Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

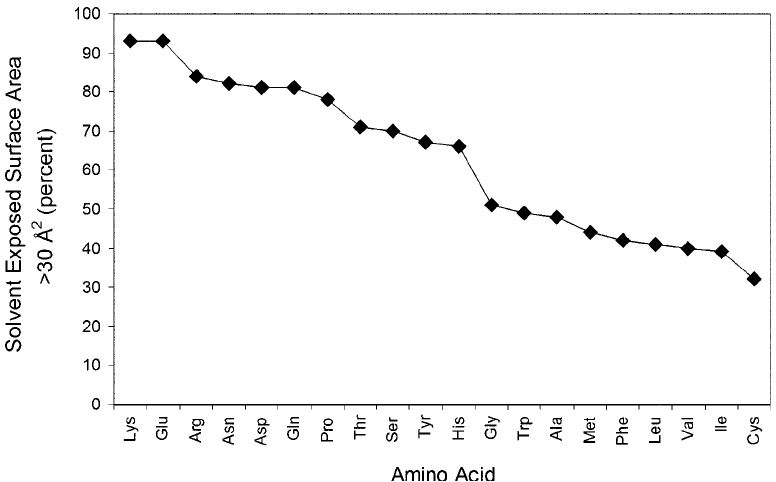
asparagine, glutamine, and tyrosine often have large areas accessible to the solvent, as do the
charged amino acids aspartic acid, glutamic acid, lysine, histidine, and arginine. Surprisingly,
proline also falls in the highly accessible group, which is not as expected, because it doesn ’t carry
a charge, nor is it a highly polar amino acid. However, proline does have a unique characteristic
that may explain its appearance on the surface of proteins: it cannot freely rotate about its imino
group as other amino acids in a peptide chain can do at amide bonds. This effect results in a
kink in the polymer (called a beta-turn), and these sharp turns in a peptide backbone probably
occur most often near the surface. Thus proline is found to be frequently accessible to the sol-
vent environment despite its hydrophobic nature.
The non-polar amino acids glycine, alanine, valine, leucine, isoleucine, methionine, pheny-
lalanine, and cysteine have lower exposure to the solvent environment than charged or polar
residues. However, the frequency at which these groups are found to have an SEA of greater
than 30 Å
2
is much higher than one would expect based solely upon consideration of their
hydrophobicity. In fact, nearly 30–50 percent of the time non-polar amino acids in a protein
can be found at the surface.
At the two extremes, lysine is observed as the amino acid most accessible on the surface of
proteins while cysteine is the least exposed amino acid. The inaccessibility of cysteine prob-
ably stems from the fact that disulfi des are typically buried within the polypeptide structure of
proteins, whether they are intrachain or interchain in nature, and proteins rarely contain many
reduced cysteine thiols.
30 1. Functional Targets
Figure 1.21 Comparison of the solvent exposed surface area of amino acids in proteins. Data are plotted as a
percentage of each amino acid in a protein having greater than a 30 Å
2
exposure to the aqueous environment.
Charged and polar amino acids are seen to have the most solvent exposure, while uncharged, aromatic, or
aliphatic amino acids have the least exposure.
It is clear from this data that proteins have complex hydrophilic and hydrophobic regions
on their surfaces that determine their potential interactions, binding sites, and active centers.
For bioconjugation purposes, targeting of an amino acid even with a high SEA for modifi cation
or crosslinking may not result in every residue being modifi ed that is theoretically present in a
protein based only on knowledge of its amino acid composition. Even when coupling to very
polar or charged groups, such as lysine, there are varying degrees of accessibility to a given rea-
gent, because of the complex folding of the polypeptide chains at the protein surface.
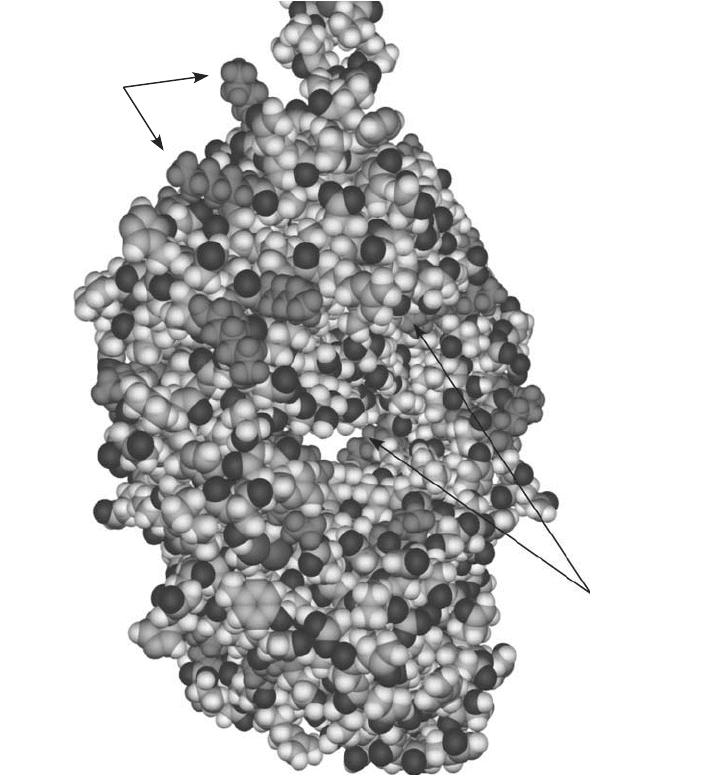
Figure 1.22 shows the globular structure of an immunoglobulin (IgG) Fc region to illustrate
this point. In this space-fi lling model, the lysine residues are highlighted in solid gray to easily
1. Modifi cation of Amino acids, Peptides, and Proteins 31
Lysines between
heavy chains or
somewhat buried
Fc region of
IgG molecule
Highly accessible
lysine groups
Figure 1.22 The solvent accessibility of lysine residues in the Fc region of an antibody is illustrated by high-
lighting the lysine groups in solid gray. Some lysine -amine groups are extremely accessible to conjugation,
while others are only partially exposed, making them diffi cult to modify in bioconjugation reactions.
32 1. Functional Targets
show their locations within the two polypeptides of the heavy chains. Notice that some of the
-amino groups at the ends of the side chains are protruding far out into the solvent and are
therefore highly accessible for modifi cation. Some of these groups, however, are less exposed
even though they are still near the surface, and a few lysines are seen to be between the heavy
chain regions where it would be diffi cult to modify them due to crowding.
Figure 1.64 in this chapter provides data to validate this effect. The reaction of the thiolat-
ing reagent SATA ( N-succinimidyl S-acetylthioacetate) with IgG resulted in only a percentage
of the available lysines being modifi ed. As the molar ratio of SATA to IgG was increased, the
yield of lysine modifi cation actually became lower. This result can be explained by the relative
accessibility of each lysine in the immunoglobulin structure. Some residues are easily acces-
sible and they get modifi ed with high yield even with low molar ratios of SATA-to-IgG. As the
molar ratio is increased, it gets more diffi cult to modify those lysines that are less accessible to
the solvent environment or are partially obscured by another polypeptide chain. Thus, the sol-
vent accessibility of particular amino acids is a major factor in whether they can be effectively
targeted and modifi ed with a given bioconjugate reagent.
1.2. Protein Crosslinking Methods
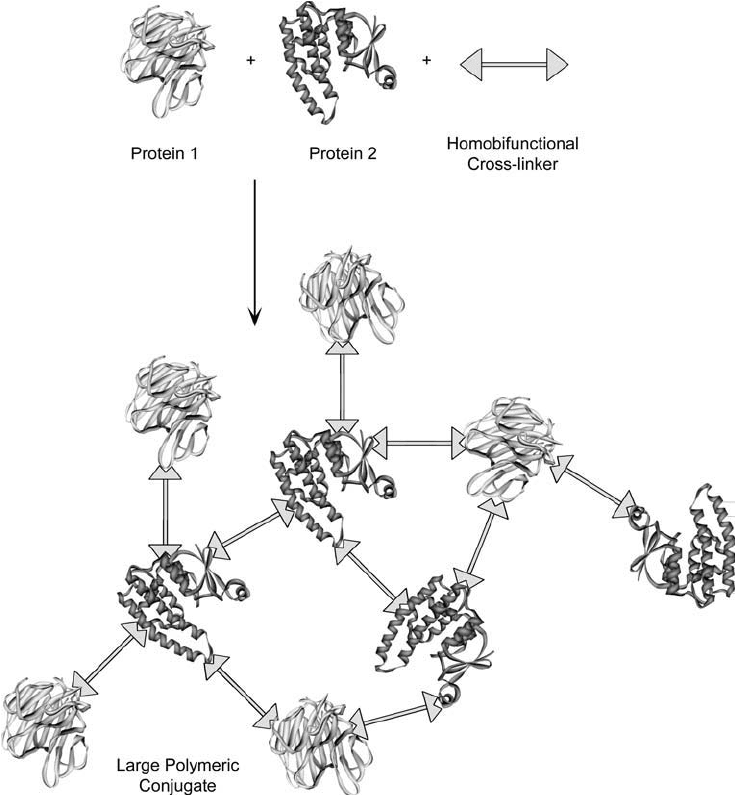
The crosslinking of two proteins using a simple homobifunctional reagent (Section 2.2) poten-
tially can result in a broad range of conjugates being produced (Avrameas, 1969). The reagent
initially may react with either one of the proteins, forming an active intermediate. This activated
protein may then form crosslinks with the other protein or with another molecule of the same
protein. The activated protein also may react intramolecularly with other functionalities on part
of its own polypeptide chain. Other crosslinking molecules may continue to react with these
conjugated species to form various mixed products, including severely polymerized proteins that
may fall out of solution ( Figure 1.23 ).
The problems of indeterminate conjugation products are amplifi ed in single-step reaction
procedures using homobifunctional reagents (Chapter 4). Single-step procedures involve the
addition of all reagents at the same time to the reaction mixture. This technique provides the
least control over the crosslinking process and invariably leads to a multitude of products, only
a small percentage of which represent the desired or optimal conjugate. Excessive conjugation
may cause the formation of insoluble complexes that consist of very high-molecular-weight poly-
mers. For example, one-step glutaraldehyde conjugation of antibodies and enzymes (Chapter 20,
Section 1.2) often results in signifi cant oligomers and precipitated conjugates. To overcome this
shortcoming, multi-step reaction procedures have been developed using both homobifunctional
and heterobifunctional reagents (Chapter 5). Controlled, multi-step conjugation protocols alle-
viate the polymerization problem and form relatively low molecular weight, soluble antibody–
enzyme complexes (Chapter 20, Section 1.1).
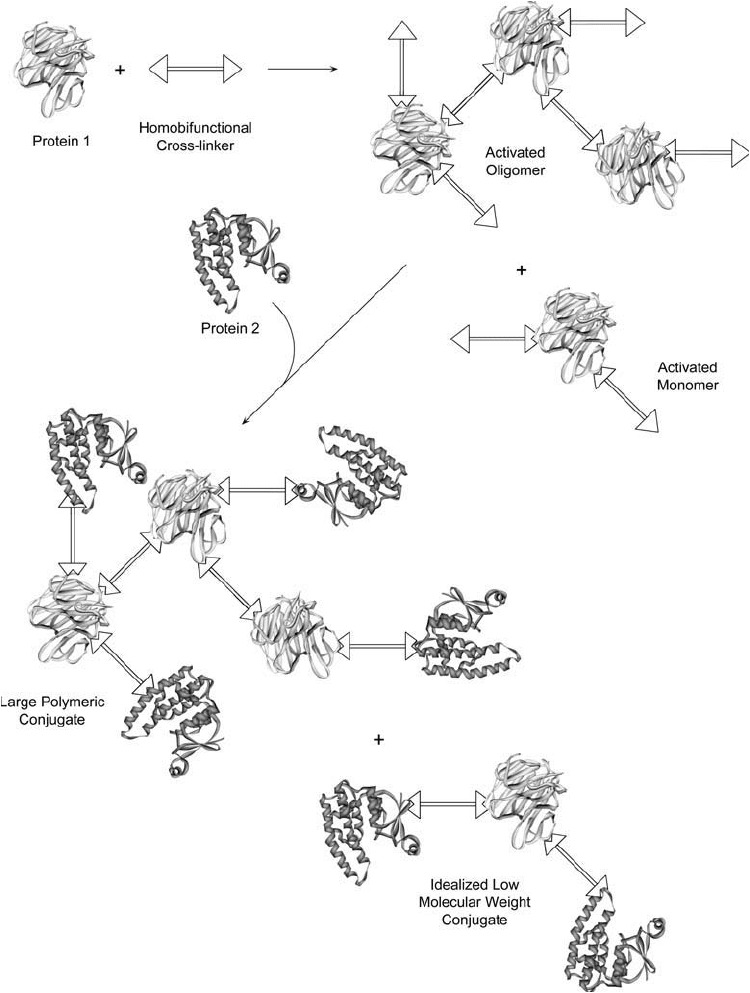
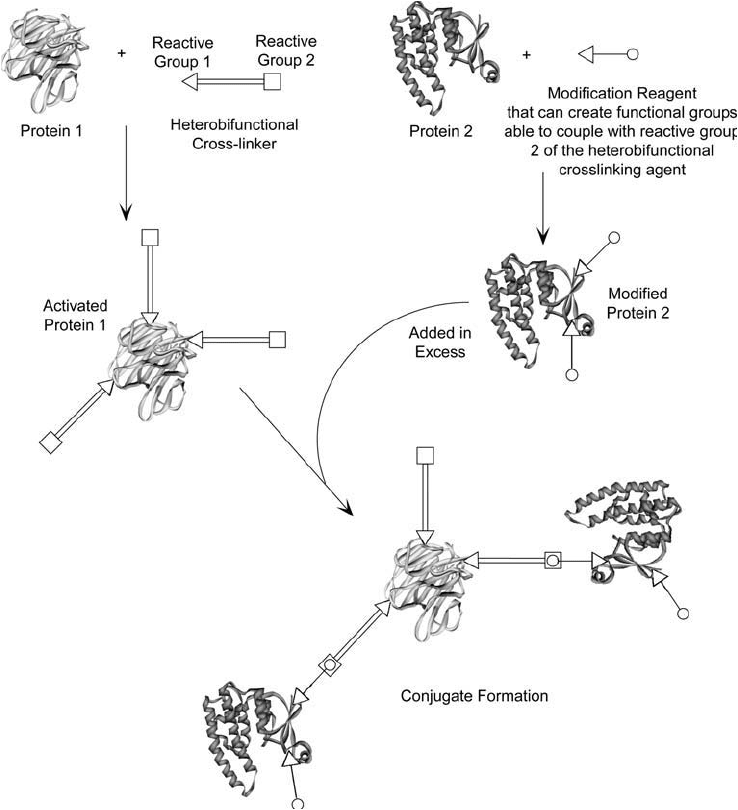
In two-step protocols, one of the proteins to be conjugated is reacted or “activated” with a
crosslinking agent and excess reagent and by-products are removed. In the second stage, the
activated protein is mixed with the other protein or molecule to be conjugated, and the fi nal
conjugation process occurs ( Figure 1.24 ).
The use of homobifunctional reagents in two-step protocols still creates many of the
problems associated with single-step procedures, because the fi rst protein can crosslink and
polymerize with itself long before the second protein is added. Homobifunctional reagents by
defi nition have the same reactive group on both ends of the crosslinking molecule. Since the
protein to be activated has target functionalities on every molecule that can couple with the
reactive groups on the crosslinker, both ends of the reagent potentially can react. This inherent
potential to uncontrollably polymerize unfortunately is characteristic of all homobifunctional
reagents, even in multi-step protocols.
The greatest degree of control in crosslinking procedures is afforded using heterobifunc-
tional reagents (Chapter 5). Since a heterobifunctional crosslinker has different reactive groups
on either end of the molecule, each side can be directed specifi cally toward different functional
1. Modifi cation of Amino acids, Peptides, and Proteins 33
Figure 1.23 Protein crosslinking reactions done using homobifunctional reagents can result in large polymeric
complexes of multiple sizes and indefi nite structure.
34 1. Functional Targets
Figure 1.24 A two-step protocol using a homobifunctional crosslinking agent offers more control than single-
step methods, but still may result in oligomer formation.
2. Modifi cation of Sugars, Polysaccharides, and Glycoconjugates 35
groups on proteins. Using a multi-step conjugation protocol with a heterobifunctional reagent
can allow one macromolecule to be activated, excess crosslinker removed, and then a second
macromolecule added to induce the fi nal linkage. Directed conjugation will occur as long as
the fi rst protein that is activated doesn ’t have groups able to couple with the second end of the
crosslinker, whereas the second molecule does possess the correct functionalities.
Occasionally, the second protein doesn ’t naturally have the target groups necessary to cou-
ple with the second end of the crosslinker. In such cases, a specifi c functionality usually can be
created to make the conjugation successful (Chapter 1, Section 4). In such three-step systems,
the fi rst protein is activated with the heterobifunctional reagent and purifi ed away from excess
crosslinker. The second protein is then modifi ed to contain the specifi c target groups required
for the second stage of the conjugation. Finally, in step three, the two modifi ed proteins are
mixed to cause the coupling reaction to happen ( Figure 1.25 ).
Two- and three-step protocols using heterobifunctional crosslinkers often are designed around
amine-reactive and sulfhydryl-reactive chemical reactions. Many of these reagents utilize NHS
esters on one end for coupling to amine groups on the fi rst protein and maleimide groups on the
other end that can react with sulfhydryls on the second protein. The NHS ester end is reacted
with the fi rst protein to be conjugated, forming an activated intermediate containing reactive
maleimide groups. Fortunately, the maleimide end of such crosslinkers is relatively stable to deg-
radation, thus the activated protein can be isolated without loss of sulfhydryl coupling ability.
Additionally, if the second protein does not contain indigenous sulfhydryls, these can be created
by an abundance of methods (Chapter 1, Section 4.1). After mixing the maleimide-activated
protein with the sulfhydryl-containing protein, conjugation can occur only in one direction.
Control of the products of conjugation increases as the protocols progress from single-step
to multi-step reactions. Likewise, control of the chemistry of conjugation increases as the rea-
gent systems evolve from simple homobifunctional to site-directed heterobifunctional. It may
appear to be a paradox, but often as the method of conjugation gets more complex the result
is less potential for side reactions and therefore fewer products being formed. Therefore, multi-
step processes using advanced heterobifunctional reagents are the best combination to assure
that the protein conjugate formed is indeed the one desired.
2. Modifi cation of Sugars, Polysaccharides, and Glycoconjugates
The basic units of food energy for cells and living organisms consist of polysaccharides or sim-
ple sugars, principally glucose and its derivatives. Biological molecules themselves often con-
tain carbohydrate or are made exclusively of such components. Complex carbohydrate “ trees ”
frequently project off the surface of cells, providing specifi c points of attachment or sites of rec-
ognition. Lipids and proteins that contain these components may possess them to give identity
or partial hydrophilicity to their parent structures.
Many of the macromolecules that are the subject of modifi cation or conjugation reactions
contain signifi cant proportions of carbohydrate. Reactions can be designed to target directly
these polysaccharide portions, either selectively modifying them with small, detectable com-
pounds or using them as conjugation bridges to couple with other macromolecules. The reac-
tivity of carbohydrate molecules in such derivatizations is an important factor in the success of
many bioconjugate techniques.
36 1. Functional Targets
Figure 1.25 Heterobifunctional crosslinking agents used in multi-step protocols result in the best control over
the products formed.
This section describes the basic chemical attributes of carbohydrate molecules. Principal sites
of reactivity on carbohydrates are discussed with the aim of developing a rational approach to
using them in modifi cation and conjugation procedures.
2.1. Carbohydrate Structure and Functionality
Carbohydrates are characterized by the presence of polyhydroxylic aldehyde or polyhydroxy-
lic ketone structures or polymers made of such units. Sugars and polysaccharides have defi nite

three-dimensional structures that are important for many biological functions. They are
hydrophilic and thus easily accessible to aqueous reaction mediums. The chemistry of biocon-
jugation using carbohydrate molecules begins with an understanding of the building blocks of
polysaccharide molecules.
Basic Sugar Structure
The simplest carbohydrate, called a monosaccharide, is composed of a structure that cannot be
hydrolyzed to simpler polyhydroxylic compounds. A disaccharide is a carbohydrate that con-
tains two of these basic units, and a polysaccharide contains many polyhydroxylic monomers.
A monosaccharide that contains an aldehyde group is called an aldose, and one that contains
a ketone group is a ketose. Monosaccharides are further classifi ed by the number of carbon
atoms they contain. Thus, a fi ve-carbon sugar is known as a pentose and a six-carbon sugar, a
hexose. All monosaccharides containing accessible aldehyde or ketone functionalities are reduc-
ing sugars—that is they are able to reduce Fehling ’s or Tollen ’s reagent.
The aldehyde or ketone group of monosaccharides can undergo an intramolecular reac-
tion with one of its own hydroxyl groups to form a cyclic, hemiacetal, or hemiketal structure,
respectively ( Figure 1.26 ). In aqueous solutions, this cyclic structure actually predominates. The
open-chain aldehyde or ketone form of monosaccharides is in equilibrium with the cyclic form,
but the open structure exists less than 0.5 percent of the time in aqueous environments. It is the
2. Modifi cation of Sugars, Polysaccharides, and Glycoconjugates 37
Figure 1.26 Carbonyl groups and hydroxyls may react to form acetal or ketal products. Sugars naturally
undergo these reactions to form ring structures in aqueous solution.
38 1. Functional Targets
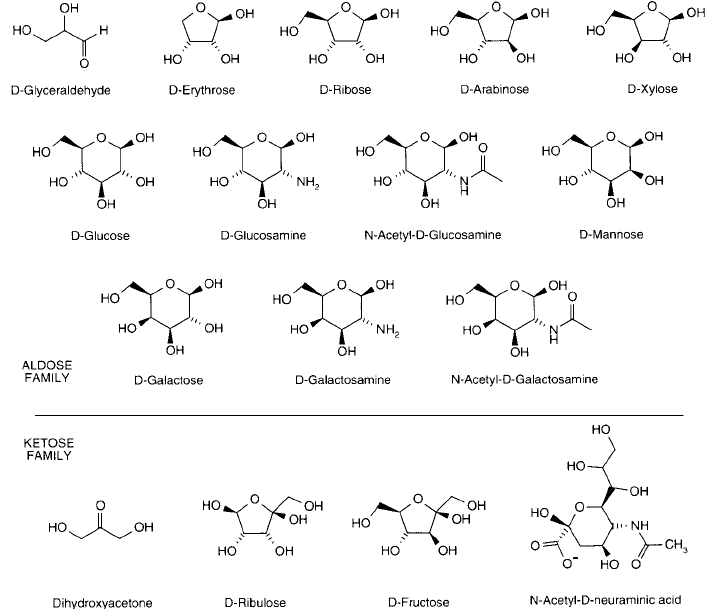
Figure 1.27 Common monosaccharides of the aldose and ketose families found in biological molecules.
open form that reduces Fehling ’s or Tollen ’s reagent. However, due to this predominance of the
cyclic structure of monosaccharides, they do not have the capability of reacting with bisulfi te
or Schiff ’s reagent, as do normal unblocked aldehydes and ketones. Thus, the carbonyl func-
tionalities of sugars have reduced reactivity, because of hemiacetal and hemiketal formation.
Figure 1.27 shows the structures of some of the most common monosaccharide molecules:
D-glyceraldehyde, D-erythrose, D-ribose, D-arabinose, D-xylose, D-glucose, D -glucosamine,
N -acetyl- D-glucosamine, D-mannose, D-galactose, D-galactosamine, N -acetyl- D -galactosamine
of the aldose family and dihydroxyacetone, D-ribulose, D-fructose, D-N-acetylneuraminic acid of
the ketose family. Formation of the cyclic structure of each of these sugars can result in one of
two stereoisomers, designated and , depending on the orientation of the aldehyde group or
ketone group during hemiacetal formation. For aldoses, the form is drawn in the standard
Haworth projection with the No. 1 carbon hydroxyl pointing down. For ketoses, the form
consists of the No. 2 carbon hydroxyl pointing down. All the common monosaccharide struc-
tures shown in Figure 1.27 are in the -stereoisomer form.
Since in aqueous solutions the cyclic form of monosaccharides is in equilibrium with their
corresponding open forms, the and structures continually interconvert. At equilibrium, one
form usually predominates. For instance, glucose dissolved in water consists of about a 2:1
2. Modifi cation of Sugars, Polysaccharides, and Glycoconjugates 39
ratio of - D-glucose to - D-glucose. Although their chemical constituents are identical, the bio-
chemical properties between the and forms can be quite different. Monosaccharides linked
together to form disaccharides and polysaccharides cannot continue to interconvert and are
therefore frozen in the or forms. Changing one monosaccharide in a complex carbohydrate
to its opposite stereoisomer form can produce radical structural changes in the polysaccharide
chain and signifi cantly alter its biochemical properties.
Sugar Functional Groups
Monosaccharide functional groups consist of either a ketone or an aldehyde, several hydrox-
yls, and the possibility of amine, carboxylate, sulfate, or phosphate groups as additional
constituents. Amine-containing sugars may possess a free primary amine, but often are mod-
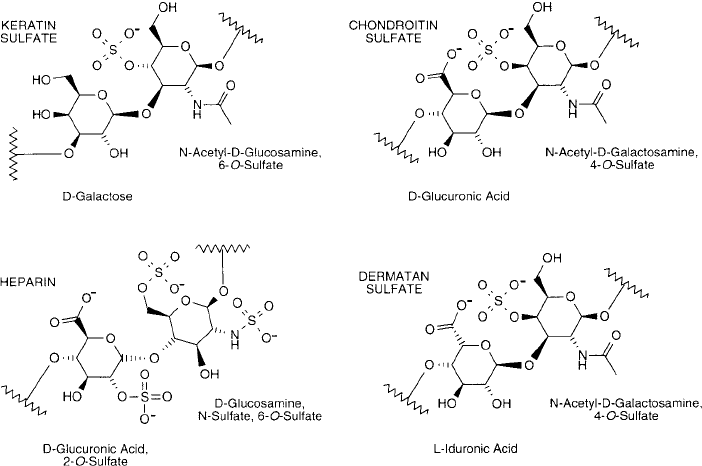
ifi ed to the N-acetyl derivative, such as the N-acetyl glucosamine residue of chitin. Sulfate-
containing monosaccharides frequently are found in certain mucopolysaccharides, including
chondroitin sulfate, dermatan sulfate, heparin sulfate, and keratin sulfate ( Figure 1.28 ).
Carboxylate-containing sugars include sialic acid as well as many aldonic, uronic, oxoaldonic,
and ascorbic acid derivatives ( Figure 1.29 ). Phosphate-containing monosaccharides are almost
exclusively created in metabolic processes involving energy utilization, such as in the produc-
tion of glucose-1-phosphate formed during glycogen breakdown and glucose-6-phosphate pro-
duced during glycolysis. Perhaps the most common phosphate sugar derivative, however, is
the 5 -phosphate of
D-ribose or D-2-deoxyribose found as a repeating component of RNA and
DNA, respectively.
Figure 1.28 Common sulfonated polysaccharides of biological origin.
