Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.

2.2 Expression of cloned genes
Once a gene is cloned it is necessary to convert the information contained in it into a
functional protein. There are a number of steps in gene expression: (i) transcription of
DNA into mRNA; (ii) translation of the mRNA into a protein sequence: and (iii) in
some instances, post-translational modification of the protein. In discussing these steps
in more detail, expression of a cloned insulin gene will be used as an example.
2.2.7 Transcription
Transcription of DNA into mRNA is mediated by the enzyme RNA polymerase. The
first stage is binding of the RNA polymerase to recognition sites on the DNA which are
called promoters. After binding, the RNA polymerase proceeds along the DNA molecule
until a termination signal is encountered. It follows that a gene which does not lie
between a promoter and a termination signal will not be transcribed. This would be the
case with a cloned insulin gene, since neither a cDN A gene nor an artificially synthesized
gene will carry a promoter. The solution is to clone the gene into a vector close to a
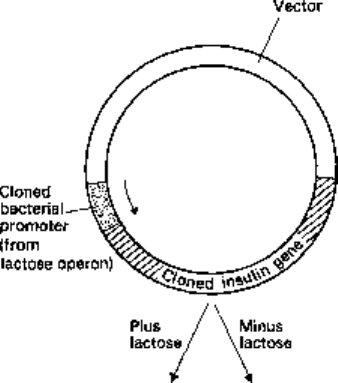
bacterial promotor. An example is shown in Fig. 24.5.
2.2.2 Translation
Translation of mRNA into protein is a complex process which involves interaction of
the messenger with ribosomes. One prerequisite for this is that the mRNA must carry a
ribosome binding site (RBS) in front of the gene to be translated. After binding, the
ribosome moves along the mRNA and initiates protein synthesis at the first AUG codon
it encounters. A synthetic insulin gene will lack an RBS and if a cDNA is used as the
starting material the RBS may be lost in the process of cloning. The solution is to
TRANSCRIPTION NO TRANSCRIPTION
Recombinant DNA technology 457
Fig. 24.5 Insertion of a cloned
insulin gene into a vector
carrying a bacterial promoter.
The arrow indicates the direction
of transcription. If we suppose
the bacterial promoter is derived
from the lactose operon then
transcription will be initiated
only in the presence of lactose.
DNA
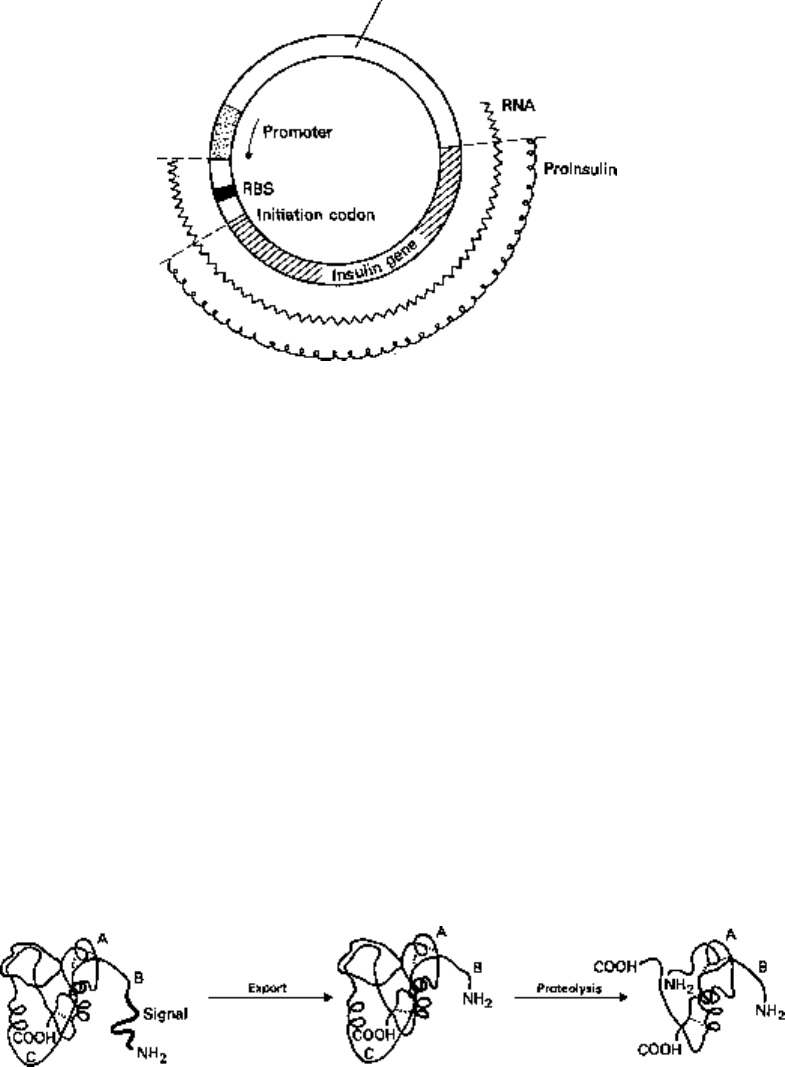
Fig. 24.6 The use of a vector carrying a promoter and adjacent ribosome binding site (RBS) and
initiation codon to obtain synthesis of proinsulin from a synthetic gene. The arrow indicates the
direction of transcription.
utilize a vector in which the insulin gene can be inserted downstream from a promoter
and RBS (Fig. 24.6).
2.2.3 Post-translational modification
A number of proteins undergo post-translational modifications and insulin is one of
these. Proteins which are to be secreted are synthesized with an extra 15-30 amino
acids at the N-terminus. These extra amino acids are referred to as a signal sequence
and a common feature of these sequences is that they have a central core of hydrophobic
amino acids flanked by polar or hydrophilic residues. During passage through the
membrane the signal sequence is cleaved off (Fig. 24.7). If the insulin gene were cloned
by the cDNA method then the signal sequence would be present and, in Escherichia
coli at least, the insulin would be transported through the cytoplasmic membrane
{exported). Using the synthetic gene approach, a signal sequence would be present on
the protein only if the corresponding coding sequence had been incorporated at the
Human preproinsulin Human proinsulin Human insulin
Fig. 24.7 The conversion of preproinsulin to insulin by sequential removal of the signal peptide and
the C fragment.
458 Chapter 24
time of construction. Sometimes it is desirable for the bacterium to export the protein,
in which case a signal sequence is incorporated; with other proteins it may be desirable
that they are retained within the cell.
In E. coli cells, the presence of a signal sequence usually results in export of a
protein into the periplasmic space rather than into the growth medium. Unfortunately,
many recombinant proteins are rapidly and extensively degraded in the periplasmic
space because of the presence there of numerous proteases. In Gram-positive bacteria
and eukaryotic microorganisms, signal sequences direct proteins into the growth
medium. Filamentous organisms such as fungi or actinomycetes might be particularly
favourable for export because of their high surface area to volume ratio.
A small number of proteins, and again insulin is an example, are synthesized as
pro-proteins with an additional amino acid sequence which dictates the final three-
dimensional structure. In the case of proinsulin, proteolytic attack cleaves out a stretch
of 35 amino acids in the middle of the molecule to generate insulin. The peptide that is
removed is known as the C chain. The other chains, A and B, remain crosslinked and
thus locked in a stable tertiary structure by the disulphide bridges formed when the
molecule originally folded as proinsulin. Bacteria have no mechanism for specifically
cutting out the folding sequences from pro-hormones and the way of solving this problem
is described in a later section.
Another modification which can be made in vivo is glycosylation, for example that
of (3 and y interferons, although the biological role of the sugar residues is not known.
Bacteria cannot glycosylate the products of cloned mammalian genes. These non-
glycosylated proteins retain their pharmacological activity but their pharmacokinetics
and in vitro stability may be different. Yeast cells can glycosylate proteins but the
pattern of glycosylation may well be different from that seen in the normal host of the
gene. Non-glycosylated or wrongly glycosylated proteins may provoke the formation
of antibodies following administration.
2.3 Maximizing gene expression
From a commercial point of view it is desirable to maximize the yield of protein in a
fermentation. This means maximizing gene expression and important factors are:
1 the number of copies of the plasmid vector per unit cell (copy number);
2 the strength of the promoter;
3 the sequences of the RBS and flanking DNA;
4 proteolysis.
The limiting factor in expression is the initiation of protein synthesis. Increasing
the copy number of the plasmid increases the number of mRN A molecules transcribed
from the cloned gene and this results in increased protein synthesis. Similarly, the
stronger the promoter (see Fig. 24.5), the more mRNA molecules are synthesized. The
base sequence of the RBS (see Fig. 24.6) and the length and sequence of the DNA
between the RBS and the initiating AUG codon are so important that a single base
change, addition or deletion, can affect the level of translation up to 1000-fold.
Proteolysis does not affect transcription and translation but by degrading the desired
product it influences the apparent rate of gene expression. Although proteolysis can be
reduced it is difficult to eliminate it completely. One approach is to use protease-deficient
Recombinant DNA technology 459
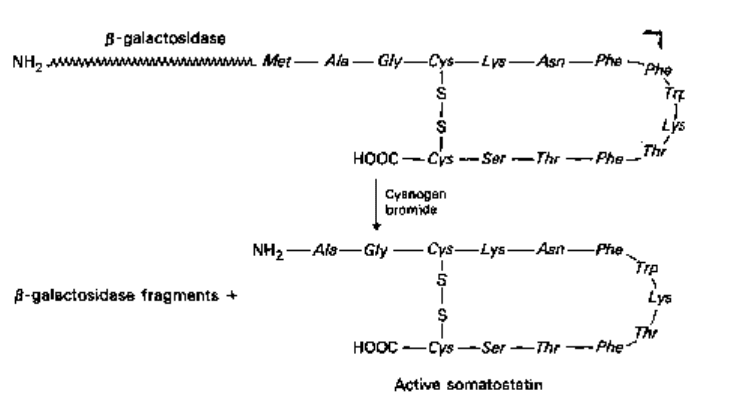
Fig. 24.8 Release of somatostatin from a hybrid protein by cyanogen bromide cleavage.
Somatostatin can be purified free of cyanogen bromide and fragments of /J-galactosidase.
mutants and another is to protect the desired protein by fusion to an E. coli protein (see
below).
Somatostatin was the first human peptide to be synthesized in a bacterial cell. It is
only 14 amino acids long and genes for polypeptides of this size are very amenable to
direct chemical synthesis. However, small peptides are rapidly degraded in E. coli and
for this reason the synthetic gene was fused to the 5' end of the /3-galactosidase gene.
This results in the synthesis of a fusion protein which is relatively stable in E. coli.
Somatostatin does not contain any methionine residues, so the synthetic gene was
constructed in such a way that a methionine was incorporated at the junction of the
fusion peptide. By treatment with cyanogen bromide, which breaks proteins into
polypeptide fragments at methionine residues, authentic somatostatin could be recovered
(Fig. 24.8). Although in this particular instance, and in the case of insulin and fi-
endorphin, the fusion protein contained a remnant of the E. coli /3-galactosidase gene
at the N-terminus, other bacterial proteins have been used, for example tryptophan
synthetase, j3-lactamase, etc.
2.4 Choice of cloning host
A number of cloning hosts are in widespread use. Escherichia coli is still the most
popular organism for initial genetic manipulations and is used for the commercial
production of a number of therapeutic proteins. Bacillus subtilis has not lived up
to its initial promise of high-level protein secretion and interest in it is declining.
Saccharomyces cerevisiae is widely used but faces competition from recombinant animal
cells: progress with the latter has been impressive and high-level expression and secretion
systems are available. Good progress has also been made in developing cloning
systems for filamentous fungi and actinomycetes, two groups of organisms which
have long been used in the production of low molecular weight pharmaceuticals.
More recently, there has been growing interest in the development of cloning systems
460 Chapter 24
for the more unusual organisms used in the pharmaceutical industry. The advantages
and disadvantages of the main microbial cloning systems are shown in Table 24.1.
Recombinant proteins can be produced in plants and animals as well as microbes.
For example, a number of important human proteins, e.g. a,-antitrypsin, have been
produced in rats and mice and in some instances can be engineered to be secreted in the
breast milk. Clearly, small mammals are not desirable as production vehicles. However,
good expression can also be obtained with animals such as sheep and goats. Given the
history of large mammals as sources of antitoxins for human therapy they also may be
acceptable for the production of recombinant proteins. A large number of recombinant
proteins also have been produced in plants, e.g. proteins toxic to insect larvae, antibody
fragments, etc. Already some of these recombinant plants are grown commercially,
and are being consumed, so there is no reason why they cannot be sources of protein
drugs as well. In this context it is worth noting that the pharmaceutical industry is used
to manufacturing drugs from plants, as plants are the source of many of the older
medicines still in use.
3 Production of medically important
polypeptides and proteins
The overproduction of a wide variety of proteins has now been achieved in E. coli and
other cloning hosts. Many of these proteins are in clinical trials and, as indicated earlier,
over a dozen are already on the market. The current status of many of these proteins is
summarized in Table 24.2. The efficacy of many of the proteins listed remains to be
determined because until the advent of recombinant DNA technology sufficient
quantities were not available to enable clinical trials to be undertaken. It should be
noted that clinical efficacy alone is not sufficient. Market size is just as important since
it can cost up to £50 million to bring a new drug to the market place and company
shareholders expect a good return on their investment.
One of the advantages of recombinant DNA technology is that is enables analogues
of human proteins to be produced. Thus, numerous groups have produced a-a and
a-/3 hybrid interferons. Some of these hybrids have altered properties in vitro but whether
this will translate into a clinical benefit remains to be determined. In some instances
the analogues have only a single amino acid change. Thus, changing cysteine residue
17 in interferon-/3 to a serine residue yields a protein with improved half-life and in
vitro stability. Changing methionine residue 358 in a,-antitrypsin to valine yields a
more oxidation-resistant enzyme.
4 Authenticity and efficacy of drugs produced by
recombinant DNA technology
To demonstrate the safety and efficacy of any polypeptide drug, regardless of whether
it is made by recombinant DNA technology, organic synthesis or extraction from a
natural source, a number of quality criteria need to be met. Not only must the protein
be produced in accordance with good manufacturing practice but it must also meet
specification. Although the absolute specification will vary depending on the identity
of the protein, the therapeutic target and the route and period of administration, certain
Recombinant DNA technology 461

462 Chapter 24
Table 24.1 Comparison of different
Organism
Escherichia coil
Bacillus subtilis
Saccharomyces cerevisiae
Filamentous fungi
Actinomycetes
Mammalian cells
organisms as cloning hosts
Advantages
Ease of manipulation
Promoters and gene regulation
well understood
Easy to culture on large scale
Already used in manufacture of
insulin, interferon and
human somatotrophin
Many proteins naturally
exported into growth
medium
Non-pathogenic
Easy to culture
Some Bacillus enzymes
excreted at high level
(>5gM)
Widely used industrial
organism which is easy to
culture
Glycosylates proteins
Can get export into growth
medium of heterologous
proteins
High-level expression systems
developed
Heterologous proteins inside
cell do nofform inclusions
Large surface area to volume
ratio should favour protein
export
Have been used in industrial
microbiology for over 40
years
Large surface area to volume
ratio should favour protein
export
Widely used in industrial
microbiology
Good expression systems being
developed
Get export of proteins
Get desired post-translational
modifications and products
not likely to be immunogenic
to humans
Good expression systems
available
Disadvantages
Do not usually get export of
proteins into growth medium
Over-expressed foreign
proteins often form
aggregates ('inclusions') of
denatured protein
Many foreign proteins rapidly
degraded
Many post-translational
modifications do not occur
Still not much known about
gene regulation
Good, high-level expression
vectors lacking
High-level export of
heterologous proteins not
achieved
Much still to be learned about
control of gene expression
Post-translational modifications
of proteins not necessarily
the same as those in the
animal cell
Promoters/gene regulation
poorly understood but may
be similar to yeast
Good expression systems
lacking
Rheology of fermentations
important
Promoters/gene regulation still
poorly understood
Rheology of fermentations
important
Large-scale growth of animal
cells costly
Great care needed to avoid
contamination of cultures

Table 24.2 Current status of selected recombinant proteins
Protein
Human insulin
Human somatotropin
lnterferon-a
2a
lnterferon-a
2b
Interferon-/
Tissue plasminogen
activator
Relaxin
a-Antitrypsin
Size/structure
Two peptide chains:
A, 21 amino acids
long, and B, 30
amino acids long
191 amino acids
166 amino acids
143 amino acids,
glycosylated
530 amino acids,
glycosylated
53 amino acids;
insulin-like (two
protein chains)
394 amino acids.
glycosylated
Expression system
E. coli
E. coli
E. coli
E. coli
E. coli
Yeast
Animal cells
E. coli
E. coli
Yeast
Clinical indications
Juvenile onset
diabetes
Pituitary dwarfism
Various cancers and
viral diseases
Chronic
granulomatous
disease
Acute mycocardial
infarct
Pulmonary embolism
Facilitates childbirth
Treatment of
emphysema
Comments
Approved for sale
A and B chains made separately as
fusion proteins and joined in vitro
Compared with animal insulins some
undesirable side-effects have been
noted
Approved for sale
If useful in treatment of osteoporosis
then market size will be much larger
Has additional methionine residue at
N-terminus, but technology for
removing this now available
Approved for sale
Over 80% success in treatment of hairy
cell leukemia; success with other
cancers lower and more variable
Market size may be limited
Unpleasant ('flu-like') side-effects
Approved for sale
In clinical trials for treatment of cancer
and viral diseases
Approved for sale
Animal cell culture most effective way
of producing active enzyme
Prepares endometrium for parturition
and reduces fetal distress
Pig relaxin shown to be clinically
effective
Prevents cumulative damage to lung
tissue caused by leucocyte elastase
In clinical trials
Continued on p. 464

Table 24.2 Continued
Protein
lnterleukin-2
Tumour necrosis factor
Human serum albumin
Factor VIII
Factor IX
Erythropoietin
Hepatitis B surface
antigen
Granulocyte colony
stimulating factor
Granulocyte-macrophage
colony stimulating
factor
Size/structure
133 amino acids
157 amino acids
582 amino acids; 17
disulphide bridges
2332 amino acids
415 amino acids
glycosylated;
modified residues
166 amino acids
glycosylated
Monomer has 226
amino acids
127 amino acids
127 amino acids
Expression system
E. coli
Animal cells
E. coli
Animal cells
Yeast
Mammalian cells
Mammalian cells
Mammalian cells
Yeast
Mammalian cells
E. coli
E. coli
Clinical indications
Treatment of cancer
Treatment of cancer
Plasma replacement
therapy
Treatment of
haemophilia
Treatment of
Christmas disease
Treatment of
anaemia
associated with
dialysis and
AZT/AIDS
Vaccination
Adjunct to cancer
chemotherapy
Improved bone
marrow transplant
Comments
Approved for sale
Very toxic and side-effects severe
Normally obtained from plasma but
now concern over potential
contamination with AIDS virus
Normally obtained from plasma but
now concern over potential
contamination with AIDS virus
Approved for sale
Must be made in mammalian cells since
glycosylation and conversion of first
12 glutamate residues to
pyroglutamate essential for activity
Approved for sale
Without glycosylation protein is cleared
very quickly from plasma
Approved for sale
Monomer self-assembles into structure
resembling virus particles
Approved for sale
By stimulating white blood cell
formation, aids recovery
Approved for sale

HPLC, high-performance liquid chromatography; ELISA, enzyme-linked immunosorbent assay
method; RIA, radioimmunoassay method.
quality guidelines have been adopted by most countries. This core specification is shown
in Table 24.3.
Although many of the quality control tests used are designed to assess purity they
often give data which confirms the identity of the protein, e.g. chromatographic
behaviour (high-performance liquid chromatography), electrophoretic mobility and
amino acid composition. However, most of the analytical techniques in current use
give no indication of the three-dimensional structure of the protein and hence no
indication of biological activity. Thus, the absolute specific activity of the protein needs
to be determined in a biological test. Determination of the specific activity is particularly
important with proteins overproduced in E. coli, for such proteins exist in aggregates
with nucleic acid often called 'inclusions'. The protein in these aggregates has to be
extracted with denaturing agents such as urea, sodium dodecyl sulphate or guanidinium
hydrochloride and then renatured, a process akin to recreating native egg white from a
meringue.
As indicated earlier, recombinant DNA technology can be used to deliberately
produce desired analogues of natural proteins. However, undesirable analogues may
also be produced inadvertently during the production process. For example, when the
human somatotrophin gene is expressed in E. coli, the resultant protein has an additional
methionine residue at the N-terminus. Other foreign gene products may or may not
carry this additional methionine residue. Recently, methods have been developed for
enzymatically removing this N-terminal methionine and for mediating another post-
translational modification, C-terminal amidation.
Another undesirable modification is removal of some amino acids residues from
the C-terminus and/or the N-terminus by microbial exoproteases. Care needs to be
taken during the fermentation and extraction stages to minimize proteolytic damage,
and any 'nibbled' molecules should be removed during purification.
Recombinant DNA technology 465
Table 24.3 Specification of therapeutic proteins to be administered parenterally
Criterion
Greater than 95% pure
Microheterogeneity below specified level
Endotoxin below specified level
Contaminating DNA below specified
level (<10pg dose
-1
)
Toxic chemicals used in purification
below specified level
IgG below specified limit (if monoclonal
antibodies used in purification)
Absence of microorganisms
Appropriate analytical methods
Gel electrophoresis; HPLC
Polyacrylamide gel electrophoresis
C- and N-terminal analysis. Amino
acid composition
Limulus amoebocyte lysate method
(see also Chapter 18)
Hybridization
Appropriate methods
ELISA or RIA
Sterility test (see also Chapter 23)
5 Future trends with protein pharmaceuticals
Already over a dozen recombinant-derived therapeutically useful proteins are being
marketed and at least as many more are in clinical trials. So, what of the future? There
are two disadvantages with developing proteins^as therapeutic entities. First, most of
them are not active when given orally, and parenteral administration is almost de rigeur.
Some proteins can be administered in other ways, e.g. insulin can be given per rectum
but this is not a route which is favoured in many countries outside of France and Japan.
Other proteins may be active if given sublingually or if administered by aerosol. Clearly,
much work needs to be done in developing new dosage forms particularly suited to the
administration of proteins. Second, most of the proteins being marketed or currently in
clinical trials were obvious candidates for development, e.g. insulin and interferons.
Identifying the next generation of therapeutically useful proteins will be much more
difficult. There are hundreds of human proteins of which relatively little is known but
only a few are likely to be worth developing. For example, a factor which promotes
bone growth would have many clinical benefits but the candidate proteins have yet to
be identified.
5.1 Small-molecule drugs
One trend which has become obvious is that many pharmaceutical companies are turning
their attention to the application of recombinant DNA technology in the production of
small molecules. Microorganisms are widely used in the production of drugs, e.g.
antibiotics and steroid transformations. Where the rate-limiting step in production has
been identified, cloning the relevant gene could well facilitate synthesis and give
increased yields and/or decreased production times. In addition, novel metabolic
pathways can be introduced into microorganisms and this could eliminate more
conventional but complex production processes involving plants.
5.2 Anti-sense agents
Many drugs treat the symptoms of a disease rather than the cause of the disease, e.g. the
different classes of drug for the treatment of hypertension. Where the primary cause of
the disease is overexpression then anti-sense agents may be of value. These are nucleic
acids which are complementary to the 5' region of a mRNA molecule and bind to it. In
this way the translation of the mRNA into protein is reduced or eliminated and hence
the anti-sense molecule modulates expression. There are two major problems with
anti-sense nucleic acids as therapeutic entities. First, small single-stranded nucleic acids
are rapidly degraded inside cells. The solution to this problem is to use modified nucleic
acids, e.g. ones in which the phosphodiester backbone is replaced with a peptide chain
('peptide nucleic acids'). The second issue is delivering enough of the anti-sense
molecules to the target cells. This can be achieved with proper formulation, and a
number of anti-sense drugs are in clinical trials.
466 Chapter 24
