Higman Chris Gasification (Газификация угля)
Подождите немного. Документ загружается.

30
Gasification
3.1.1 Devolatilization
The first step, heating up of the coal particles, is in one sense the simplest part of the
process. Nonetheless, the speed at which it takes place has an influence on the
subsequent steps, so that it is of great importance in any accurate model.
Devolatilization takes place already at low temperatures (350–800°C) and in parallel
with the heating up of the coal particles. The rate of heating of the coal particles
influences the way in which the devolatilization takes place (Jüntgen and van Heek
1981, p. 65). The rate of devolatilization is dependant not only on the rate of heating,
however, but also on the particle size and the rate of gasification by the water gas
reaction, and hence on the reaction temperature and the partial pressure of steam.
The interplay between pyrolysis and gasification under different heating conditions
is shown in Figure 3-2. If the heating up is slow then the pyrolysis reactions set in
from about 350°C. The gasification reaction of both volatiles and char with steam is
very slow at this temperature. The concentration of volatiles outside the particle
increases rapidly, and gasification only sets in after devolatilization is complete. If,
however, the rate of heating is high, then both pyrolysis and gasification take place
simultaneously, so that a high concentration of volatiles is never allowed to build
up. This is one reason why high-temperature entrained-flow reactors produce a clean
gas in such a short time. Compare this with a counter-current moving-bed process
where lump coal is used. The heating up rate is slow and a high volatiles concentration
is built up and removed unreacted from the reactor by the syngas.
For finely pulverized coal particles at high temperature the residence time is
very short (10–200 ms) (Smoot and Smith 1985, p. 55 ff). The extent of devolatil-
ization is highly dependant on the final temperature and can vary considerably
from that found by performing a proximate analysis in accordance with ASTM
(American Society for Testing and Materials), DIN (Deutsches Institut für Normung),
or other standard methods. The product distribution of the devolatilization process
also varies significantly with changes in the pyrolysis temperature and the speed
of heating up.
Although devolatilization processes during gasification and combustion are
thought to be generally similar, the fact that many gasification processes operate at
SOLID
CARBONACEOUS
MATERIAL
(COAL, BIOMASS)
CO, H
2
, CH
4
, CO
2
,
H
2
O
CO, H
2
, CH
4
, CO
2
,
H
2
O
AND CRACKING
PRODUCTS
(
CRACKING, REFORMING,
COMBUSTION, CO SHIFT)
GAS PHASE REACTIONS
OXYGENATED
COMPOUNDS
(PHENOLS, ACID)
TAR, OIL, NAPHTHA
PYROLYSIS GASES
(CO, H
2
, CH
4
, H
2
O,
etc.)
PYROLYSIS
(GASIFICATION,
COMBUSTION, CO SHIFT)
CHAR-GAS REACTIONS
CHAR
Figure 3-1
Figure 3-1Figure 3-1
Figure 3-1. Reaction Sequence for Gasification of Coal or Biomass (Source:
Adapted from Reimert and Schaub 1989)

The Kinetics of Gasification and Reactor Theory
31
higher pressures has to be taken into account. The weight loss due to devolatilization
can be of the order of 10% less at typical gasifier pressures of 30 bar.
3.1.2 Volatiles Combustion
The devolatilization of coal produces a variety of species, including tars, hydrocar-
bon liquids, and gases, including methane, CO, CO
2
, H
2
, H
2
O, HCN, and so forth.
(Smoot and Smith 1985). This material reacts with the oxidant surrounding the coal
particle. The extent to which the oxidant is completely or only partially depleted
depends on the amount of volatiles produced.
In a combustion environment, where there is an overall excess of oxygen, the
combustion of the volatiles is complete. In a gasification environment, this is not
necessarily the case, especially where the coal has a high volatiles content. There is a
recirculation of synthesis gas in many gasification reactors, not only in fluid-bed but
also to some degree in entrained-flow reactors. To the extent that this occurs in the
vicinity of the burner, the effects are very different from the combustion situation.
Recirculated combustion flue gas consists mainly of carbon dioxide, water vapor
and (in the air-blown situation) nitrogen. The carbon dioxide and water vapor have
COAL
SLOW
TEMPERATURE
RISE
PYROLYSIS &
GASIFICATION
GASIFICATION
PYROLYSIS
(SOFTENING)
PYROLYSIS
PRODUCT
GASIFICATION
AGENT
HEAT FLOW
GASIFICATION
PRODUCT
FAST
Figure 3-2.
Figure 3-2.Figure 3-2.
Figure 3-2. Influence of Heating Rate on Gasification Process (Source: Jüntgen
and van Heek 1981)
32
Gasification
a moderating effect, thus reducing temperature. In the case of gasification the
recycled gas contains significant quantities of carbon monoxide and hydrogen (up to
90% for an oxygen-blown gasifier) and will cause locally very high temperatures,
should it come into contact with the oxidant.
There is not much kinetic data available on volatiles combustion. It is, however,
clearly established that this process, being a reaction between gases, is much more
rapid than the heterogeneous char gasification where mass transport limitations play
a more important role.
3.1.3 Char Gasification
The slowest reactions in gasification, and therefore those that govern the overall
conversion rate, are the heterogeneous reactions with carbon, namely the water gas,
Boudouard, and hydrogenation reactions already discussed in Chapter 2. The rates
of reaction for the water gas and Boudouard reactions with char are comparable and
are several orders of magnitude faster than for the hydrogenation reaction (Smoot
and Smith 1985, p. 79).
There are several different models describing the Boudouard and water gas reactions
(Williams et al. 2000). A widely utilized model for the Boudouard reaction is attribu-
table to Ergun (1956) and proposes a two-step process.
Step 1 C
fas
+CO
2
C(O) +CO
Step 2 C(O) → CO + C
fas
In the first step, CO
2
dissociates at a carbon free active site (C
fas
), releasing carbon
monoxide and forming an oxidized surface complex (C(O)). In the second step the
carbon-oxygen complex produces a molecule of CO and a new free active site. The
rate limiting step is the desorption of the carbon-oxygen surface complex.
The model for the water gas reaction is basically similar:
Step 1 C
fas
+H
2
OC(O)+H
2
Step 2 C(O) → CO + C
fas
In this case the first step is the dissociation of a water molecule at a carbon-free
active site (C
fas
), releasing hydrogen and forming an oxidized surface complex
(C(O)). In the second step, the carbon-oxygen complex produces a molecule of
CO and a new free active site. In some models the rate-limiting step is the desorption
of the carbon-oxygen surface complex as for the Boudouard reaction. Other models
include the possibility of hydrogen inhibition by the inclusion of a third step:
Step 3a C
fas
+H
2
C(H)
2
←
→
←
→
←
→
The Kinetics of Gasification and Reactor Theory
33
or
Step 3b C
fas
+½ H
2
C(H)
whereby some of the sites can become blocked by hydrogen.
Fundamental work continues in this field to develop a detailed understanding of
the mechanisms of gasification reactions (Williams et al. 2000, p. 237).
Rate of Reaction. For the Boudouard reaction (2-4),
C + CO
2
2CO
being an equilibrium reaction, the reaction rate of carbon conversion,
(3-1)
is assumed to be proportional to the concentration of CO
2
in the gas, so that
(3-2)
where k
m
is the mass-related reaction-rate constant,
c
CO
2
the concentration of CO
2
in
the gas and the order of the reaction may be assumed to be 1.
The temperature dependency of the rate constant can be expressed in Arrhenius
form as
(3-3)
where
A
is a pre-exponential factor and
E
is the activation energy for the reaction.
This can be expressed alternatively as
(3-4)
which provides a convenient form for comparing the reactivities of different
chars.
Comparison of Different Types of Solid Feedstocks. The reactivity of different
coals and chars depends on a number of factors, in particular
• The porosity of the coal, that is, its inner structure, surface, and active sites.
• The crystal structure of the fixed carbon.
• Catalytic effects of ash components in the coal.
Young (low-rank) coals such as browncoal have a large specific surface and thus a
high reactivity. On the other hand, older coals, particularly anthracitic coals, have a
poor reactivity. Reactivity is enhanced by alkalis, particularly potassium.
←
→
←
→
r
m
dC
dt
-------
=
r
m
k
m
c
co
2
⋅=
k
m
Ae
E–
RT
-------
⋅=
k
m
()ln
E
R
---
–
1
T
---
⋅
A
()ln+=

34
Gasification
In a systematic review Bürkle (1998) has plotted the reactivity of different chars
from various biomasses, coals, and other materials, as shown in Figure 3-3.
Effective Reactivities. In all cases it is necessary to distinguish between the physical
and chemical steps involved and which effects control the measurable rate of reaction
in different temperature zones.
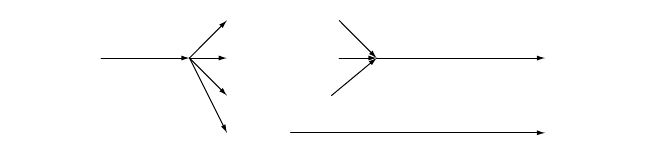
Zone I, the low-temperature zone, in which the chemical reaction is the rate-
controlling step and the experimentally observed activation energy is the
true activation energy.
Zone II, a medium-temperature zone, in which the rate of chemical reaction is
higher, but is limited by internal diffusion of the gaseous reactants
through the pores of the individual particles. The observed activation
energy is only about half the true value.
Zone III, a high-temperature zone in which external, bulk surface diffusion of the
gaseous reactants is rate controlling and the apparent activation energy is
very small.
This is illustrated in Figure 3-4.
Data for coal gasification is presented in Figure 3-5, from which it can be
concluded that in any solid-fuel gasification process the progress of the reaction is
determined by the mass transfer phenomena.
Another indication of the importance of mass transfer is given in Figure 3-6
where the time required for the gasification of a solid fuel is plotted as a function of
the particle size.
−11
−9
−7
−5
−3
−1
1.121.071.020.970.920.870.820.770.72
BLACK COAL
ln(r
m
) [.]
1/ T [K
−1
]
BLACK LIQUOR
CHARCOAL
LIGNITE
BIOMASS
PETCOKEPVC CHAR
CARBON BLACK
600
70080090010001100
T[°C]
Figure 3-3.
Figure 3-3.Figure 3-3.
Figure 3-3. Reactivity of Various Materials as a Function of Temperature (Source:
Bürkle 1998)
The Kinetics of Gasification and Reactor Theory
35
3.2 REACTOR THEORY
Classical chemical engineering theory teaches us about a variety of idealized reactor
types. The oldest type of chemical reactor is the batch reactor, of which the coke
oven is currently the only example that is relevant to gasification (Thoenes 1994;
REACTION
RATE
1/T [K
−1
] (PARTICLE TEMPERATURE)
BULK SURFACE
DIFFUSION
PORE
DIFFUSION
LOG (REACTION RATE)
IIIIII
Figure 3-4.
Figure 3-4.Figure 3-4.
Figure 3-4. Effective Reaction Rates in Temperature Zones
0.01
0.1
1
10
100
500 700 900 1100 1300 1500
Temperature [°C]
Overall reaction rate
Mass transfer
Overall rate
Chemical reaction
Figure 3-5.
Figure 3-5.Figure 3-5.
Figure 3-5. Overall Gasification Reaction Rate as a Function of Temperature
(Source: Hedden 1961 with permission from Elsevier)

36
Gasification
and Westerterp, van Swaaij, and Beernackers 1987). In the coke oven a batch of
coal is indirectly heated via the side walls in a relatively flat vertical oven where
devolatilization takes place. Such a batch process can also be made semicontinuous,
as is the case in a coal stove for domestic use and a moving-bed gasifier (see Section
5.1), or fully continuous, as in large-scale grate-type furnaces as applied in power
stations and industry. For the coal or other solid fuel the fully continuous version is
a typical example of a so-called plug flow reactor.
Plug Flow Reactors. The idealized plug flow reactor (PFR) is characterized by the
following properties:
• There is a continuous flow through the reactor.
• There are no radial gradients.
• There is no axial mixing, that is, there is no exchange of material or heat.
• In addition, a plug flow reactor will generally be operated in a steady state manner.
For a first-order kinetics and the generalized component A, equations 3-1 and 3-2
can be expressed as:
(3-5)
which after integration yields for the concentration after time
t
:
(3-6)
and for the conversion of component
A
:
(3-7)
These reactions also hold for a batch reactor.
0.01 0.1 1 10 100
Particle size [mm]
Residence time [s]
1
10
100
1000
10000
Figure 3-6.
Figure 3-6.Figure 3-6.
Figure 3-6. Time Required for the Gasification of a Solid Fuel as a Function of
Particle Size
dc
A
dt
--------
–
kc
A
=
c
A
c
A 0,
---------
e
kt–
=
c
A 0,
c
A
–
c
A 0,
-------------------- -
1
e
kt–
–=
The Kinetics of Gasification and Reactor Theory
37
Continuously Stirred Tank Reactors. The ideal continuously stirred tank reactor
(CSTR) has the following characteristics:
• There is a continuous flow through the reactor.
• The contents of the reactor are ideally mixed; thus the conditions are the same at
all points in the reactor. This implies that the effluent has the same composition as
anywhere else in the reactor. In the CSTR the mixing time should be about two
orders of magnitude lower than the average residence time to achieve this.
When we set the average residence time equal to τ we derive the relation from for-
mula 3-5 for a first-order reaction at constant density:
(3-8)
and so:
(3-9)
For the conversion of component
A
we then get:
(3-10)
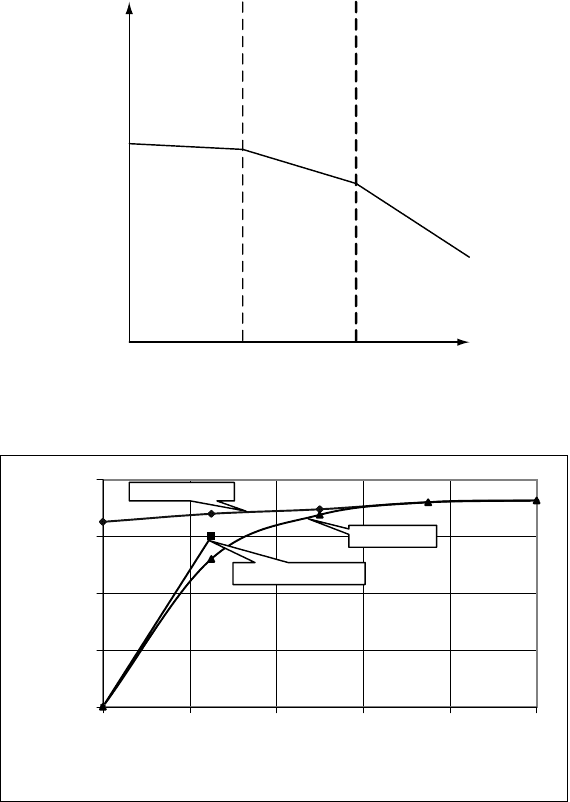
Equations 3-9 and 3-10 show that increasing the residence time τ will increase the
conversion. In some cases, this can lead to impractically large reactors. In order to
avoid this the single CSTR can be replaced by two or more CSTRs in series, which
each have the same volume and residence time, but that have a combined average
residence time and volume that is equal to the single stage CSTR. For N stages we
then obtain:
(3-11)
As N→∞, this yields equation 3-6. An infinite number of CSTRs hence gives the
same results as a PFR with the same overall residence time. In practice a number of
about four CSTRs already gives results that approximate a PFR with the same
residence time, provided the conversions required are not excessive. In Figure 3-7,
some comparative results are given for various values of N.
The result that a large number of CSTRs gives the same result as a PFR can easily
be understood when we consider that in a single stage CSTR there is a small chance
that reactants leave the reactor without having the time to react. Adding a second
CSTR diminishes this chance, although the overall residence time remains the same.
Adding subsequent CSTRs virtually eliminate the chance of “short circuiting” of
reactants, and for the conversions given in Figure 3-7 we see that the results of the
PFR can hardly be distinguished from that of eight CSTRs in series having the same
overall residence time.
c
A 0,
c
A
–
kc
A
τ=
c
A
c
A 0,
---------
1
k
τ 1+
-------------- -
=
c
A 0,
c
A
–
c
A 0,
-------------------- -
k
τ
k
τ 1+
-------------- -
=
c
AN,
c
A 0,
----------
1
k
τ
N
--- -
⋅+
N–
=

38
Gasification
3.3 APPLICATIONS TO REACTOR DESIGN
If we include coke ovens as a typical batch reactor in our definition of gasification,
then practically all of these idealized reactor forms have been applied at some time
or other in the search for an optimum gasifier design. Of course, what is optimum in
any particular case is heavily dependant on the application in hand (chemical or
power), the nature of the feedstock, the size of the plant, and a number of other
factors as well as the classical trade-off between investment and operating costs. It is
therefore not surprising that representatives of most reactor forms still find commer-
cial application today. The various basic gasification processes are discussed against
this background and that of the basic theoretical models of PFR and CSTR in
Chapter 5, where the implications for some of the commercial processes are also
shown.
3.3.1 Modeling
The systems described in this chapter are extremely complex, which has to date
placed limitations on the application of kinetic models to commercial gasification
reactor design. Nonetheless “the availability of increasingly powerful computers with
a deeper understanding of the physical and chemical processes of coal combustion
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
024 68
kτ
C
AN
/C
A,0
N=1
N=2
N=4
N=8
PFR
1
3
5
7
Figure 3-7.
Figure 3-7.Figure 3-7.
Figure 3-7. Relative Conversion as a Function of the Dimensionless Time kτ
The Kinetics of Gasification and Reactor Theory
39
now enables the development and use of greater sophistication in the models
employed” (Williams et al. 2002). In particular, applications are emerging where
their incorporation into computational fluid dynamics (CFD) is proving to be of
practical material benefit.
The most important practical applications to date from the knowledge of actual
coal kinetics are in the field of combustion, particularly in the field of NO
x
pre-
diction. However, although CFD already has an important place as a design aid in
describing the combustion of coal in utility furnaces, increasing demands are
being made to provide quantitative rather than qualitative results (Williams et al.
2002).
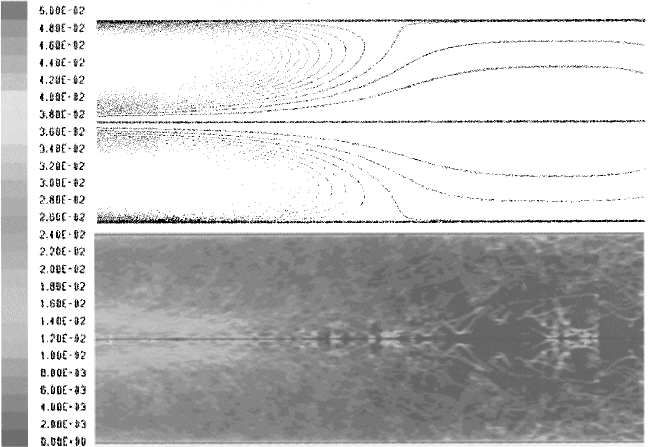
Progress is now being made in applying CFD to gasification. In their discussion
of the application of CFD to burner developments for Lurgi’s MPG oil gasification
burner, Hofmockel, Liebner, and Ulber (2000) describe including kinetic models for
bulk and pore diffusion. (This is further detailed in Ulber, 2002). An example from
their results of CFD is given in Figure 3-8.
Bockelie et al (2002) have presented initial results for various entrained-flow coal
gasifiers, illustrating, for example, performance variations when using different
feedstocks. This work is part of an ongoing program to investigate generic improve-
ments for the operation and design of such gasifiers.
The complexity of kinetic calculations ensures that any practical quantitative
results are only likely to be accessible via CFD and will therefore remain the realm
Figure 3-8.
Figure 3-8.Figure 3-8.
Figure 3-8. CFD Model of a Gasification Burner Outlet Zone (Source: Hofmockel,
Liebner, and Ulber 2000)
