Hillert M. Phase Equilibria, Phase Diagrams and Phase Transformations: Their Thermodynamic Basis
Подождите немного. Документ загружается.

14.6 Transformations in steel under quasi-paraequilibrium 315
α
γ
quasi-paraequilibrium
paraequilibrium
x
C
x
M
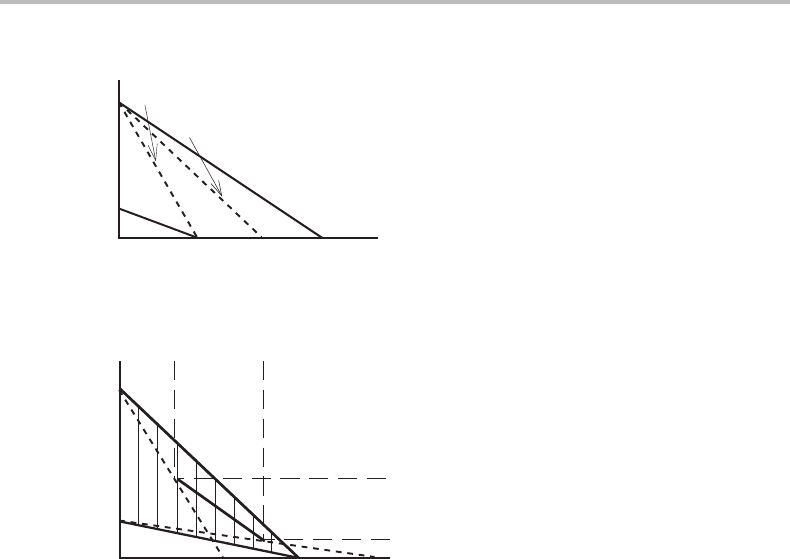
Figure 14.13 Comparison of two partitionless growth conditions for the γ → α transformation in
a ternary system with a very mobile component C.
α
γ
x
C
a
M
Figure 14.14 Solution to Exercise 14.5.
Hint
Find tie-lines in Fig. 14.11(c) by projection from Fig. 14.11(a) and (d).
Solution
The α + γ two-phase field under paraequilibrium is a line in Fig. 14.11(b) and there are
no tie-lines. A tie-line in Fig. 14.11(c) is shown in Fig. 14.14.
14.6 Transformations in steel under quasi-paraequilibrium
In a steel with carbon and some substitutional alloying elements it often happens that
anew phase forms with the same alloy content as the parent phase but with a different
carbon content. Such a phase transformation may occur under local paraequilibrium at
the migrating interface, or under quasi-paraequilibrium, or in between. In this section we
shall examine the quasi-paraequilibrium case, using results from the preceding section.
As a simple example we shall first discuss the γ → α transformation. In a u
M
,u
C
phase
diagram all products of a paratransformation or a quasi-paratransformation will fall on
the same horizontal line as the parent phase. In the phase diagram we can easily find
a point representing the composition of a growing phase, because it must fall on the
correct level of alloy content but also on the appropriate phase boundary, the α/γ phase
316 Partitionless transformations
α
γ
γα
u
M
u
C
u
M
alloy
u
M
d
(a) (b)
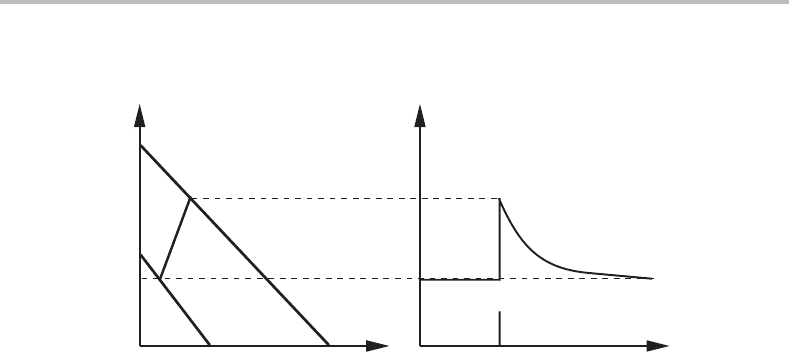
Figure 14.15 Construction yielding the conditions at the phase interface for a
quasi-paratransformation γ → α in a ternary system with a mobile component C.
boundary in the present case. Having located that intersection we have found a tie-line
representing the local equilibrium conditions at the migrating α/γ interface. We can
thus construct a composition profile for the alloying element. In order to do this we shall
plot u
M
on the ordinate axis (see Fig. 14.15).
From the composition profile we may conclude that there is a thin spike of the alloying
element in front of the migrating interface. This is similar to the quasi-diffusionless case
in a binary alloy. According to the mathematics of diffusion, we can estimate the width
of the spike to D
M
/υ,where υ is the rate of migration of the interface and D
M
is
the diffusion coefficient of the alloying element M relative to Fe. The width is usually
extremely small and the alloy content in the spike originates from a transient stage of
growth at a very early time.
One may normally expect that the rate is governed by the rate of long-range diffusion
of carbon. In order for the transformation to proceed, carbon must diffuse from the α/γ
interface and into the interior of the γ phase because the growing α phase has less carbon
than γ.Itisthus necessary that the carbon potential is higher at the interface than in
the interior of the γ phase. The critical limit for the position of the initial γ phase is
thus found on the intersection of the level of alloy content, u
alloy
M
, and the isoactivity line
for carbon in γ which goes through the γ end-point of the tie-line. This construction
is shown in Fig. 14.16. Naturally, this isoactivity line must be extrapolated below the
γ/(α + γ) boundary. The critical limit is represented by a circle in the diagrams and falls
on the line for quasi-paraequilibrium in Fig. 14.13. The rapid, quasi-paratransformation
can only occur on the left-hand side of that critical line, i.e. in this case below the
line.
Suppose the carbon activity for a γ phase is initially lower than for the isoactivity
line in Fig. 14.16. The γ → α transformation can then start in the way described above.
However, during the growth of α, the γ phase will accumulate more and more carbon.
Its carbon activity will increase and eventually reach the value for the isoactivity line in
Fig. 14.16. The rapid growth will then stop and the transformation can only continue at
a much slower rate which permits the sluggish alloying element M to be redistributed
14.6 Transformations in steel under quasi-paraequilibrium 317
α
γ
o
α
γ
rapid
γ→α
o
u
M
u
C
u
C
u
M
alloy
u
M
isoactivity line
for C in γ
(a) (b)
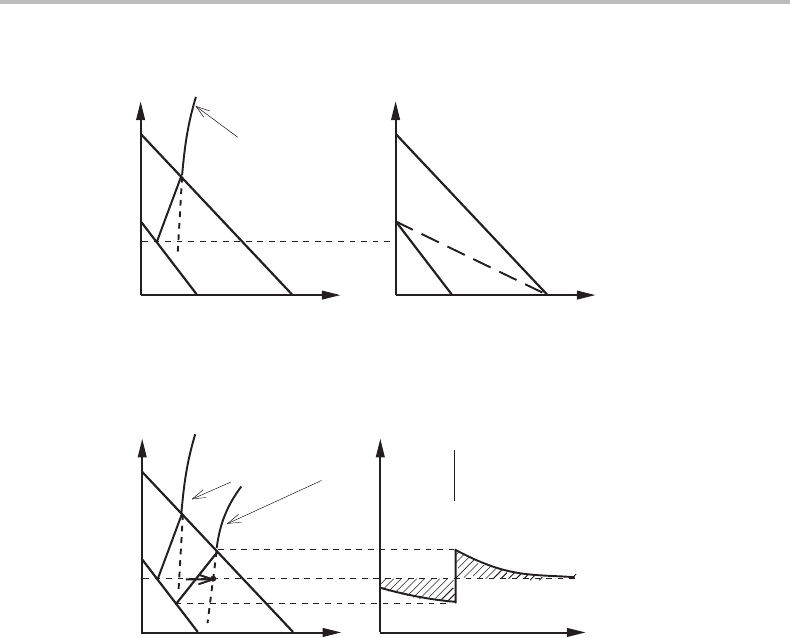
Figure 14.16 Construction yielding the critical limit for a quasi-paratransformation γ → α in a
ternary alloy with a mobile component C.
α
o
αγ
.
u
M
u
C
u
M
alloy
isoactivity
lines for C in γ
d
(a) (b)
Figure 14.17 Growth conditions of α from γ at a stage where diffusion of the sluggish alloying
element M is required.
relative to Fe. During this stage of slow growth there will be sufficient time for the mobile
carbon to equilibrate inside the system and at each moment all the γ phase present may
fall practically on a common isoactivity line for carbon in γ.Ifweknow the momentary
composition of the interior of the γ phase we can easily find the γ end-point of a tie-line
representing the equilibrium conditions on the migrating α/γ interface. We can thus
construct the composition profile for the alloying element M during any stage of very
slow growth; see Fig. 14.17 in which the filled circle represents the present composition
of the interior of the γ phase and the arrow indicates that its carbon content is gradually
increasing during this growth. In the composition profile the spike has now widened to
a considerable thickness, which may be evaluated by using the fact that the two shaded
areas represent the same amount of M. The rate of growth is now governed by diffusion
of the alloying element down the spike.
Let us now examine the more complicated case where the γ phase transforms to the
eutectoid mixture of α and cementite, (Fe,M)
3
C, which is called pearlite. In order for
that reaction to be governed by the rate of carbon diffusion it is necessary for both α and
cementite to inherit the alloy content of the parent γ. Each one of the two growing phases
must fall on the correct side of its critical line. We shall first illustrate this by two separate
phase diagrams in Fig. 14.18(a) showing the α/γ equilibrium and (b) showing the
318 Partitionless transformations
α
γ
rapid
γ→α
γ
α+γ
rapid
γ→cementite
γ+cementite
u
M
u
M
u
C
u
C
(a)
(b)
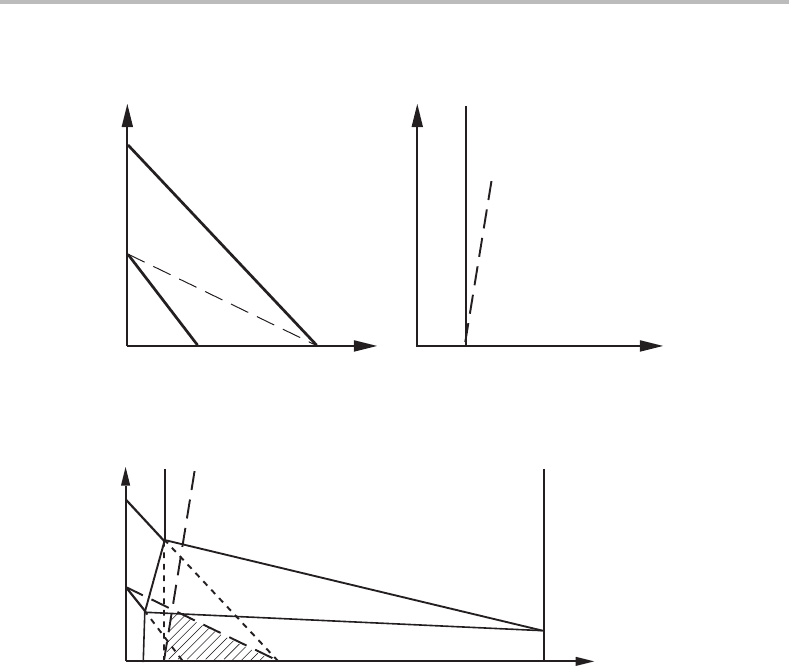
Figure 14.18 The critical limit for the quasi-paratransformation of γ to (a) α; and (b) cementite in
an Fe–M–C alloy.
α
γ
γ+cementite
α+γ+cementite
α+cementite
α+γ
cementite
u
M
u
C
Figure 14.19 Phase diagram for an Fe–M–C system, illustrating the conditions for a rapid
formation of α + cementite (so-called pearlite) under quasi-paraequilibrium (see shaded
triangle).
γ/cementite equilibrium. The position of the region for rapid precipitation of cementite,
relative to the full equilibrium phase boundary, has been drawn in agreement with the
general rule that the paraequilibrium phase boundaries lie inside the full equilibrium
two-phase field.
If the alloy is at a temperature where the γ → α + cementite transformation is pos-
sible, the two two-phase regions α + γ and γ + cementite must overlap to some degree
and there must be a three-phase α + γ + cementite region, as illustrated in Fig. 14.19.
This phase diagram resembles the Fe–Ni–C phase diagram because Ni prefers to
dissolve in γ rather than in α or cementite. The metastable parts of the phase boundaries
have been drawn with dotted lines. The two critical lines form a small triangular region
which we may regard as a critical triangle. Rapidly growing quasi-parapearlite can be
expected to form from a γ phase situated inside the critical triangle. A γ phase situated
to the left of the triangle should first precipitate so-called proeutectoid α and thus move
into the triangle where pearlite can start forming. A γ phase situated to the right of
the triangle should first precipitate proeutectoid cementite and then pearlite. However, a
requirement is that the level of alloy content falls below the top of the triangle. Otherwise,

14.7 Transformations in steel under partitioning of alloying elements 319
the rapid, proeutectoid precipitation will stop at its critical line. The transformation can
continue only by the slow rate of diffusion of the alloying element into the interior of the
austenite because both α and cementite must dispose of nickel into the parent γ. That
should normally be a very slow reaction and pearlite formed under such conditions has
not been reported. In solidification, the growth conditions for a eutectic may be similar
but the rate of diffusion in the liquid phase is so rapid that the eutectic reaction is not
inhibited above the triangle for the rapid eutectic transformation.
Exercise 14.6
An Fe–Ni alloy with u
Ni
= 0.01 is first in the state of γ (fcc) at 1273 K but then it
is carburized and cementite (Fe
3
C) forms isothermally. Suppose one would like to try
to produce homogeneous cementite with u
Ni
= 0.01. Estimate what carbon activity is
required during the carburization. It is known that the γ + cementite equilibrium in the
binary Fe–C system is 1.01 at 1273 K. It is also known that the distribution coefficient
for Ni between cementite and γ is K
cementite/γ
Ni
= 0.26.
Hint
Inspiration can be obtained from Fig. 14.18(b) because formation of cementite with the
initial Ni content can only occur under a rapid reaction. However, in order to calculate
the necessary carbon activity we can go to Section 11.3. The carbon content of cementite
is u
C
= 1/3 and for γ it is about 0.073.
Solution
If the growing cementite has u
Ni
= 0.01 and there is local equilibrium (quasi-
paraconditions) then the adjoining γ has u
Ni
= 0.01/0.26 = 0.038. Equation (11.23)
gives ln(a
C
/a
binary
C
) =(1 −0.26)/[(1/3) −0.073] ×0.038 =0.108; a
C
=1.01 ×1.114 =
1.125. The carbon activity must be higher than this value.
14.7 Transformations in steel under partitioning of alloying elements
In the preceding section we concluded for Fe–Ni–C that, for Ni contents falling above the
top of the critical triangle, pearlite could grow only by nickel diffusing into the remaining
γ because both growing phases, α and cementite, require that the nickel content is lower
than in the adjoining γ. The situation will be quite different if one of the phases, α or
cementite, can grow with a higher alloy content than the adjoining γ. Then it would be
sufficient that the alloying element diffuses side-wise and distributes itself between the
two growing phases. This process can take place with an observable speed by diffusion
inside the pearlite/γ interface. Interfacial diffusion can be orders of magnitude faster
than volume diffusion.
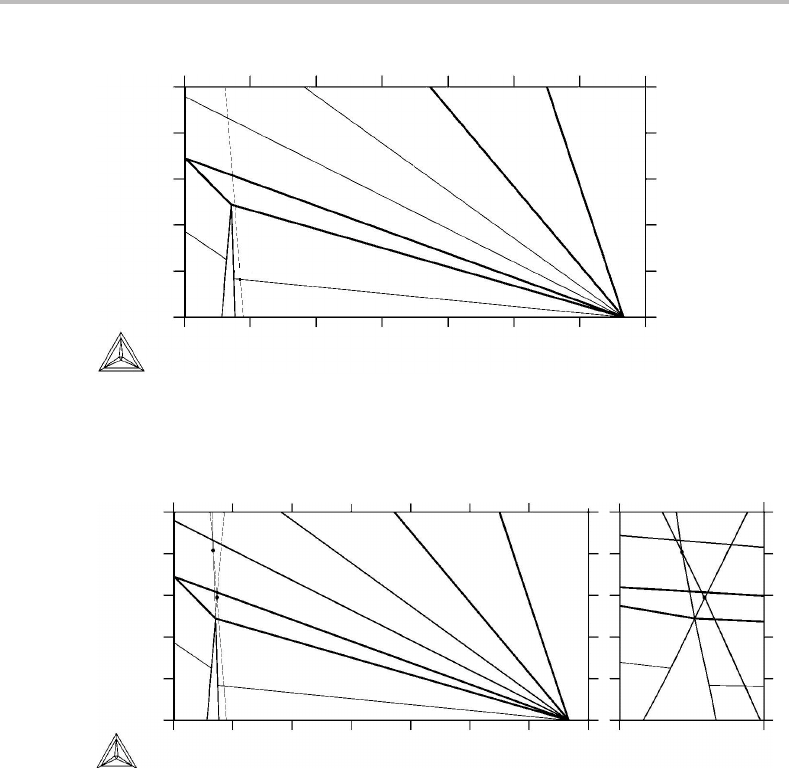
320 Partitionless transformations
0.05
0.04
0.03
0.02
0.01
0.05 0.10 0.15 0.20 0.25 0.30 0.35
0
0
α + γ
α+cementite
γ+cementite
u
C
u
Si
+
Figure 14.20 See Exercise 14.7.
0.05
0.04
0.03
0.02
0.01
0
0 0.05 0.10 0.15 0.20 0.25 0.30 0.025 0.045
γ+cementite
+
+
γ
α+cementite
α+γ
u
C
u
C
u
Si
(a) (b)
Figure 14.21 Solution to Exercise 14.7.
Examples of alloying elements allowing pearlite to grow under partitioning between
α and cementite are manganese and silicon. Cementite will attract manganese and α will
attract silicon.
Exercise 14.7
Suppose a steel is first transformed to homogeneous γ at a high temperature and then
cooled to 1023 K where it is represented by the cross in the Fe–Si–C phase diagram
(Fig. 14.20). The thin line is an isoactivity line for γ going through the cross. Examine if
there would be a positive difference in Si content inside γ, u
γ
Si
,todrive the redistribution
of Si between α and cementite and thus allow γ to transform to pearlite. If that is the
case, evaluate the fractions of α and cementite in that pearlite.

14.7 Transformations in steel under partitioning of alloying elements 321
Hint
First find the intersections with the isoactivity line and the extrapolated phase boundaries
for γ. Then try to find the other end-points on the corresponding tie-lines.
Solution
The construction is shown in Fig. 14.21(a) and again, with an expanded u
C
scale, in
(b). The intersection of the isoactivity line with the two phase boundaries will give the
compositions of γ at the interfaces to α and cementite if there is full local equilibrium.
The difference u
γ
Si
= u
γ/cementite
Si
− u
γ/α
Si
is positive and will thus make silicon diffuse
away from cementite to α.Pearlite can thus grow but it must have the same average
Si content as the initial γ, u
Si
= 0.01. Since cementite has practically no Si and α has
about 0.035, we find that the fraction of α is 0.01/0.035 = 0.3. The fraction of cementite
is thus 0.7 and the carbon content of this pearlite is extremely high. It requires a large
supply of carbon to be drawn from the interior of the γ phase.

15
Limit of stability and critical
phenomena
15.1 Transformations and transitions
In Chapter 12 we were mainly concerned with the question whether a transformation
is sharp or gradual. The difference between those cases is very practical and straight-
forward. It is based on a one-dimensional phase diagram where the only axis represents
the quantity that is being varied. If that diagram shows a two-phase field of some exten-
sion between the two one-phase fields, then the transformation between the two phases
will be gradual. If the two-phase field has no extension, then the transformation will be
sharp. For a unary system with a transformation this will happen if one varies a potential,
e.g. T. The Gibbs energy is a continuous function of T across the sharp transformation
but its derivatives, yielding S and V, show discontinuous jumps. This is why the phase
boundaries separate when a molar axis is introduced (see Figs 9.1 and 9.2). In other
cases there is no such separation because the first-order derivatives are zero. A typical
example is found in a ferromagnetic substance, which gradually loses its magnetization
as the temperature is increased. At the Curie temperature it reaches zero and the sub-
stance has thus become paramagnetic. There is no temperature where ferromagnetic and
paramagnetic regions coexist in a pure substance, not even if one varies a molar quantity.
As a consequence, there is not really a two-phase field between the two one-phase fields
and this fact is indicated by the use of a dashed line to separate the one-phase fields
(see Fig. 15.1 where the two phases are denoted by β and β
in order to emphasize their
close relationship). If there is no discontinuous jump in the first-order derivatives but
there is one in the second-order derivatives, then one calls this a second-order transi-
tion as distinguished from a first-order transition when there is a jump in the first-order
derivatives.
When considering a T, P diagram and calculating the locus of a sharp transformation
between two phases in Section 8.3,weapplied the Gibbs–Duhem relation to each of
the phases, obtaining two equations for dµ
A
.Byrequiring that µ
A
must change in the
same way for the two phases along their line of coexistence, it was possible to calculate
the slope of that line, (see Fig. 8.6). In this way dP/dT was obtained as a function of
discontinuous jumps in the two first-order derivatives, S and V . This method does
not work in the present case because the two equations become identical on the line we
want to calculate, and S and V both go to zero there. This problem was solved by
Ehrenfest [27]who instead used the fact that on the line there is no difference between
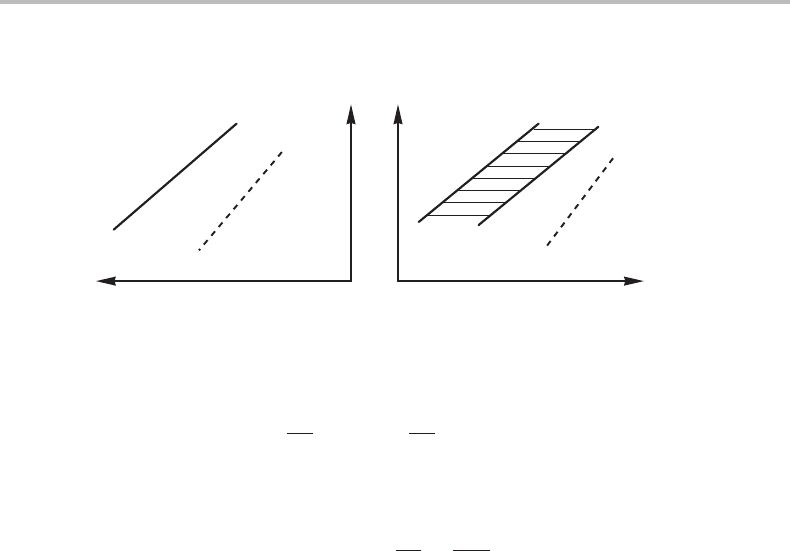
15.1 Transformations and transitions 323
P
V
γ
γ
β
β
β′β′
T
(a) (b)
Figure 15.1 Characteristics of a second-order transition β ↔ β
. The γ ↔ β transition is first
order.
V of the two states. The expression for dV,
dV =
∂V
∂T
P
dT +
∂V
∂ P
T
dP = V α dT − V κ
T
dP, (15.1)
must give the same value in both states. Because V itself also has the same value in both
states but α and κ
T
do not, we get, by taking the difference between the two states,
dP
dT
=
α
κ
T
(15.2)
It is also possible that the discontinuous jumps first occur in the third-order deriva-
tives of G and the corresponding transition would be of third order, etc. In practice, it
is often difficult to decide by experimental measurements whether α and κ
T
differ
from zero, as they should for a second-order transition. Sometimes the individual values
of the second-order derivatives appear to go to infinity at the transition point and it is
not meaningful to try to evaluate their difference. Ehrenfest’s expression for dP/dT is
then of little practical use. Of course, it can be used when one investigates a particular
model that gives definite values for second-order derivatives. In view of these complica-
tions, it is common to call all transitions with continuous first-order derivatives, second
order.
It should be emphasized that nothing really happens in a system when it passes a
second-order transition point. It does not really transform. The only difference is that
it starts behaving in a new way. The real changes in the system occur gradually as the
system moves away from the point of transition. In that sense, the second-order transition
is just the start of a gradual transformation. Figure 15.2 illustrates different possibilities
for a pure substance at constant pressure. The internal variable ξ is some measure of the
arrangements of the atoms or electrons. Curve (a) with its discontinuous jump represents
a first-order transition. Curve (b) shows a discontinuous jump but also a gradual change.
In curve (c) the jump has disappeared but the curve is horizontal at the break point. In
curve (d) the curve never turns horizontal.
The words transformation and transition are often used as synonyms. One word is
favoured by the experts in some fields and the other word in other fields. There is a need
for two words with different meanings. For our purposes, it would seem most natural
to use transition in much the same way as above but use transformation to describe the
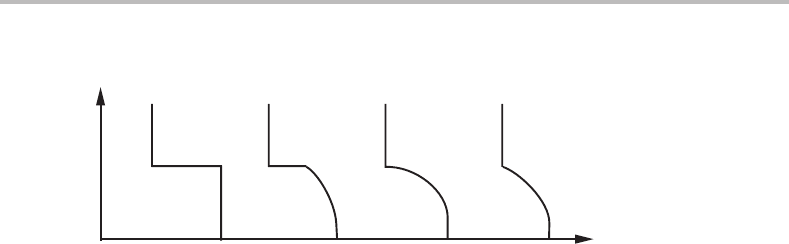
324 Limit of stability and critical phenomena
T
ξ
(a) (b) (c) (d)
Figure 15.2 Different cases of transitions and transformations. (a) A first-order transition that
occurs with a sharp transformation. (b) A first-order transition where the transformation is
partly sharp, partly gradual. (c) and (d) Second-order transitions with completely gradual
transformations.
progress of the real changes occurring in a system. The break points in all the curves in
Fig. 15.2 would thus be regarded as the transition point and the transition is the change
occurring at the transition point, whether it is a real change or a change in behaviour
that will reveal itself as the system moves away from the transition point. In curve (a)
the first-order transition yields a sharp transformation if T, being a potential, is varied.
In curve (b) part of the transformation would be sharp and occur at the transition point,
and another part would be gradual and occur below the transition point. In curves (c)
and (d) the transformation would be completely gradual and start as the transition point
is crossed. Thus, a transition point yielding a transformation that is at least partly sharp,
would be of first order. Otherwise, it would be of second order. It should be emphasized,
however, that there are many cases where the concept of first- and second-order transition
does not appear to be very useful. This will be demonstrated in Section 15.4.
The strict difference between first-order and second-order transitions is of consider-
able theoretical interest but from a practical point of view it may sometimes be of less
importance whether there is a small discontinuous jump or no jump at all.
In the previous chapters, only the word transformation has been used. From now on,
an attempt will be made to apply both terms and with the definitions given here. In view
of the conclusion drawn in Section 12.2,asharp transformation, which must always be
a first-order transition, will turn gradual when the variable is changed from a potential
to a molar quantity. From the theoretical point of view this is a trivial effect and should
not affect the classification of the transition. The theoretical study of phase transitions is
thus carried out without involving any molar quantity.
A phase transition is often caused by a tendency of an ordered arrangement to disorder.
Such transitions are called order–disorder transitions and the driving force comes
primarily from the increasing configurational entropy. In other cases, the cause may
be the lowering of the energy by deformation of the structure, e.g. by decreasing the
tetragonality, without changing the configurational entropy. Such transitions are called
displacive transitions.Ofcourse, the characteristics of order–disorder and displacive
can be applied to the corresponding transformation, as well. In both cases, the progress
of the change can be expressed by some internal variable, e.g. the degree of long-range
order or the tetragonality. For simplicity, all such variables are sometimes called ‘order
parameters’ and, in principle, all internal variables could play this role.
