Higman Chris Gasification (Газификация угля)
Подождите немного. Документ загружается.

230
Gasification
van Liere, J., Bakker, W. T., and Bolt, N. “Supporting Research on Construction Materials and
Gasification Slag.” Paper presented at VGB (Technische Vereinigung der Großkraftwerkstbe-
treieber) Conference, “Buggenum IGCC Demonstration Plant,” Maastricht, November 1993.
van Loon, W. “De vergassing van koolstof met zuurstof en stoom.” (The gasification of
carbon with oxygen and steam). Ph.D. diss., Delft University, 1952.
Visconty, G. J. “Method of Transporting Powder into Advanced Pressure Zone.” U.S. Patent
2,761,575, 1956.
Weigner, P., Martens, F., Uhlenberg, J., and Wolff, J. “Increased Flexibility of Shell Gasification
Plant.” Paper presented at IChemE Confernce, “Gasification: The Clean Choice for Carbon
Management,” Noordwijk, April 2002.
Weiss, M. M. “Selection of the Acid Gas Removal Process for IGCC Applications.” Paper pre-
sented at IChemE Conference, “Gasification Technology in Practice,” Milan, February 1997.
Wildermuth, E., Stark, H., Ebenhöch, F. L., Kühborth, B., Silver, J., and Rituper, R. “Iron
Compounds” In Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed., vol. A14, p. 596.
Weinheim: VCH Verlagsgesellschaft, 1990.
Wilhelm, S. M. “Effect of Mercury on Engineering Materials Used in Ammonia Plants.”
Paper presented at AIChE Ammonia Plant Safety Symposium, San Diego, 1990.
231
Chapter 7
Applications
7.1 CHEMICALS
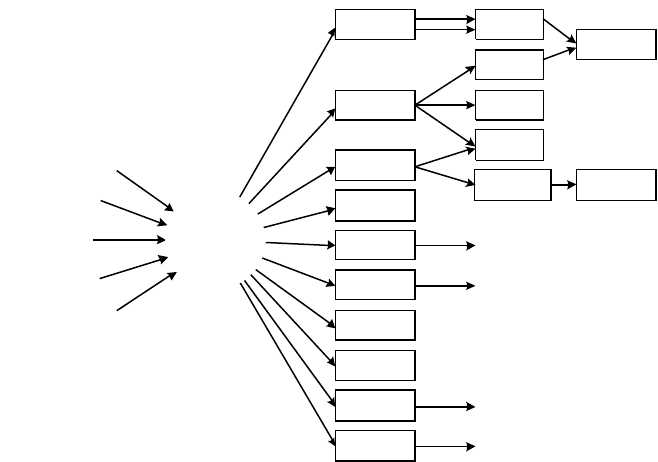
The two chief components of synthesis gas, hydrogen and carbon monoxide, are the
building blocks of what is often known as C1 chemistry. The range of products
immediately obtainable from synthesis gas extends from bulk chemicals like ammonia
and methanol, through industrial gases, to utilities such as clean fuel gas and
electricity. Furthermore, there are a number of interesting by-products, such as CO
2
and steam. As can be seen from Figure 7-1, many of these direct products are only
intermediates toward other products closer to the consumer market, such as acetates
and polyurethanes.
Synthesis gas is an intermediate that can be produced by gasification from a wide
range of feedstocks and can be turned into an equally wide range of products. And
although every combination of gasifier feed and end product is technically possible,
this does not mean that every combination makes economic or even technical sense.
In North Dakota, synthesis gas generated from coal is successfully processed to
manufacture synthetic natural gas (SNG). In Malaysia, partial oxidation of natural
gas is used to generate the synthesis gas feed for a synthetic liquid fuels operation.
Yet it would clearly make no sense to generate synthesis gas by partial oxidation of
natural gas to manufacture SNG.
Given that this broad range of products is available from the single intermediate
of synthesis gas, there is no technical reason why one could not produce more than
one product from the same gas source. In fact, many operators of gasification plants
do precisely this. This is known, in an analogy with co-generation (electricity and heat),
as polygeneration. Some even go a step further and install surplus downstream cap-
acity compared with the available syngas generation capacity. In this manner, such
operators are able to “swing” production from one product, say ammonia, to
another, say methanol, or peaking power in accordance with market demand and are
thus in a position to optimize revenue from the gasification plant. In a reverse manner,
there are other operators using different feedstocks, and even where appropriate differ-
ent technologies, to generate their syngas. In such a case, the opportunity is to work
with the cheapest feedstocks, topping up with more expensive ones only as required.
This inherent flexibility associated with syngas production and use provides a
multitude of choices that is increased by the variety of utility systems, in particular
232
Gasification
the broad possibilities for steam system configuration. It is therefore useful to look
at some typical gas processing designs for a number of the commoner applications
and review the considerations behind them.
7.1.1 Ammonia
Market
Over 90% of the world’s ammonia production capacity of 160 million t/y in 2001 is
based on steam reforming of natural gas or (in India) naphtha. Almost all the rest,
some 10 million t/y, is based on gasification of either coal or heavy oil.
The worldwide production of ammonia is by most measures the largest of any bulk
chemical. The principle use of ammonia is as nitrogenous fertilizer for agriculture.
Typical plant sizes today are 1500–2000 t/d. Process licensors are currently
revealing plans for plants up to 4000 or 5000 t/d size (Davey, Wurzel, and Filippi
2003; Parkinson 2001).
Synthesis Gas Specification
Ammonia synthesis takes place at high pressure over a catalyst that is usually iron,
although one process uses ruthenium according to the reaction:
AMMONIA
METHANOL
CARBON
MONOXIDE
GAS
TURBINES
REDUCTION
GAS
TOWN GAS
SNG
HYDROGEN
OXO
ALCOHOLS
FISCHER
TROPSCH
UREA
FORMAL-
DEHYDE
RESINS
MTBE
ACETIC
ACID
PHOSGENE
POLY-
URETHANE
DETERGENTS, PLASTICIZERS
FUELS, WAXES, OTHERS
COAL
LIQUID
RESIDUES
NATURAL GAS
BIOMASS
WASTE
METALS
ELECTRIC POWER
GASIFICATION
NH
3
CO
2
Figure 7-1.
Figure 7-1.Figure 7-1.
Figure 7-1. Applications for Synthesis Gas
Applications
233
N
2
+3 H
2
2NH
3
−92 MJ/kmol– N
2
(7-1)
A typical specification for ammonia synthesis gas is (Mundo and Weber 1982):
Ammonia plants based on gasification technologies normally surpass these specifi-
cations when a liquid nitrogen wash is the last stage of purification.
Most ammonia plants are built in conjunction with urea plants, the CO
2
from the
ammonia plant being used directly for urea production. According to the reaction
2 NH
3
+CO
2
NH
2
CONH
2
+H
2
O (7-2)
one mole of CO
2
is required for every two moles of ammonia. Typical requirements
for the CO
2
are as follows:
CO
2
>98.5 mol%
H
2
S + COS <2 mg/Nm
3
H
2
<0.15mol%
Methanol <10 ppmv
Design Considerations
To process a raw synthesis gas to conform to this specification a number of different
tasks must be completed:
• Tar and volatiles removal (if present in raw gas)
• Desulfurization
• CO shift (CO +H
2
O = >H
2
+CO
2
)
• CO
2
removal
• Final removal of carbon oxides and water
• Adjustment of N
2
:H
2
ratio
N
2
:H
2
1:3 (For some modern processes nitrogen excess is
required)
CO + CO
2
<30 ppmv (As sum of total oxygen containing species)
H
2
O (in principle as for CO and CO
2
, but it can be
washed out with product ammonia in the synthesis
unit itself)
Sulfur <1 ppmv
Phosphorus, Arsenic,
Chlorine
(These are also poisons. Appl [1999] gives an
upper limit of 0.1 ppm for chlorine)
Inerts (including
methane)
<2%
minimum
←
→
←
→
234
Gasification
The order in which these tasks are performed may be modified to the extent that
there is a choice between performing the desulfurization before or after the CO shift.
Whereas older plants may have used a copper liquor wash for CO removal, most
modern gasification-based ammonia plants combine the tasks of CO removal and
nitrogen addition in a liquid nitrogen wash. In addition, the gas needs to be com-
pressed to the operating pressure of the synthesis, which can vary between 90 and
180 bar.
Prior to developing a block flow diagram, two (or perhaps three) key decisions
have to be taken.
First, a decision on the overall pressure profile of the plant needs to be determined.
In general, there is an energy advantage to running the gasifier at the highest possible
pressure, since the energy required to compress the synthesis gas is considerably
more than that required for compression of the feed materials. However, there are
three variables that one needs to consider:
•
The maximum pressure of the gasifier selected
. With oil feed, Texaco has numerous
references with 80 bar gasification. Shell can also operate SGP at this pressure,
but generally recommends operation at closer to 60 bar to reduce organic acid
formation in the system. For coal gasifiers, current commercial experience is limited
to 40 bar with one single unit at about 60 bar.
•
The oxygen compression system
. Here the choice is between compression of
gaseous oxygen and liquid oxygen pumping. Many 900 t/d or larger ammonia
plants operating with a gasifier at 60 bar have centrifugal compressors. Plants
significantly smaller than this would require the use of a reciprocating machine
with installed spare. Most of the 80 bar plants use liquid oxygen pumping. The
overall compression energy demand for liquid pumping (i.e., air plus oxygen
plus nitrogen) is some 3–7% higher than for the equivalent compression system.
This cancels some of the syngas compression advantages of an 80-bar system.
There are some safety arguments claimed in favor of liquid pumping, but the
excellent record of oxygen turbo-compressors in this service does not argue
against their use. All in all, there is not that much to choose between the two
systems.
•
The synthesis pressure
. In the 1970s and 1980s, typical operating pressures in the
ammonia synthesis were 220 bar. Today, most plants using conventional magnetite
catalyst are designed to operate at 130–150 bar, occasionally up to 180 bar.
Kellogg now uses a ruthenium catalyst operating at about 90 bar. The energy
demand for the synthesis does not change much over the range 130–180 bar, since
syngas compression gains made by operating at the lower pressure tend to be
compensated for by an increased refrigeration demand. Furthermore, at higher
pressures the volume of catalyst can be reduced, making the converter smaller
and, despite the increased design pressure, also cheaper. Often, therefore, the opti-
mization consists of selecting pressure levels for the gasifier and synthesis loop,
which minimize the number of casings on the synthesis gas compressor but
exploit that minimum number to the maximum extent.
Applications
235
• An additional factor to be aware of, even if it will not substantially influence the
choice of operating pressure, is that with increased pressures physical solvents for des-
ulfurization and CO
2
removal, gas losses through coabsorption of CO and H
2
will tend
to increase. In the range up to 80 bar, however, this remains within acceptable limits.
The second important decision is the selection of the acid-gas removal system and
its integration with the CO shift.
• When reviewing alternative gas treatment systems, the one immutable parameter
is the specification of the syngas. For ammonia production from gasification it is
not only a matter of eliminating carbon oxides, as is the case downstream of a
steam reformer. One needs to remove other components, some of which are
general to all gasification systems such as ammonia and HCN, others of which
may be feedstock or gasifier specific, such as the hydrocarbons produced by low-
temperature gasifiers. The system, which has proved itself capable of producing
on-spec gas behind practically all gasification processes, is Rectisol, which uses
cold methanol as a wash liquor. As a low-temperature process Rectisol is expensive.
However, in the ammonia environment this is not as serious as in other applications,
since a number of synergies can be achieved. All ammonia syntheses use some
refrigeration to condense the ammonia from the loop gas, and the product ammonia
is often stored in low-pressure tanks at a temperature of −33°C. The integration of
the refrigeration systems for Rectisol and ammonia synthesis allows some savings
compared with the stand-alone case.
Similarly, it is possible to integrate the refrigeration demand of Rectisol and the
liquid nitrogen wash, which operates at a temperature of −196°C. An additional
advantage of a physical wash is that CO
2
required for urea production can in part be
recovered under pressure, thus saving energy in CO
2
compression.
• One solution that is typical for use in conjunction with a syngas cooler is an imme-
diate desulfurization of the raw gas. After desulfurization the gas is “clean” but
also dry. In order to perform the CO shift reaction, it is necessary to saturate the
gas with water vapor in a saturator tower using water preheated by the exit gas of
the shift converter. CO
2
removal takes place in a separate step. The gas has thus to
be cooled and reheated twice in the process of acid-gas removal and CO shift. The
necessary heat-exchange equipment causes considerable expense and pressure
drop, a fact that has to be counted as a disadvantage of this system.
The raw gas from gasification of a typical refinery residue can contain about
1% H
2
S and say 4% CO
2
. A simple desulfurization of this raw gas will provide a
sour gas of about 20% H
2
S, sufficient for direct treatment in an oxygen-blown SRU.
Concentration of the H
2
S content up to 50% does not require much additional
expense. Furthermore, the CO
2
formed in the shift is free from sulfur and can be
used for urea production or emitted to the atmosphere without further treatment.
• When operating with a quench reactor, the gas emerges saturated with water vapor
at about 240°C. This temperature is too low to be able to generate high-pressure
steam, so it makes sense to utilize the water vapor immediately in a raw gas
236
Gasification
shift. In addition to saving the saturator tower, this has a number of minor advan-
tages, for example, that COS in the raw gas is also converted to H
2
S, and that HCN
is hydrogenated to ammonia (BASF undated). The sour gase components, H
2
S and
CO
2
, are then removed all in a single step. Attractive as this appears, it is not with-
out its difficulties. In this case the “natural” sour gas has an H
2
S:CO
2
ratio of about
1:50. This requires considerable expense to concentrate the H
2
S to an acceptable
level for a Claus furnace and to clean the CO
2
before emission to the atmosphere.
Ultimately, however, there is not much to choose between these two systems.
A detailed study performed in 1971 (Becker etal.) compared a 60 bar scheme using
syngas cooling and clean gas shift with a 90 bar scheme using quench and raw gas shift.
It showed very little difference between the two routes. There was a slight energy
advantage for the syngas-cooler route, and the difference in investment amounted to
only 2%—lower than the estimating tolerance. This result continues to be valid to this
day, as can be judged by the commercial and operating success of both schemes.
Example
In order to elucidate these matters in more detail, we provide a worked example of a
1000 t/d ammonia plant based on heavy oil feed and using a syngas cooler and clean
gas shift. Based on the selected gasifier pressure of 70 bar and an overall pressure
drop of 15 bar over the gas treatment train, the syngas compressor suction would be
55 bar. This would allow a synthesis loop at 135 bar. For the sake of the example, it
is assumed that a liquid oxygen pump will be applied.
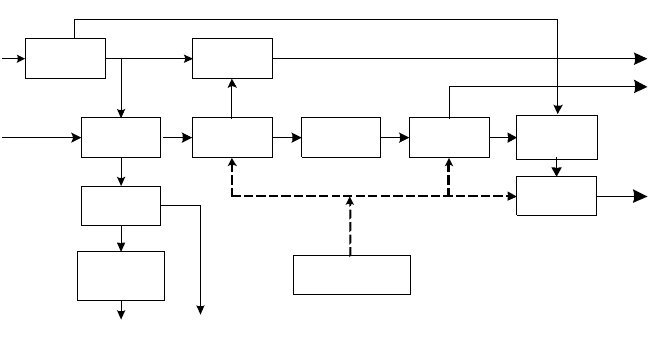
Before entering into a detailed description of the block diagram in Figure 7-2, there
are a number of further design considerations that need reviewing:
ASU CLAUS
POX
WASTE
WATER
TREATMENT
CARBON
HANDLING
REFRIGERATION
UNIT
AMMONIA
SYNTHESIS
LIQUID
NITROGEN
WASH
CO
2
RECTISOL
CO SHIFT
H
2
S
RECTISOL
NITROGEN
SULFUR
CO
2
AMMONIA
VANADIUM
CONCENTRATE
RESIDUE
WASTE
WATER
OXYGEN
CARBON SLURRY
H
2
S
AIR
Figure 7-2.
Figure 7-2.Figure 7-2.
Figure 7-2. Residue-Based Ammonia Plant
Applications
237
•
Oxygen quality
. Considering the fact that the final ammonia synthesis gas contains
25 mol% nitrogen, it is worth reviewing the extent to which this could and should
be brought into the system with the oxygen. The energy required for oxygen pro-
duction shows a flat minimum between about 90% and 95% purity. On the other
hand, an increase of nitrogen in the oxygen decreases the cold gas efficiency or
yield of H
2
+CO per kg residue. A detailed study will show an optimum at around
95% O
2
. This conclusion is also valid for an IGCC application where nitrogen in
the gas turbine burner is in any case required for NO
x
suppression. For many other
chemical applications (e.g., methanol), however, this would not be true, since N
2
is an unwanted inert in the synthesis loop.
•
Steam system
. The high-pressure steam must for safety reasons be capable of enter-
ing the gasification reactor under all conditions, and it is logical to generate steam
in the syngas cooler at the same selected pressure. For our example plant, we will
use 100 bar as the saturation pressure of the high-pressure steam and allow a
pressure drop of 8 bar across the superheater and controls. Provision will be made
for 25-bar medium-pressure steam and 10-bar low-pressure steam.
•
Compressor drivers
. With a syngas cooler installed after the gasifier, there is usually
sufficient high pressure steam to satisfy the demand of the CO shift, the syngas
compressor, and the nitrogen compressor. An external energy source is required
for the air compressor and (in this case) the nitrogen circulator required for oxygen
evaporation in the ASU. In the event of using an oxygen compressor, this would
substitute for the latter. An external energy source is also required for a refrigera-
tion compressor. Generally, two alternatives are available, electric power (pro-
vided the grid is stable enough to cope with starting a 12 MW electric motor) and
steam, which is generated in an auxiliary boiler on site. This is an economic
decision, but it should be borne in mind that start-up steam is required in any case.
Furthermore, having a substantial boiler in operation all the time can help in stabi-
lizing the overall steam system.
•
Refrigeration system
. The ammonia synthesis will require refrigeration capacity at
typically about 0°C. For final product cooling to atmospheric storage and for the
Rectisol acid-gas removal unit, additional refrigeration capacity at about −33°C is
required. Ammonia compression and absorption systems are available for this
duty. Absorption systems are generally more expensive in capital cost, but they
can operate on low-level waste heat that might otherwise remain unrecovered.
Studies performed in the context of ammonia plants like our example have shown
that it is more economical to use waste heat in boiler feed-water preheat than in
absorption refrigeration. On the other hand, if the waste heat really is “waste,”
then it pays to invest in an absorption system. Not only have both systems been
used in various locations, but they have also been built in combination, where an
absorption system was used to bring the refrigerant to 0°C and a booster compressor
was used for the −33°C duty.
Looking at the results of this discussion in Figure 7-2 together with the material
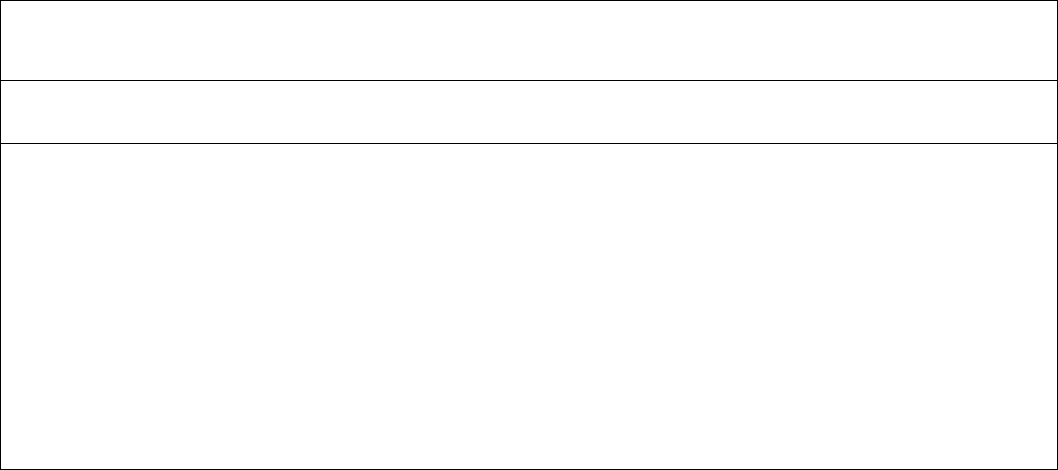
balance in Table 7-1 we see the following:
238
Gasification
Table 7-1
Mass Balance for 1000 t/d Ammonia Plant
Oxygen Steam
Raw
Gas
Desulf.
Gas
Sour
Gas
Shift
Gas Raw H
2
Pure
CO
2
N
2
to
LNW
NH
3
Syngas
CO
2
, mol% 3.67 4.16 66.71 33.82 0.00 99.96
CO, mol% 49.28 49.46 4.37 3.20 4.74 0.02
H
2
, mol% 45.65 45.92 0.88 62.66 94.81 0.02 75.00
CH
4
, mol% 0.27 0.27 0.08 0.19 0.26
N
2
, mol% 0.10 0.09 0.09 0.02 0.06 0.09 100.00 25.00
A, mol% 0.40 0.10 0.10 0.00 0.07 0.10
H
2
S, mol% 0.90 26.75
COS, mol% 0.04 1.19
O
2
, mol% 99.5
H
2
O, mol% 100.00
Dry gas (kmol/h) 1014 801 4250 4223 143 6116 3940 496 1321 4942
Pressure (bar) 65 72 55.1 52.5 1.5 49.5 47.6 1.5 50.0 44.5
Temp. (°C) 120 380 45 35 10 45 −60 20 45 35
Applications
239
Oxygen and nitrogen are manufactured in the ASU, where the compressors are
all driven by condensing steam turbines. The oxygen is pumped in the liquid
phase to a pressure of 80 bar and evaporated with gaseous nitrogen, which
returns the cryogenic cold to the cold box. The vacuum residue is gasified in
the partial oxidation reactor with oxygen and steam at 70 bar and about
1300°C. The raw gas from the reactor contains soot and ash, which is removed
in a water wash.
The raw gas, freed of solid matter is cooled down to about −30°C in the Rectisol
unit, where it is washed with cold methanol to a residual total sulfur content of
less than 100 ppbv. The sulfur-free gas is then heated up and saturated with water
at about 220°C in a saturator tower in the CO shift. Additional steam is added that
reacts over the catalyst with carbon monoxide to form hydrogen and CO
2
. The
gas at the outlet of the CO shift has a CO slip of about 3.2% and a CO
2
content of
about 34%. This gas reenters the Rectisol unit and is washed again with cold
methanol, this time at about −60°C. The CO
2
content is reduced to about
10 ppmv. The resulting gas is a raw hydrogen with about 92% H
2
and about 5%
CO, the rest being nitrogen, argon, and methane. This gas is cooled down to about
−196°C and washed with liquid nitrogen.
Simultaneously, the amount of nitrogen required for the ammonia synthesis is
added. The gas is then compressed to the pressure required for the synthesis loop.
The mass balance in Table 7-1, based on gasifying 32 t/h visbreaker residue in the
partial oxidation unit to produce 1000 t/d ammonia, illustrates this gas treatment
scheme. The feed quality is as described in Table 4-10.
7.1.2 Methanol
Market
Approximately 3.3 million metric tons per year, or about 9% of the estimated world
methanol production, is based on the gasification of coal or heavy residues.
Methanol is an important intermediate and, as can be seen from Figure 7-3,
approximately two-thirds of the production goes into the manufacture of formalde-
hyde and MTBE (methyl tertiary-butyl ether). The demand for methanol has varied
substantially from year to year, creating some dramatic price swings when supply
has failed to keep up with demand. At the time of this writing, the future of MTBE
as a component in reformulated gasoline is still uncertain, and this has its effects on
the market.
During the 1990s the typical size of world-scale natural gas–based plants
increased from 2000 to about 3000 mt/d. Current designs go up to 5000 t/d, and three
plants of this size are in the design stage (Göhna 1997). Most plants based on gasifi-
cation technologies are somewhat smaller. The largest, in the Leuna refinery in
Germany, has a nameplate capacity of 2060 mt/d.
