); add these inhibitors to buffers just before use.
3. Method
3.1. Homogenization and centrifugation
One round of ultracentrifugation processes 25–30g of brain tissue. Four times this
amount (100 g) can be processed in a day by doing four rounds of ultracentrifugation.
The following is our standard procedure for the isolation of soluble proteoglycans
from 100–120 g of rat brain.
1. Chill four 250-ml centrifuge bottles in a freezer. Set up a Polytron homogenizer in a cold
room (see Note 2).
2. Weigh pieces of frozen brain tissues and distribute 25–30 g of tissues per centrifuge bottle.
Keep the bottles on ice.
3. Take one bottle and add buffer A up to ~1 cm below the neck of the bottle.
4. Insert the probe of a Polytron homogenizer into the centrifuge bottle and homogenize at
speed 4–5 for 30 s. Keep the bottle on ice during homogenization. Put the bottle back on ice.
Repeat step 4 for the other three bottles.
5. Homogenize each bottle again for 30 s.
6. Adjust the balance of bottles with buffer A. Centrifuge in a GSA rotor at 12,400g for
30 min at 4°C.
38 Yamaguchi
7. Transfer supernatants into a beaker on ice.
8. Fill eight 30-ml ultracentrifuge tubes with the supernatant. Balance precisely. Every
tube must be filled up to the neck, otherwise the tube will break during ultracentrifuga-
tion and become almost impossible to remove from the rotor. Use buffer A to balance
and fill the tubes.
9. Ultracentrifuge in the Sorvall T865 rotor at 60,000 rpm (~380,000g ) for 60 min at 4ºC.
10. Collect supernatants (= soluble fraction) in a beaker on ice. Collect pellets from the bottom of
the ultracentrifuge tubes with a spatula and keep them on ice until solubilization (see step 13).
11. Repeat steps 8–10 for the rest of the homogenates.
12. Combine supernatants from four rounds of ultracentrifugation (~1000 mL). Clarify by
filtration through a 0.2 µm pore filter, if necessary. If the supernatants are not applied to
DEAE column on the same day, add CHAPS at 0.5% to prevent aggregation during storage.
13. For isolation of the membrane fraction, combine pellets from step 10 in a Dounce or a
Teflon-on-glass homogenizer, add ~50 mL of buffer B, and homogenize. Incubate
homogenates for 1–4 h at 4ºC, if necessary. After solubilization, remove insoluble aggre-
gates by centrifugation at 380,000g for 1 h (= membrane fraction).
3.2. Isolation of Proteoglycan-Enriched Fractions
by Anion-Exchange Chromatography
The following procedure is for the isolation of a fraction enriched for soluble
proteoglycans. For the membrane fraction, the size of the column and the volume of
buffers should be reduced based on the volume of the sample. We perform the entire
process of chromatography in a cold room.
1. Apply the combined soluble fraction onto a column of DEAE-Sepharose Fast Flow
preequilibrated with buffer C. For 1000 mL extract, a column with 25 to 30-mL bed volume
is sufficient. It takes several hours to load this volume of extracts onto the column. We
usually let the extract pass through the column overnight by gravity flow.
2. Wash the column with 10 column volumes of buffer C. A peristaltic pump may be used
during the washing and elution steps. For a 25 to 30-mL column of DEAE Fast Flow, set the
pump at around 3 mL/min.
3. Wash the column with 10 column volumes of buffer D.
4. Wash the column with 10 column volumes of buffer E.
5. Wash the column with 5 column volumes of buffer F.
6. Wash the column with buffer G until pH returns to ~8.
7. Elute with 5–6 column volumes of 0.2–1 M NaCl linear gradient in (e.g., 75 mL of buffer
G and 75 mL of buffer H for a column of 25-mL bed volume). Collect fractions of 2 mL.
8. Determine protein concentration in each fraction.
9. Analyze fractions by SDS-PAGE. Proteoglycans appear as diffuse smears. To identify
what types of proteoglycans are present in the fractions, treat them with carrier-free
chondroitinase ABC (protease-free chondroitinase ABC; Seikagaku America, Rockville,
MD) and analyze by SDS-PAGE. Bands appear after chondroitinase digestion represent
CSPG core proteins (see Note 3). See Fig. 1 for a representative result.
4. Notes
1. Unlike Triton X-100, CHAPS has a small micelle size and is rather easy to remove from the
sample if necessary. However, CHAPS is more expensive than Triton X-100. If this poses a
problem, the CHAPS in buffers C, D, E, and F can be replaced with Triton X-100. In this
Nervous Tissue Proteoglycans 39
case, the large portion of Triton X-100 will be washed out and replaced with CHAPS
during the preelution with buffer G. Note that Triton X-100 does not efficiently solubi-
lize GPI-anchored proteins, including glypicans. Also Triton X-100 interferes with
Absorbance Measurements.
2. Homogenization with a Dounce or a Teflon-on-glass homogenizer is preferable when
smaller amounts of brain tissues are processed. For a large-scale isolation, as described
here, the use of these types of homogenizers is not very practical.
3. For the analysis of proteoglycan core proteins, the use of carrier-free chondroitinase ABC
is important. Most of the commercial glycosaminoglycan lyases contain BSA as a stabi-
lizer, which migrate as a thick, 67-kDa band, rendering the identification of core proteins
in this region impossible. No carrier-free heparitinase or heparinase is available commer-
cially at present. For the analysis of heparan sulfate proteoglycan core proteins, nitrous
acid digestion may be useful. The presence of carrier BSA in heparitinase will not be a
problem when the isolated fractions are analyzed by immunoblotting or after iodination of
core proteins (1).
References
1. Herndon, M. E. and Lander, A. D. (1990) A diverse set of developmentally regulated
proteoglycans is expressed in the rat central nervous system. Neuron 4, 949–961.
2. Yamaguchi, Y. (2000) Chondroitin sulfate proteoglycans in the nervous system, in:
Proteoglycans: Structure, Biology and Molecular Interactions, Iozzo, R. ed., Marcel
Dekker, New York, NY, in press.
3. Yamaguchi, Y. (2000) Lecticans: organizers of the brain extracellular matrix. Cell. Mol.
Life Sci., 57, 276–289.
4. Iwata, M. and Carlson, S. S. (1993) A large chondroitin sulfate proteoglycan has the character-
istics of a general extracellular matrix component of adult brain. J. Neurosci. 13, 195–207.
5. Yamada, H., Fredette, B., Shitara, K., Hagihara, K., Miura, R., Ranscht, B., Stallcup, W.
B., and Yamaguchi, Y. (1997) The brain chondroitin sulfate proteoglycan brevican associ-
ates with astrocytes ensheathing cerebellar glomeruli and inhibits neurites outgrowth from
granule neurons. J. Neurosci. 17, 7784–7795.
6. Schmalfeldt M., Dours-Zimmermann M. T., Winterhalter K. H., and Zimmermann D. R.
(1998) Versican V2 is a major extracellular matrix component of the mature bovine brain.
J. Biol. Chem. 273, 15,758–15,764
7. Stipp, C. S., Litwack, E. D., and Lander, A. D. (1993) Cerebroglycan: an integral mem-
brane heparan sulfate proteoglycan that is unique to the developing nervous system and
expressed specifically during neuronal differentiation. J. Cell. Biol. 124, 149–160.
8 Yamada, H., Watanabe, K., Shimonaka, M., and Yamaguchi, Y. (1994) Molecular cloning
of brevican, a novel brain proteoglycan of the aggrecan/versican family. J. Biol. Chem.
269, 10,119–10,126.
9. Yamada, H., Watanabe, K., Shimonaka, M., Yamasaki, M., and Yamaguchi, Y. (1995)
cDNA cloning and the identification of an aggrecanase-like cleavage site in rat brevican.
Biochem. Biophys. Res. Commun. 216, 957–963.
10. Rauch, U., Gao, P., Janetzko, A., Flaccus, A., Hilgenberg, L., Tekotte, H., Margolis, R.
K., and Margolis, R. U. (1991) Isolation and characterization of developmentally regu-
lated chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of brain identified
with monoclonal antibodies. J. Biol. Chem. 266, 14,785–14,801.
11. Miura, R., Aspberg, A., Ethell, I. M., Hagihara, K., Schnaar, R. L., Ruoslahti, E., and
Yamaguchi, Y. (1999) The proteoglycan lectin domain binds sulfated cell surface gly-
colipids and promotes cell adhesion. J. Biol. Chem. 274, 11,431–11,438.

Proteoglycans and Glycosoaminoglycans from Drosophila 41
41
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
6
Analysis of Proteoglycans
and Glycosaminoglycans from
Drosophila
William D. Staatz, Hidenao Toyoda, Akiko Kinoshita-Toyoda,
Kimberlly Chhor, and Scott B. Selleck
1. Introduction
The fruitfly, Drosophila melanogaster, has provided a powerful model organism
for the study of development. Analysis of patterning in a variety of species from the
nematode, C. elegans, to frogs, chicks, and mice have demonstrated that the funda-
mental mechanisms of morphogenesis are conserved among diverse species. In recent
years genetic studies in Drosophila have shown that proteoglycans (PGs) and their
associated glycosaminoglycans (GAGs) are critical for normal development. While
there exists a sophisticated set of genetic and molecular tools for the study of Droso-
phila, methods for the analysis of PGs and GAGs are not nearly as well developed. We
have begun to divise such methods in order that genetic and biochemical studies can
be merged to understand better the function of PGs during development at the
molecular level.
We describe here methods for the analysis of PGs from Drosophila larvae and tis-
sue culture cells, as well as quantitative methods for characterizing GAGs from differ-
ent Drosophila developmental stages. The PG methods are scaled-down modifications
of those used for vertebrate tissues and cultured cells (1) and are sufficiently sensitive
to detect PGs immunochemically in DEAE column fractions from 25–50 third-instar
larvae (see Fig. 1). The most practical and perhaps the only quantitative approach to
studying GAG structure is the analysis of the unsaturated disaccharides derived enzy-
matically from GAGs. The most common detection method used for this type of analy-
sis on material from vertebrate sources is ultraviolet absorption at 232 nm. This method
is not sensitive enough for the microdetermination of samples derived from 7–10
third-instar Drosophila larvae or comparable amounts of other Drosophila tissue. The
postcolumn fluorometric detection method using 2–cyanoacetamide, which we
describe here, is especially well suited for analyzing unsaturated disaccharides from
Drosophila GAGs (2) (see Fig. 2).

42 W. D. Staatz et al.
2. Materials
2.1. Purification of Proteoglycans from Drosophila
1. Homogenization buffer: 50 mM Tris HCl, 0.15 M NaCl, pH 8.0 1% CHAPS 2 × Complete
Protease Inhibitors (Roche Molecular Biochemicals) (see Note 1). (6 M urea may be added
to all buffers except the pH 8.0 wash and the high-salt buffer, or it may be added as a
separate urea wash buffer [see Note 2]).
2. Column buffer: 50 mM Tris-HCl, 0.15 M NaCl, pH 8.0, 0.5% CHAPS, 1 × Complete
Protease Inhibitors. (6 M urea: see Note 2.)
3. Low-salt buffer: 50 mM Tris-HCl, 0.25 M NaCl, pH 8.0, 0.1% Triton X-100, 1 × Com-
plete Protease Inhibitors. (6 M urea: see Note 2.)
4. Urea wash buffer (used only if no urea is in the previous buffers [see Note 2]). 50 mM
Tris-HCl, 0.25 M NaCl, pH 8.0, 6 M urea, 0.1% Triton X-100, 1 × Complete Protease
Inhibitors.
5. pH 3.5 buffer: 50 mM Sodium formate, 0.25 M NaCl, pH 3.5, 0.1% Triton X-100, 2 mM
PMSF, 10 mM EDTA, 6 M urea. Urea is always included in this buffer (see Note 2).
6. pH 8.0 wash buffer : 50 mM Tris-HCl, pH 8.0, 0.5% CHAPS, 2 mM PMSF, 10 mM
EDTA.
7. High-salt buffer: 50 mM Tris-HCl, 2 M NaCl, pH 8.0, 0.5% CHAPS, 2 mM PMSF, 10 mM
EDTA.
8. A 0.5 × 3 cm chromatography column.
Fig. 1. Isolation of the proteoglycan Dally from third-instar Drosophila larvae by chroma-
tography on DEAE Sepharose. Forty third-instar larvae were homogenized and fractionated on
a DEAE column as described under Subheading 3.1. Thirty micrograms of protein from each
fraction were separated by SDS-PAGE, blotted onto PVDF membrane (Millipore), and
immunostained using a polyclonal anti-Dally antiserum at 1:15,000 dilution followed by goat
anti-rabbit-HRP conjugate at 1:5000 and ECL (Amersham Pharmacia Biotech) according to the
manufacturer’s instructions. The lanes contain (1) flow-through, (2) column buffer (first wash),
(3) column buffer (second wash), (4) low salt buffer, (5) 6 M urea, (6) pH 3.5 wash. (7) pH 8.0
wash, (8) 2 M NaCl (first wash), (9) 2 M NaCl (second wash). Dally elutes in the 2 M NaCl
fractions as a smear between 90 and 200 kDa.
Proteoglycans and Glycosoaminoglycans from Drosophila 43
9. 0.3 mL of DEAE Sepharose Fast Flow (Amersham Pharmacia Biotech). (see Note 3).
10. A supply of Drosophila. For the volumes used in this chapter, initial experiments should
be done with 40 pupae or third-instar larvae, 60 adults, 80 second-instar larvae, or 400
embryos or first-instar larvae. These numbers will have to be optimized for the needs of
each individual experiment. (see Note 3).
2.1.1. For the Alternative Stepped NaCl Gradient Elution
1. Aliquots of column buffer (above) with total NaCl concentrations of 0.25, 0.5, 0.75, 1.0,
1.5, and 2.0 M. Because it is significantly less expensive, 0.1% Triton X-100 may be
substituted for CHAPS in these buffers.
2.2. Purification of Proteoglycans from Drosophila S-2 Cells
1. CellFECTIN Reagent
®
(Life Technologies) (see Note 4).
2. Insect tissue culture medium. HQ-CCM3 serum-free insect cell culture medium (Hyclone)
or Shields and Sang M-3 insect cell culture medium (Life Technologies, Sigma, or Hyclone)
are both suitable. The latter must be supplemented with 12.5% fetal calf serum (Life Tech-
nologies, Sigma, or Hyclone) (see Note 5).
3. Transfection vector F449 (3) with the gene of interest under the control of the Hsp70
promoter. See Note 6.
4. To generate stable cell lines, a selection plasmid, such as pH8C0 (4) or pHGC0 (5), which
confers methotrexate resistance (see Note 6).
5. Log-phase cultures of S-2 cells. S-2 cells are routinely maintained in dishes or flasks as
nonadherent cells with a mean generation time of 24 h, and are split 1/5 every 5 days.
To ensure optimum transfection rates, the cells should be split 1/1 in fresh medium the
day before transfection. A thorough review of Drosophila cell culture can be found in
Cherbas et al. (6).
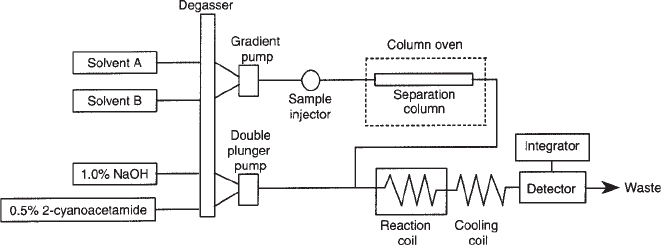
Fig. 2. Flow diagram of the postcolumn HPLC system used for analyzing the unsaturated
disaccharides from Drosophila glycosaminoglycans. The sample is injected into the solvent
pathway and separated on the column at 55° C with a NaCl gradient formed by the gradient pump.
The column eluate is then mixed with 2-cyanoacetamide and NaOH, supplied by the double-
plunger pump, and reacted at 125°C to form fluorescent products that, after cooling, are detected
by the fluorescence detector. All solvents are degassed before they enter the separation and
detection pathways. Solvent A is 1.2 mM tert-n-butylammonium hydrogen sulfate in 8.5%
acetonitrile. Solvent B is 0.2 M NaCl in solvent A.

44 W. D. Staatz et al.
6. Growth medium containing 10
-7
M methotrexate, if stable cell lines are being generated.
7. TBS: 50 mM Tris-HCl, 0.15 M NaCl, pH 7.4, containing Complete Protease Inhibitor
coctail (Roche Molecular Biochemicals).
8. 2 mL of DEAE Sepharose Fast Flow (Amersham Pharmacia Biotech).
9. A 1.4 × 4 cm glass chromatography column (Bio-Rad). A disposable polyproplyene col-
umn with a 3-mL capacity would also work.
10. The column and buffer materials under Subheading 2.2.
2.3. Extraction of Glycosaminoglycans from Drosophila
1. GAG extraction solution: 0.5% SDS, 0.1 M NaOH, 0.8% NaBH
4
.
2. 1.0 M sodium acetate.
3. 1.0 M HCl.
4. 80% Ethanol.
5. Ethanol.
6. 200- to 300-µm pore disposable filter column (Fisher Scientific).
7. Drosophila or Drosophila tissue sample.
2.4. Microdetermination of Chondroitin Sulfate in Drosophila
1. Crude GAG solution (see Subheading 3.3.).
2. Digestion buffer: 0.2 M Tris–acetate buffer (pH 8.0).
3. Chondroitinase ABC (E.C. 4.2.2.4) at 10 IU/mL (Seikagaku America).
4. Chondroitinase ACII (E.C. 4.2.2.5) at 10 IU/mL (Seikagaku America).
5. Unsaturated disaccharide standards from chondroitin sulfate (Seikagaku America). See
Table 1.
2.5. Microdetermination of Heparan Sulfate in Drosophila
1. Crude GAG solution (see Subheading 3.3.).
2. Ultrafree MC Durapore microcentrifugal filtration units with 0.45 cm pores (Millipore).
3. Ultrafree MC Biomax–5 microcentrifugal filtration units with a nominal molecular-
weight exclusion of 5000 daltons (Millipore).
Table 1
Disaccharide Standards from Chondroitin Sulfate
