Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

protein structure. In many cases, simply re-introducing the needed group into the surrounding
medium can restore full activity.
In addition to small organic molecules or metal ions, proteins may have other components
tightly associated with them. Nucleoproteins, for instance, contain noncovalently bound DNA
or RNA, as in some of the structural proteins of viruses. Lipoproteins contain associated lipids
or fatty acids and may also carry cholesterol, as in the high-density and low-density lipopro-
teins in serum.
During modifi cation or conjugation reactions, prosthetic groups and other associated mol-
ecules may be lost or damaged. Metal ions temporarily may be removed by the inclusion of
a chelating agent added to maintain sulfhydryl stability during coupling through the SH
groups of a protein. To restore activity after conjugation, it is necessary to remove the chelator
and add the required metal salts. Other changes to the prosthetic carriers may not be so easily
corrected. For instance, heme-containing molecules are sensitive to the presence of agents that
can form a coordination complex with or modify the oxidation state of the chelated metal ion.
Some reagent systems may permanently inactivate the heme-containing protein.
Thus, loss of activity can occur not only through changes to the amino acid constituents
of a protein, but through prosthetic group or cofactor loss or damage as well. Most of these
potential diffi culties can be overcome through careful selection of the reaction conditions and
through knowledge of the cofactor dependencies that are critical to the activity of the protein
being modifi ed.
Post-translational modifi cations to protein structure are covalent changes that occur as the
result of controlled enzymatic reactions or due to chemical reactions not under enzymatic regu-
lation. One of the most common cellular modifi cations performed on proteins after ribosomal
synthesis is glycosylation. Proteins newly synthesized on ribosomes, may be transported to the
Golgi apparatus where specifi c glycosyl transferases catalyze the coupling of carbohydrate resi-
dues to the polypeptide chains. Glycoproteins and mucoproteins are formed by the coupling
of polysaccharides through o-glycosidic linkages to serine, threonine, or hydroxylysine and
through N -glycosidic linkages with the amide side chain group of asparagine.
The structure of most glycoprotein carbohydrate is branched with the sugars mannose,
N-acetyl glucosamine, sialic acid, galactose, and
L-fucose being prevalent. Asparagine-linked
polysaccharides are well characterized and are known to be constructed of a core unit consist-
ing of three mannose residues and two N-acetyl glucosamine (GlcNAc) residues. The GlcNAc
residues are bound to the Asp side chain amide nitrogen through a 1 linkage (Kornfi eld and
Kornfi eld, 1985). The three mannose groups then usually form the fi rst branch point in the oli-
gosaccharide chain (Chapter 1, Section 2).
The content by weight of carbohydrate in glycoproteins may vary from only a few per-
cent to over 50 percent in some proteins in mucous secretions. Although the function of the
polysaccharide in most glycoproteins is unknown, in some cases it may provide hydrophilicity,
recognition, and points of noncovalent interaction with other proteins through lectin-like affi n-
ity binding.
The presence of carbohydrate on protein or peptide molecules can provide important points
of attachment for modifi cation or conjugation reactions. Coupling exclusively through polysac-
charide chains often can direct the reaction away from active centers or critical points in the
polypeptide chain, thus preserving activity. Polysaccharides can be specifi cally targeted on
glycoproteins through mild sodium periodate oxidation. Periodate cleaves adjacent hydroxyl
groups in sugar residues to create highly reactive aldehyde functionalities (Chapter 1, Section 4.4).
20 1. Functional Targets
The level of periodate addition can be adjusted to selectively cleave only certain sugars in the
polysaccharide chain. For instance, a concentration of 1 mM sodium periodate at temperatures
less than 4 °C specifi cally oxidizes sialic acid residues to contain aldehydes, leaving all other
monosaccharides untouched. Increasing the concentration to 10 mM and doing the reaction
at room temperature, however, will cause oxidation of other sugars in the carbohydrate chain,
including galactose and mannose. The generated aldehydes then can be used in coupling reac-
tions with amine or hydrazide containing molecules to form covalent linkages. Amines can
react with formyl groups under reductive amination conditions using a suitable reducing agent
such as sodium cyanoborohydride. The result of this reaction is a stable secondary amine link-
age (Chapter 2, Section 5.3). Alternatively, hydrazides spontaneously react with aldehydes to
form hydrazone linkages, although the addition of a reducing agent increases the effi ciency of
the reaction (Chapter 2, Section 5.1).
Another form of post-translational modifi cation that may add carbohydrate to a polypep-
tide is non-enzymatic glycation. This reaction occurs between the reducing ends of sugar mole-
cules and the amino groups of proteins and peptides. See Section 2.1 in this chapter for further
details and the reaction sequence behind this modifi cation.
Protecting the Native Conformation and Activity of Proteins
The goal of most protein modifi cation or conjugation procedures is to create a stable product
with good retention of the native state and activity. Ideally, any derivatization should result in a
protein that performs exactly as it would in its unmodifi ed form, but with the added functionality
imparted by whatever is conjugated to it. Thus, an antibody molecule tagged with a fl uorophore
should retain its ability to bind to antigen and also have the added functionality of fl uorescence.
One of the best ways to ensure retention of activity in protein molecules is to avoid doing
chemistry at the active center. The active center is that portion of the protein where ligand,
antigen, or substrate binding occurs. In simpler terms, the active center (or active site) is that
part that has specifi c interaction with another substance (Means and Feeney, 1971). For the
preparation of enzyme derivatives, it is important to protect the site of catalysis where conver-
sion of substrate to product happens. For instance, when working with antibody molecules, it
is crucial to stay away from the two antigen binding sites.
The best chemical procedures avoid the active site by selecting functional groups away from
that area or by protecting the site through the incorporation of additives. In some cases, the
inclusion of substrates, cofactors, ligands, inhibitors, or antigens in the modifi cation reaction
will protect the active site. Addition of the appropriate substance can bind the active site and
mask it from modifi cation by crosslinking agents. In enzyme derivatization procedures, this
is often just a matter of adding a reversible inhibitor or substrate analog. For instance, when
working with alkaline phosphatase merely doing the reaction in phosphate buffer protects the
active center from chemical modifi cation, since phosphate ions bind in the catalytic site. With
trypsin, the incorporation of benzamidine similarly masks and protects the active site.
However, protecting the antigen binding sites on an antibody molecule by using this method
is often more diffi cult. Inclusion of antigen to mask the binding sites is effective in blocking
these areas, but it also may cause irreversible crosslinking of the antigen to the antibody. This
is especially true when the antigen is a peptide or a protein having the same chemical function-
alities as the antibody. Any modifi cation reactions that are directed at the antibody may modify
the antigen as well. Therefore, only use this method if the antigen is lacking in the chemical
1. Modifi cation of Amino acids, Peptides, and Proteins 21
targets that are going to be used on the antibody. For instance, if the polysaccharide chains on
the antibody are targeted for modifi cation, then using a protein antigen that does not contain
carbohydrate to block the antigen binding sites may work well.
An equally effective method of protecting the activity of a protein is by using site-directed
reactions that result in modifi cations away from the active center. In some cases, specifi c func-
tionalities are known to be present only at restricted sites within the three-dimensional struc-
ture of a protein. If these functionalities are not present close to the active site, then using them
exclusively for modifi cation reactions should assure good retention of activity. For instance,
sulfhydryl groups or carbohydrate chains are often present in limited quantity and in specifi c
regions on a protein. Selecting reagent systems that target these groups assures derivatization
only at restricted sites within the protein molecule, thus potentially avoiding the active center.
Surprisingly, the goal of some protein crosslinking schemes is to somewhat alter the native
presentation of the conjugate. This is especially true in hapten–carrier conjugation as used for
immunogen or vaccine preparation. In this case, the main objective is to modify the environ-
ment of the hapten to create an immunological response in vivo. A hapten is usually a small
molecule that is not able to generate an immune response on its own, but can react with the
products of such a response once generated. Most often these products are antibodies having
binding specifi city for the hapten.
The complexities involved in achieving a successful conjugation strategy are best illustrated
in the problems and concerns dealing with hapten–carrier conjugation. In order to produce the
initial immune response to a small molecule, the hapten is typically coupled to a larger protein
that can generate a response on its own. In simple terms, the larger carrier protein confers
immunogenicity to the smaller hapten. The native presentation of the hapten is altered toward
the immune system, thus creating the immune response.
The site of attachment of the hapten to the carrier and the nature of the crosslinker are both
important to the specifi city of the resultant antibodies generated against it. For proper recogni-
tion, the hapten must be coupled to the carrier with the appropriate orientation. For an anti-
body subsequently to recognize the free hapten without the attached carrier, the hapten–carrier
conjugate must present the hapten in an exposed and accessible form. Optimal orientation is
often achieved by directing the crosslinking reaction to specifi c sites on the hapten molecule.
With peptide haptens, this is typically done by attaching a terminal cysteine residue during syn-
thesis. This provides a free thiol group on one end of the peptide for conjugation to the carrier.
Crosslinking through this group provides hapten attachment only at one end, therefore ensur-
ing consistent orientation.
In hapten–carrier conjugation, the goal is not to maintain the native state or stability of
the carrier, but to present the hapten in the best possible way to the immune system. In reach-
ing this goal, the choice of conjugation chemistry may control the resultant titer, affi nity, and
specifi city of the antibodies generated against the hapten. It may be important in some cases to
choose a crosslinking agent containing a spacer arm long enough to present the antigen in an
unrestricted fashion. It also may be important to control the density of the hapten on the sur-
face of the carrier. Too little hapten substitution may result in little or no response. A hapten
density too high actually may cause immunological suppression and decrease the response. In
addition, the crosslinker itself may generate an undesired immune response. Fortunately, for
the majority of hapten–carrier conjugation problems, a few main crosslinking techniques pro-
vide a workable compromise to solving all these concerns and ultimately generating an effec-
tive immune response (Chapter 19).
22 1. Functional Targets
Oxidation of Amino Acids in Proteins and Peptides
The modifi cation of amino acids in proteins and peptides by oxidative processes plays a major
role in the development of disease and in aging (Halliwell and Gutteridge, 1989, 1990; Kim
et al., 1985; Tabor and Richardson, 1987; Stadtman, 1992). Tissue damage through free radi-
cal oxidation is known to cause various cancers, neurological degenerative conditions, pulmo-
nary problems, infl ammation, cardiovascular disease, and a host of other problems. Oxidation
of protein structures can alter activity, inhibit normal protein interactions, modify amino acid
side chains, cleave peptide bonds, and even cause crosslinks to form between proteins.
Due to their abundance in cells relative to other biological molecules, proteins are one of
the primary targets of oxidation in vivo. However, sometimes oxidation reactions involving
proteins and peptides are thought of solely as the creation of disulfi des from thiols on cysteine
residues. This is certainly an important form of oxidation that can affect protein structure
and function or even cause problems relevant to bioconjugation reactions. The presence of an
accessible free thiol on a protein in an aqueous solution can be highly unstable to rapid oxida-
tion unless precautions are taken to prevent disulfi de formation. Dissolved oxygen and other
potentially catalytic components, such as certain metal salts, quickly can result in disulfi des
being formed within a protein or between different protein molecules.
From a broader perspective, protein oxidation can result in covalent modifi cation at many
sites other than just at cysteine thiols. The earliest reports on protein oxidation date from the
fi rst decade of the twentieth century, but it took many more years to characterize these reac-
tions and their products (Dakin, 1906).
The signifi cance of protein oxidation became paramount with the advent of recombinant
protein biologics used as human therapeutics. Careful characterization of protein stability is
essential to maintaining the effi cacy of protein pharmaceuticals. If even a single side chain
amino acid residue becomes oxidized, then a protein therapeutic may not have the same activ-
ity in vivo as the unmodifi ed protein.
Oxidation of proteins can result from exposure to oxidative species from many sources: reac-
tive oxygen intermediates caused by metabolic reactions within cells (mitochondrial electron
transport function and certain enzymes, such as oxidases, peroxidases, and P-450 enzymes),
from the by-products of oxidative stress reactions in cells (Sayre et al., 2001), or through the
presence of strongly oxidizing compounds within a solution—all of these can contribute to
selective damage or modifi cation to protein structures. Some examples of chemical agents that
oxidatively can modify proteins include hydrogen peroxide (H
2
O
2
) and other peroxy com-
pounds, such as perborate and peroxycarbonate; hydroperoxyl radical (
HO
2
); superoxide anion
(
O
2
); singlet oxygen (
1
O
2
); hydroxyl radical (
·
OH), periodate (IO
4
); metal salts in the pres-
ence of oxygen species, such as those of iron (Fe
3
and Fe
2
) and copper (Cu
2
); ozone (O
3
);
peroxynitrite (ONOO
); Hypobromous acid (HOBr); hypochlorous acid (HOCl); performic
acid (HC(O)OOH); trichloromethylperoxyl radical (CCl
3
OO
·
); under the right conditions
metal-chelating compounds, such as porphyrins, texaphyrins, and FeBABE; and gamma radia-
tion and UV light. For additional information see Winterbourn and Kettle (2000); Baynes and
Thorpe (2000); Greenacre and Ischiropoulos (2001); Halliwell and Gutteridge (1989, 1990);
Stadtman (1992).
Singlet oxygen (
1
O
2
) differs from the predominant oxygen molecule in that O
2
is in the
ground state or triplet state and its outer two unshared electrons have parallel spins (some-
times designated
3
O
2
), which is nearly unreactive toward other molecules, while singlet oxygen
1. Modifi cation of Amino acids, Peptides, and Proteins 23
has increased energy and has its outer electrons transformed into an opposite spin orientation,
which is highly reactive. Superoxide (
O
2
) is different from singlet oxygen in that it is a reduced
form of oxygen having an extra unpaired electron, called a radical. The presence of the radi-
cal makes superoxide extremely reactive and highly damaging to proteins and other biological
molecules.
Singlet oxygen and superoxide, in addition to their modifying effects on proteins, also are
important reactive oxygen species in biological applications, as they are intermediates used in
some detection methods and in photodynamic therapy (PDT) for the treatment of cancer. One
of the more common compounds used in PDT is Photofrin, which is a mixture of oligomers
consisting of ether and ester linkages that combine up to eight porphyrin groups (Misawa et al .,
2005). The generation of reactive oxygen species takes place by irradiation with 630 nm wave-
length laser light, which also penetrates the skin effectively during therapy. Photoactivation of
the Photofrin molecule causes radical initiation to form porphyrin-excited states. Transfer of
electrons from the porphyrin groups to molecular oxygen then generates the highly reactive
singlet oxygen species. Subsequent radical reactions also can form superoxide and hydroxyl
radicals, all of which severely damage tissue in the region of the tumor and ultimately cause
cancer cell death.
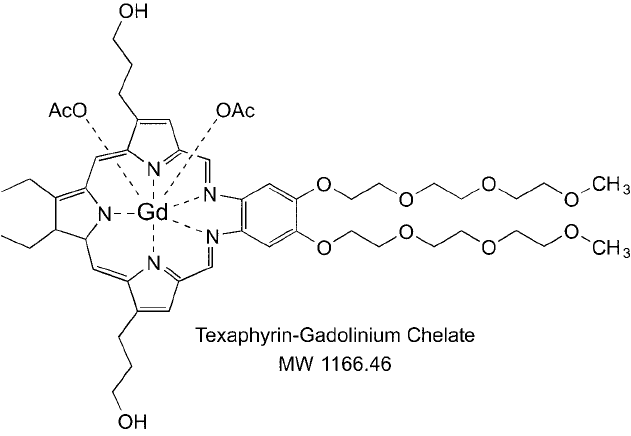
Another compound used to generate reactive oxygen species for PDT is texaphyrin, which
contains a metal-chelating ring structure resembling a porphyrin group ( Figure 1.17 ). Typically,
a gadolinium atom is chelated in the texaphyrin center and this complex becomes both a pho-
tosensitizing agent and a magnetic resonance imaging (MRI) contrast agent to better visualize
tumor locations for irradiation therapy (Donnelly et al ., 2004).
24 1. Functional Targets
Figure 1.17 The texaphyrin–gadolinium chelate structure used as a photosensitizer and MRI contrast agent in
the detection and treatment of cancer.

The reactive oxygen species involved with protein oxidation can be generally categorized
according to their relative reactivity as follows:
HO , HO O ROOH, H O O , ClO , BrO O
2
22 22
1
2
Thus, radicals are the most reactive and destructive of protein structure, followed by peroxy
derivatives, singlet oxygen, and other oxygen compounds. The oxidative reactivity of some of
these oxygen species is so high that just contact of the pure compound with paper or cotton
fabrics can cause combustion (e.g., superoxide).
In vitro studies of protein oxidation indicate that virtually all proteins and peptides are sus-
ceptible to damage by the radicals:
·
OH and
O
2
Analysis of protein modifi cation products
after oxidation indicates the presence of altered molecular weight (either fragmentation or oli-
gomerization), altered net charge, tryptophan destruction, and the formation of tyrosine dim-
ers (Davies, 1987). Even in the presence of very low concentrations of oxidants (nM), SDS
polyacrylamide gel electrophoresis of proteins can indicate multiple bands of higher and lower
molecular weight due to oxidative damage.
Transition metals in solution can catalyze the formation of reactive oxygen species that are
particularly damaging to proteins and other biomolecules. In a series of reactions, reduced tran-
sition metals, such as Fe
2
and Cu
1
, can be oxidized by oxygen to produce superoxide and
ultimately undergo a Fenton reaction to create hydroxyl radicals (Kim et al., 1985). Transition
metal-chelating groups can accelerate this reaction, as demonstrated in the process of hydroxyl
radical footprinting of protein interactions using EDTA chelates of iron (see discussion on the
reagent FeBABE in Chapter 28, Section 4).
Production of superoxide: Fe
2
O
2
→ Fe
3
O
2
Production of hydrogen peroxide: 2
O
2
2H
→ H
2
O
2
O
2
Production of hydroxyl radical: Fe
2
H
2
O
2
→ Fe
3
OH OH
The potential sites of oxidation within a protein molecule include the peptide backbone and
the side chain amino acid groups. Hydrogen atom abstraction at the -carbon of an amino
acid chain can occur upon reaction with an oxidative species to form a radical intermediate.
Subsequent reaction can result in peptide bond cleavage and fragmentation of the protein
structure, often forming carboxylic acids or carbonyls (aldehydes or ketones). This is the basic
mechanism of fragmentation caused by the bifunctional chelating reagent FeBABE. When used
in the presence of H
2
O
2
and ascorbic acid, polypeptides will fragment in the neighborhood of
interacting proteins.
Amino acid side chains can undergo oxidation through hydrogen abstraction, elimination,
or by addition reactions. In the presence of oxygen, aliphatic amino acids usually experience
oxidation to a peroxy intermediate, which causes either hydrogen atom abstraction or an elimi-
nation reaction resulting in the formation of carbonyls, hydroxyls, or other peroxides (Requena
et al., 2001) ( Figure 1.18 ). Aromatic amino acids typically undergo addition reactions following
exposure to strong oxidants. An example of this type of reaction is the nitrosation of tyrosine
groups in the presence of a peroxynitrite (ONOO
) to create o-nitrotyrosine (see Figure 1.19 ).
After exposure to an oxidant, the potential types of oxidation products in proteins and pep-
tides can be extensive (Stadtman and Levine, 2000). Cysteine and methionine undergo a vari-
ety of sulfur oxidation reactions to yield cysteine disulfi des, methionine sulfoxide, methionine
1. Modifi cation of Amino acids, Peptides, and Proteins 25
sulfone, and sulfonate products (e.g., cysteic acid) ( Figure 1.20 ). Oxidation with performic acid
can be used purposely to convert methionine and cysteine in peptides and proteins to more sta-
ble products prior to acid hydrolysis and amino acid analysis. Cysteine and methionine are
perhaps the most sensitive amino acids to oxidation, and for this reason, they are an early indi-
cator of oxidative damage to proteins.
Tyrosine also is easily modifi ed through addition reactions due to the ring activating nature
of its phenolic group. Using oxidants, tyrosine ’s ring can be chlorinated, iodinated, undergo
nitrosation or hydroxylation, and even form tyrosine–tyrosine crosslinks. The last product can
be formed purposely by use of a peroxidase in the presence of hydrogen peroxide, and this
type of reaction has been studied extensively in the manufacture of phenolic polymer resins
26 1. Functional Targets
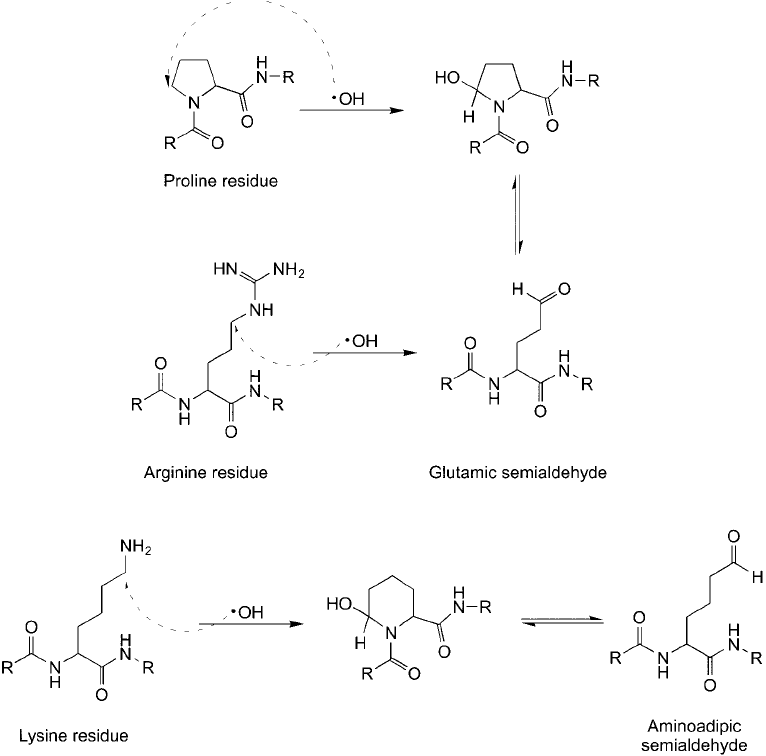
Figure 1.18 Reaction of proline, arginine, and lysine residues with hydroxyl radical results in oxidation of
side-chain structures that form carbonyls. Both arginine and proline oxidation result in the same product.
1. Modifi cation of Amino acids, Peptides, and Proteins 27
(Dordick, 1991). In addition, Fancy et al. (1996) as well as Fancy and Kodadek (1997, 1998)
have applied the oxidation of tyrosine to form dityrosine to the study of protein–protein inter-
actions using nickel-chelated 6 His tagged fusion proteins in oxidative environments.
Nitrogen-containing side chains in amino acids can be altered by oxidation forming chlo-
ramines or even become deaminated. The result is often the formation of carbonyls (e.g., alde-
hydes) and hydroxyls. Lee, S et al. (2006) found that Fe-EDTA-mediated oxidation of human
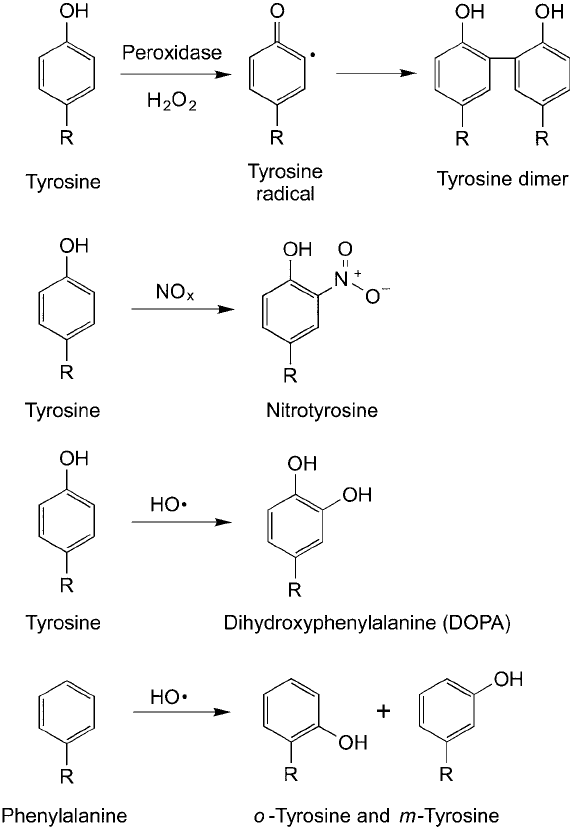
Figure 1.19 Tyrosine and phenylalanine residues can undergo oxidation to modify their phenyl side-chain
groups. Tyrosine can form covalent dimers that link two side chains together via a radical reaction. Both tyrosine
and phenylalanine can be modifi ed by oxidation to add oxygen-containing groups directly to their aromatic ring.
serum albumin resulted in extensive aldehyde and ketone formation from modifi cation of
lysine, arginine, histidine, proline, threonine, and aspartic and glutamic acids. Some groups
will oxidize and convert to another amino acid altogether. For instance, histidine can be con-
verted to asparagine, proline to hydroxyproline, and tyrosine changed to dihydroxyphenyla-
lanine (DOPA) through oxidation reactions.
It is obvious that the oxidation of protein molecules can have detrimental effects on protein
structure and function. However, there are some unique methods in bioconjugation wherein
controlled and purposeful oxidation is done to study protein–protein interactions (Chapter 28,
Section 4).
Unfortunately, there are no universal methods to detect all types of protein oxidation, because
the products formed can be so diverse in nature. However, some forms of protein oxidation can
be assayed using chemical modifi cation (Davies et al., 1999; Shacter, 2000). In particular, the
formation of carbonyl groups on proteins can be targeted using the reagent 2,4-dinitrophenyl-
hydrazine (DNPH). This compound reacts with aldehydes to form 2,4-dinitrophenylhydrazone
derivatives, which create chromogenic modifi cations that can be detected at high sensitivity in
microplate assays or Western blot analysis (Buss et al., 1997; Winterbourn et al., 1999).
28 1. Functional Targets
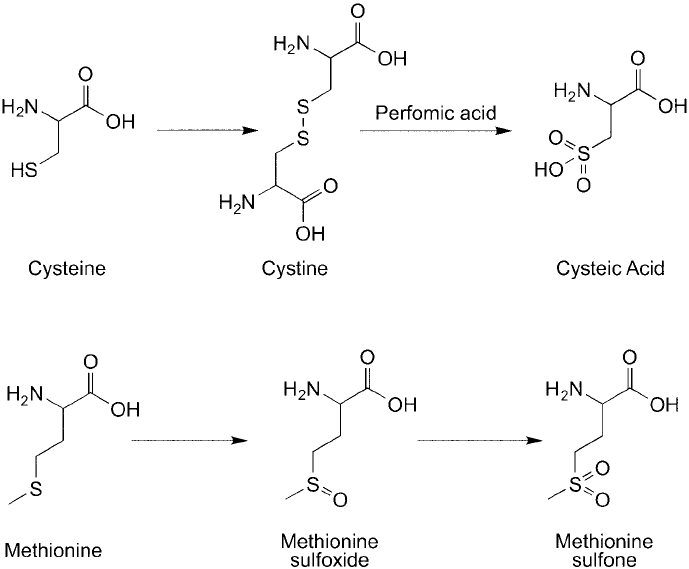
Figure 1.20
Cysteine and methionine are highly susceptible to oxidation reactions. Cysteine thiols can form
disulfi de linkages with other cysteine groups or be oxidized to cysteic acid. Methionine is oxidized very easily to
the sulfoxide or sulfone products.
In addition, a method involving mass spec analysis to determine carbonyl formation as a
result of protein oxidation was developed using a novel mass tag. The carbonyl-specifi c
Element-Coded Affi nity Mass Tag (O-ECAT) can covalently couple to aldehyde or ketone
oxidation sites using an aminoxy group to form an oxime (Lee et al., 2006; see also Chapter 16,
Section 2). The ECAT mass tag consists of a bifunctional metal-chelating group that coor-
dinates a lanthanide metal ion of specifi c mass. Proteins that have been oxidized to contain
carbonyls can be labeled with this reagent and the exact sites of modifi cation determined by
analyzing the mass spec signature of the labeled peptides after proteolysis.
Solvent Accessibility of Functional Targets in Proteins
Proteins are highly complex, folded polypeptide chains consisting of at least 20 different amino
acids that are strung together in unique sequences, which relate to structure and function.
Particular amino acids in proteins may be further modifi ed post-translationally to contain a
wide variety of covalent modifi cations normally found in native proteins. The way in which a
peptide chain is wrapped and folded governs each amino acid ’s relative exposure to the outside
environment, but post-translational modifi cations also can obscure the protein surface from
easy access to the solvent environment.
Amino acid side chains are the primary effectors of the three-dimensional structure of a pro-
tein, because their properties vary depending on the presence of charged groups, uncharged
polar components, aliphatic chains, aromatic rings, and groups able to form hydrogen bonds
with other amino acid residues. The relative hydrophilicity or hydrophobicity of an amino acid
side chain is a major factor in determining whether the group will be found on the surface of a
globular protein or buried within its globular structure.
However, just considering the individual properties of each amino acid type is not enough
to determine its accessibility to the surrounding aqueous environment. There have been many
attempts at developing analytical models with predictive value for determining buried or sur-
face accessible amino acids in a folded polypeptide chain. These studies have concluded frac-
tional assignments for each residue that relate to its accessible surface area (ASA) or its solvent
exposed area (SEA).
In most cases, there are general trends that emerge from theoretical studies in which
hydrophilic amino acids are more likely to be found on the surface of a protein and hydro-
phobic amino acids are more likely to be inside its three-dimensional structure, but we already
knew this intuitively so this conclusion is not surprising. However, a real-life study of the posi-
tions of amino acids in proteins whose structures are known is more revealing. The data for
Figure 1.21 was calculated from 55 proteins in the Brookhaven database by Bordo and Argos
(1991), and the graph was derived from the analysis as presented by the Jena Image Library
of Biological Macromolecules ( http://www.imb-jena.de/IMAGE_AA.html ). Although most of
these structures were determined using crystallographic means and thus the proteins are “ fro-
zen ” in a single structural state, the results are revealing as to how often particular amino acids
are accessible to the surrounding solvent.
Three levels of SEA are presented in the graph for each amino acid, which corresponds to
areas in Å
2
accessible to the solvent environment: greater than 30 Å
2
for highly accessible amino
acids, between 10 and 30 Å
2
for medium accessibility, and less than 10 Å
2
for those residues
that are relatively not accessible to the solvent. Only the SEA for each amino acid of 30 Å
2
is
shown in the plotted data. The graph shows that the polar amino acids such as serine, threonine,
1. Modifi cation of Amino acids, Peptides, and Proteins 29
