Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.


fungi (incubation temperature 20-25°C). Other media may be used provided they can
be shown to be suitable alternatives.
2 Membrane filtration is the technique recommended by most pharmacopoeias and
involves filtration of fluids through a sterile membrane filter (pore size =£0.45 |im),
any microorganism present being retained on the surface of the filter. After washing
in situ, the filter is divided aseptically and portions transferred to suitable culture media
which are then incubated at the appropriate temperature for the required period of
time. Water-soluble solids can be dissolved in a suitable diluent and processed in this
way.
3 A sensitive method for detecting low levels of contamination in intravenous infusion
fluids involves the addition of a concentrated culture medium to the fluid in its original
container, such that the resultant mixture is equivalent to single strength culture medium.
In this way, sampling of the entire volume is achieved.
With the techniques discussed above, the media employed should previously have
been assessed for nutritive (growth-supporting) properties and a lack of toxicity using
specified organisms. It must be remembered that any survivors of a sterilization process
may be damaged and thus must be given the best possible conditions for growth.
As a precaution against accidental contamination, product testing must be carried
out under conditions of strict asepsis using, for example, a laminar airflow cabinet to
provide a suitable environment (Chapter 22).
Both the British and European pharmacopoeias indicate that it is necessary to conduct
control tests which confirm the adequacy of the facilities by sampling of air and surfaces
and carrying out control tests using samples 'known' to be sterile. In reality, this means
Fig. 23.2 Isolators used for sterility testing. The operator works within the hood which is
suspended inside the cubicle; the hydrogen peroxide generator which is used to sterilize
the isolators is shown in the left foreground. (Courtesy of SmithKline Beecham
Pharmaceuticals.)
Sterilization control and sterility assurance 447

samples that have been subjected to a very reliable sterilization process, e.g. radiation,
or samples that have subjected to a sterilization procedure more than once. In order
to minimize the risk of introducing contaminants from the surroundings or from the
operator during the test itself, isolators are often employed which physically separate
the operator from the materials under test. These are designed on the same principle as
a glove box, but on a much larger and more sophisticated scale, so the operator works
inside a sterile cubicle but is separated from the atmosphere within it by a flexible
moulded covering (rather like a space suit) which is an integral part of the cubicle base
(Fig. 23.2).
5.2 Antimicrobial agents
Where an antimicrobial agent comprises the product or forms part of the product, for
example as a preservative, its activity must be nullified in some way during sterility
testing so that an inhibitory action in preventing the growth of any contaminating
microorganisms is overcome. This is achieved by the following methods (sections 5.2.1-
5.2.3).
5.2.1 Specific inactivation
An appropriate inactivating (neutralizing) agent (Table 23.3) is incorporated into the
culture media. The inactivating agent must be non-toxic to microorganisms as must
any product resulting from an interaction of the inactivator and the antimicrobial agent.
Although Table 23.3 lists only benzylpenicillin and ampicillin as being inactivated
by /3-lactamase (from B. cereus), other /^-lactams may also be hydrolysed by their
appropriate /3-lactamase. Other antibiotic-inactivating enzymes are also known (Chapter
9) and have been considered as possible inactivating agents, e.g. chloramphenicol
acetyltransferase (inactivates chloramphenicol) and enzymes that modify amino-
glycoside antibiotics. In addition, encouraging results have been obtained by the use of
antibiotic-absorbing resins.
448 Chapter 23
Inhibitory agents
Phenols, cresols
Alcohols
Parabens
Mercury compounds
Quaternary ammonium
compounds
Benzylpenicillint 1
Ampicillin J
Other antibioticst
Sulphonamides
* Neutralizing agents; see
f See text.
Table 23.3 Inactivating agents*
Inactivating agents
None (dilution)
None (dilution)
Dilution and Tween
-SH compounds
Lecithin + Lubrol W;
Lecithin + Tween (Letheen)
/5-Lactamase from Bacillus cereus
None (membrane filtration)
p-Aminobenzoic acid
also Table 11.4 (Chapter 11).
5.2.2
Dilution
The antimicrobial agent is diluted in the culture medium to a level at which it ceases to
have any activity, for example phenols, cresols and alcohols (see Chapter 11). This
method applies to substances with a high dilution coefficient, r|.
5.2.3 Membrane filtration
This method has traditionally been used to overcome the activity of antibiotics for
which there are no inactivating agents, although it could be extended to cover other
products if necessary, e.g. those containing preservatives for which no specific or
effective inactivators are available. Basically, a solution of the product is filtered
through a hydrophobic-edged membrane filter which will retain any contaminating
microorganisms. The membrane is washed in situ to remove any traces of antibiotic
adhering to the membrane and is then transferred to appropriate culture media.
5.3 Positive controls
It is essential to show that microorganisms will actually grow under the conditions of
the test. For this reason positive controls have to be carried out; in these, the ability of
small numbers of suitable microorganisms to grow in media in the presence of the
sample is assessed. The microorganism used for positive control tests with a product
containing or comprising an antimicrobial agent must, if at all possible, be sensitive to
that agent, so that growth of the organism indicates a satisfactory inactivation, dilution
or removal of the agent. The British Pharmacopoeia suggests the use of appropriate
strains of Staphylococcus aureus, CI. sporogenes and Candida albicans for aerobic,
anaerobic and fungal positive controls, respectively.
In practice, a positive control (media with added test sample) and a negative control
(media without it) are inoculated simultaneously, and the rate and extent of growth
arising in each should be similar. However, the negative control without the test sample,
is, in effect, exactly the same as the nutritive properties control which is also described
in the test procedure, so, for the organisms concerned, it is not necessary to do both.
All the controls may be conducted either before, or in parallel with, the test itself,
providing that the same batches of media are used for both. If the controls are carried
out in parallel with the tests and one of the controls gives an unexpected result, the test
for sterility attempt is recorded as invalid, and, when the problem is resolved, the test is
'recommenced' as if for the first time. It is important to recognize that the terms
'recommenced' and 'retest' have different meanings. A 'retest' may, under certain
circumstances, be performed when the first (and, exceptionally, even the second) valid
test shows signs of product contamination.
5.4 Specific cases
Specific details of the sterility testing of parenteral products, ophthalmic and other
non-injectable preparations, catgut, surgical dressings and dusting powders will be
found in the British and European pharmacopoeias.
Sterilization control and sterility assurance 449
5.5 Sampling
A sterility test attempts to infer the state (sterile or non-sterile) of a batch from the
results of an examination of part of a batch, and is thus a statistical operation.
Suppose that/? represents the proportion of infected containers in a batch and q the
proportion of non-infected containers. Then, p-\-q=\oxq=\-p.
Suppose also that a sample of two items is taken from a large batch containing 10%
infected containers. The probability of a single item taken at random being infected is
/? = 0.1 (10% = 0.1), whereas the probability of such an item being non-infected is
given by q = 1 -p = 0.9.
The probability of both items being infected is/?
2
= 0.01, and of both items being
non-infected, q
2
= (1 -p)
2
= 0.81. The probability of obtaining one infected item and
one non-infected item is 1 - (0.01 + 0.81) = 0.18 = 2pq.
In a sterility test involving a sample size of n containers, the probability/? of obtaining
n consecutive 'steriles' is given by q
n
= (1 -p)
n
. Values for various levels of p (i.e.
proportion of infected containers in a batch) with a constant sample size are given in
Table 23.4 which shows that the test cannot detect low levels of contamination. Similarly,
if different sample sizes are employed (based also upon (1 -p)
n
) it can be shown that
as the sample size increases, the probability of the batch being passed as sterile decreases.
The British Pharmacopoeia makes an allowance for accidental contamination which
may arise during the execution of a sterility test by allowing the test to be repeated.
Under these circumstances the following rules apply.
1 If no growth occurs with fresh samples, the batch passes the test.
2 If growth occurs, but the organism differs from that found previously, the test is
repeated on a third sample from the batch using double the number of containers of
product.
3 If no growth occurs with the third sample, the batch passes the sterility test; if,
however, any microorganism is found, the batch is treated as non-sterile, unless or
until the material has been resterilized and has passed the above tests.
In actual fact, however, these additional tests increase the chances of passing a
batch containing a proportion of infected items (Table 23.4, first retest). This may be
Table 23.4 Sampling in sterility testing
p
q
Probability P, of drawing 20
consecutive sterile items:
First sterility test*
First retestt
Infected items in
0.1
0.001
0.999
0.98
0.99
1
0.01
0.99
0.82
0.99
batch (%)
5
0.05
0.95
0.36
0.84
10
0.1
0.9
0.12
0.58
20
0.2
0.8
0.012
0.11
50
0.5
0.5
<0.00001
0.002
* Calculated from P = (1 -p)
20
= q
20
.
t Calculated from P = (1 -p )
20
[2 - (1 -p)
20
].
450 Chapter 23
deduced by using the mathematical formula
(l-/?)"[2-(l-/>)"]
which gives the chance in the first retest of passing a batch containing a proportion p of
infected containers.
It can be seen from the above that a sterility test can only show that a proportion of
the products in a batch is sterile. Thus, the correct conclusion to be drawn from a
satisfactory test result is that the batch has passed the sterility test not that the batch is
sterile.
Conclusions
The techniques discussed in this chapter comprise an attempt to achieve, as far as
possible, the continuous monitoring of a particular sterilization process. The sterility
test on its own provides no guarantee as to the sterility of a batch; however, it is an
additional check, and continued compliance with the test does give confidence as to
the efficacy of a sterilization or aseptic process. Failure to carry out a sterility test,
despite the major criticism of its inability to detect other than gross contamination,
may have important legal and moral consequences.
Acknowledgements
We are grateful to SmithKline Beecham Pharmaceuticals for permission to use
Fig. 23.2.
Further reading
Baird R.M. & Bloomfield S.F. (eds) (1996) Microbial Quality Assurance in Cosmetics, Toiletries and
Non-sterile Pharmaceuticals. London: Taylor & Francis.
British Pharmacopoeia (1993) London: HMSO.
Denyer S.R (1982) In-use contamination in intravenous therapy—the scale of the problem. In: Infusions
and Infection. The Hazards of In-use Contamination in Intravenous Therapy (ed. RF. D'Arcy), pp.
1-16. Oxford: Medicine Publishing Foundation.
Denyer S.R (1992) Filtration sterilization. In: Principles and Practice of Disinfection, Preservation
and Sterilization, 2nd edn. (eds A.D. Russell, W.B. Hugo & G.A.J. Ayliffe), pp. 573-604. Oxford:
Blackwell Science.
Denyer S.R & Baird R.M. (eds) (1990) Guide to Microbiological Control in Pharmaceuticals. Chichester:
Ellis Horwood. (Chapters 7, 8 and 9 provide additional information).
European Pharmacopoeia, 3rd edn. (1997) Maisonneuve: SA.
Gardner J.F. & Peel M.M. (1991) Introduction to Sterilisation, Disinfection and Infection Control, 2nd
edn. Melbourne: Churchill Livingstone.
Greene V.N. (1992) Control of sterilization processes. In: Principles and Practice of Disinfection,
Preservation and Sterilization, 2nd edn. (eds A.D. Russell, W.B. Hugo & G.A.J. Ayliffe), pp. 605-
624. Oxford: Blackwell Scientific Publications.
Gilbert P. & Allison D. (1996) Redefining the 'sterility' of sterile products. Eur J Parenteral Sci, 1,
19-23.
Health Technical Memorandum (1994) Sterilisers. HTM 2010. London: Department of Health.
Hodges, N.A. (1995) Reproducibility and performance of endospores as biological indicators. In:
Microbiological Quality Assurance: a Guide Towards Relevance and Reproducibility of Inocula
(eds M.R.W. Brown & P. Gilbert), pp. 221-234. New York: CRC Press.
Sterilization control and sterility assurance 451
Hoxey E.V., Soper C.J. & Davies D.J.G. (1984) The effect of temperature and formaldehyde
concentration on the inactivation of Bacillus stearothermophilus spores by LTSF. J Pharm
Pharmacol, 36, 60.
Line S.J. & Pickerell J.K. (1973) Testing a steam-formaldehyde sterilizer for gas penetration efficiency.
J Clin Pathol, 26, 716-720.
Pharmaceutical Codex (1994) London: Pharmaceutical Press.
Soper C.J. & Davies D.J.G. (1990) Principles of sterilization. In: Guide to Microbiological Control in
Pharmaceuticals (eds S.P. Denyer & R.M. Baird), pp. 157-181. Chichester: Ellis Horwood.
United States Pharmacopoeia (1995) 23rd revision. Rockville, MD: US Pharmacopeial Convention.
Production of therapeutically useful
substances by recombinant DNA
technology
1
2
2.1
2.2
2.2.1
2.2.2
2.2.3
2.3
2.4
3
Introduction
The basic principles of recombinant
DNA technology
Introduction to cloning
Expression of cloned genes
Transcription
Translation
Post-translational modification
Maximizing gene expression
Choice of cloning host
Production of medically important
4
5
5.1
5.2
5.3
6
7
Authenticity and efficacy of drugs
produced by recombinant DNA
technology
Future trends with protein
pharmaceuticals
Small-molecule drugs
Anti-sense agents
Gene therapy and gene repair
Glossary
Further reading
polypeptides and proteins
Introduction
Natural products of pharmaceutical interest are synthesized by a wide variety of
organisms, ranging from prokaryotes such as bacteria to eukaryotes such as yeast, other
fungi, flowering plants, animals and man. The commercial production of compounds
from microbes is relatively simple since the organism in question can be grown on a
large scale and high-yielding variants can be isolated following many successive rounds
of mutation and selection. A good example is penicillin production by Penicillium
chrysogenum (Chapter 7), where the wild strain yield of a few milligrams per litre
has been raised to over 20gH. Commercial production of compounds from plants
is less easy since synthesis may be tissue or organ specific and may only occur at a
certain developmental stage. If the genetics of the producing organism have not been
studied then selection of high-yielding variants is extremely difficult. Molecules of
pharmacological interest from higher animals are by definition extremely potent, for
example hormones, and so are synthesized in relatively minute quantities. This is a
serious limitation if the producing organisms are animals such as cattle or pigs, as in
the case of insulin, but production is well nigh impossible if the only source is man
himself, as with human growth hormone.
In order that demand should meet supply, or to reduce production costs, it would be
of great benefit if microorganisms could be induced to synthesize pharmacologically
active molecules whose production is normally limited to higher plants and animals.
With the advent of recombinant DNA technology, often called genetic engineering,
this is now possible and synthesis no longer is restricted to polypeptides.
The advantages of recombinant DNA technology are enormous, as the following
example shows. Somatostatin is a hormone that inhibits the secretion of pituitary growth
hormone. The researchers who first isolated somatostatin required nearly half a million
sheep brains to produce 5 mg of the substance. Using a chemically synthesized gene, 9
Recombinant DNA technology 453
24
2
2.1
litres of bacterial culture, costing just a few pounds or dollars, produced the same
amount. Development work has already led to the production of numerous biologically
active human agents in clinically significant amounts, and a number of them are
commercially available (see Table 24.2, pp. 463-464).
The basic principles of recombinant DNA technology
Introduction to cloning
Let us suppose that we wish to construct a bacterium which produces human insulin.
Naively, it might be thought that all that is required is to introduce the human insulin
gene into its new host. The fallacy with this idea is that foreign genes are not maintained
in cells, since they are not replicated. With recombinant DNA technology this problem
is solved by inserting the insulin gene into a cloning vector. The latter is simply a DNA
molecule that can replicate in vivo. Cloning vectors are usually plasmids (Chapter 9),
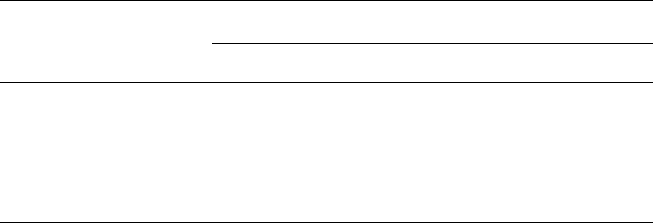
which are extrachromosomal, autonomously replicating DNA molecules (Fig. 24.1).
In order to insert foreign DNA into a plasmid, use is made of special enzymes
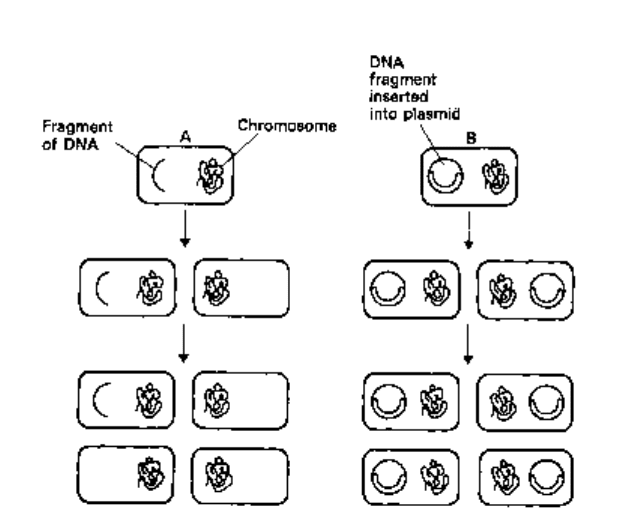
known as restriction endonucleases. These enzymes cut large DNA molecules into
shorter fragments by cleavage at specific recognition sites (Fig. 24.2), i.e. they are
highly specific deoxyribonucleases (DNases). Some of these enzymes generate
fragments with single-strand protrusions called 'sticky-ends' because their bases are
complementary. Fragments of the foreign DNA are inserted into plasmid vectors cut
Fig. 24.1 The requirement for a cloning vector: (A) fragments of DNA introduced into the
bacterium by transformation do not undergo replication and gradually are diluted out of the
population; (B) DNA fragments introduced into plasmids are inherited by both daughter progeny ;
cell division.
454 Chapter 24
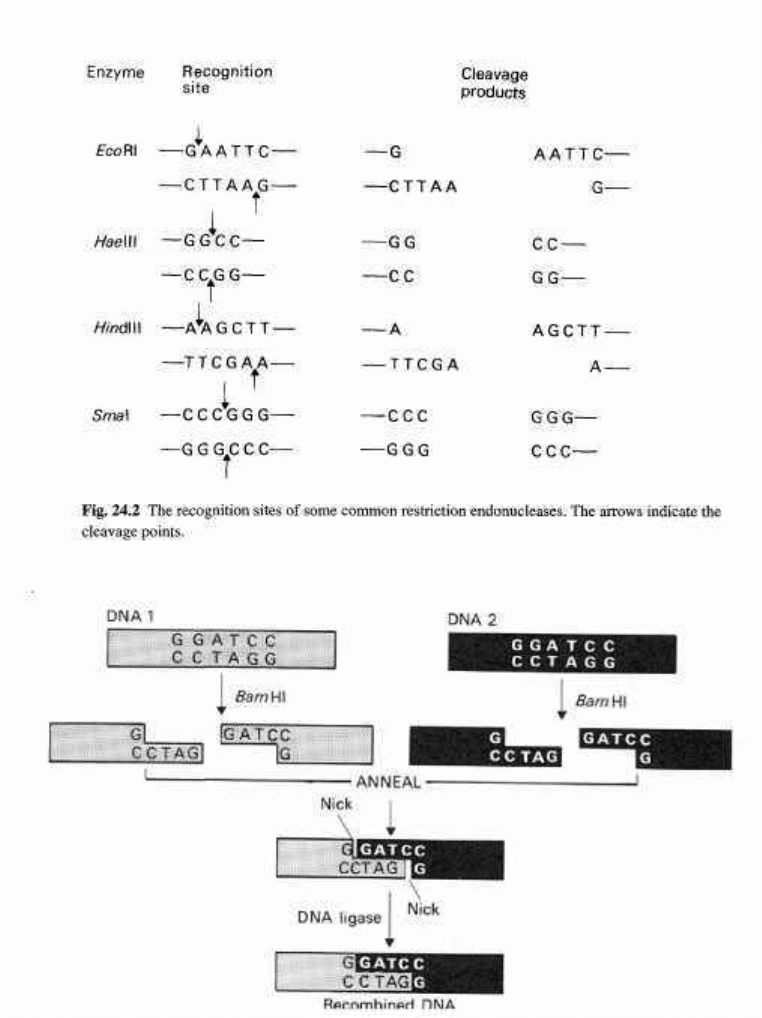
Fig. 24.3 The construction of a chimeric (or recombinant) DNA molecule by joining together two
DNA fragments produced by cleavage of different parental DNA molecules with the same restriction
endonuclease.
open with the same enzyme, which therefore have matching ends (Fig. 24.3).
The resulting recombinants or chimeras are transformed into the new host microbe.
Since each transformant may contain a different fragment of the foreign genome it is
necessary to select those with the desired gene. In practice this can be the most difficult
Recombinant DNA technology 455
step, but the screening methods used are outside the scope of this chapter. Suffice
to say that a necessary prerequisite usually is a sensitive test for the desired protein
product.
Theoretically, it is possible to clone any desired gene by 'shotgunning'. This is
done by inserting into plasmids a random mixture of fragments from total human DNA,
in the case of the insulin gene, and then selecting the appropriate clone. However, if
introduced into a bacterium this clone would not make human insulin. The reason for
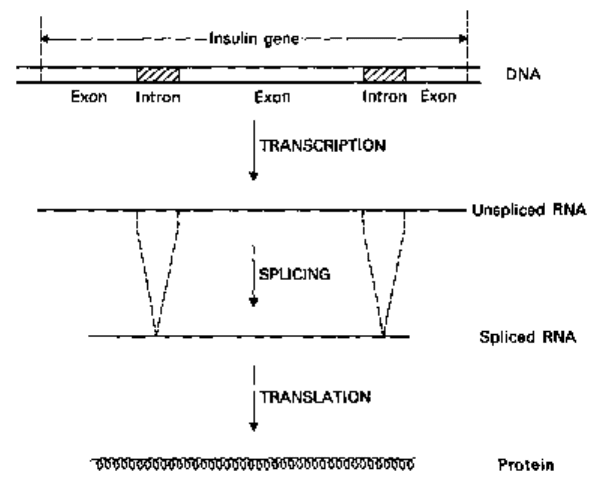
this is that many genes of eukaryotes, including the human insulin gene, are a mixture
of coding regions (called exons) and non-coding regions (called introns). In eukaryotes,
genes containing introns are transcribed into messenger RNA (mRNA) in the usual
manner but then the corresponding intron sequences are spliced out (Fig. 24.4).
Unfortunately not all bacteria can splice out introns.
A solution to the problem of introns is to isolate mRNA extracted from the human
pancreas cells that make insulin. These cells are rich in insulin mRNA from which
introns have already been spliced out. Using the enzyme reverse transcriptase it is
possible to convert this spliced mRNA into a DNA copy. This copy DNA (cDNA),
which carries the uninterrupted genetic information for insulin can be cloned. Although
yeast cells (Saccharomyces) can splice out introns it is normal practice to eliminate
them anyway by cDNA cloning.
An alternative approach is to synthesize an artificial gene in the test-tube starting
with the appropriate deoxyribonucleotides. This approach, which demands that the
entire amino acid sequence be known, has been used to clone genes encoding proteins
200 amino acids long.
Fig. 24.4 Splicing of a messenger RNA molecule transcribed from a hypothetical insulin gene
containing two introns.
