Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

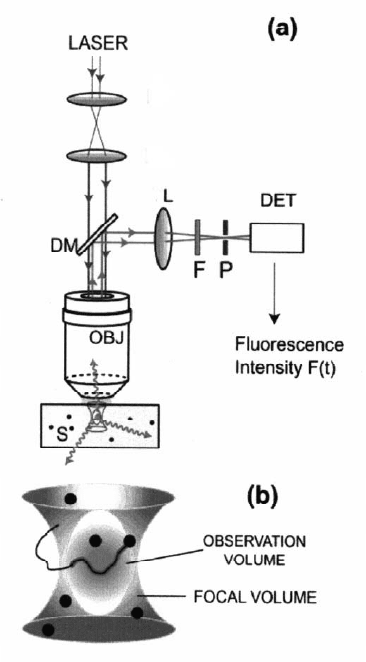
670 Gerhard N¨agele, Jan K. G. Dhont, and Gerhard Meier
Fig. 16.19. (a) Typical configuration of confocal optics as used in an FCS experi-
ment. A wide laser beam is strongly focussed by means of a high numerical aperture
objective (OBJ). The fluorescent light is collected by the same objective, which is
reflected by a dichroic mirror (DM), and focussed by a lens (L) onto a pinhole
(P). The band filter admits fluorescent light to the detector (DET). The region
from which fluorescent light is detected is limited to a small volume (the so-called
confocal volume) by means of the pinhole P. (b) A magnification of the confocal
volume. Only that part of a macromolecule that is inside the volume contributes
to the measured fluorescent intensity. After [44].
16 Diffusion in Colloidal and Polymeric Systems 671
I
0
(r) ∼ exp{−(x
2
+ y
2
)/2σ
2
1
} exp{−z
2
/2σ
2
2
} , (16.169)
where σ
1
measures the width of the confocal volume in the plane perpendic-
ular to the propagation of the beam, while σ
2
measures the length along the
propagation direction. Typically, σ
1
≈ 100−300 nm, and σ
2
≈ 200−600nm.
Similar to (16.158), the fluorescent intensity is equal to
I
f
(t) ∼
dr I
0
(r) c
d
(r,t) , (16.170)
where c
d
is the local concentration of fluorescent dye molecules, which are
(at least in part) attached to the colloidal particles. The time dependence is
now entirely due to the fact that colloidal particles diffuse in and out of the
confocal volume. What is neglected in (16.170) is the dependence of excitation
probabilities and fluorescent intensities on the orientation of dye molecules.
This is probably a good approximation when there are many dye molecules,
with random orientations, attached to the colloidal particles. Contrary to
FRAP, the volume from which fluorescent intensities are collected is assumed
to contain at most a single colloidal particle. At high concentrations this
sometimes requires to mix labelled with unlabelled colloidal particles. In a
FCS experiment, the intensity correlation function C
f
(t) ≡I
f
(t) I
f
(t =0)
is measured. From (16.170) we obtain
C
f
(t)=
dr
dr
I
0
(r) I
0
(r
) c
d
(r,t) c
d
(r
,t=0)
∼
dq
dq
I
0
(q) I
0
(q
) c
d
(q,t) c
d
(q
,t=0) , (16.171)
where in the second equation Bessel’s theorem has been applied twice, to
convert spatial integrations to wavevector integrations, which will simplify
the further mathematical analysis. Here, q-andq
-dependent functions are
understood to be spatial Fourier transforms with respect to r and r
, respec-
tively. Now suppose that there is at most a single fluorescent colloidal particle
inside the confocal volume. The Fourier transform of the dye concentration
c
0
can be written as
c
0
(q,t)=B
f
(q,t)exp{iq · r
c
(t)} , (16.172)
where r
c
is the position coordinate (the position of the center of mass) of the
colloidal particle, and the “fluorescence amplitude” B
f
is defined as
B
f
(q,t)=
V
0
(t)
dr χ
d
(r)exp{iq · r} , (16.173)
where V
0
is the volume occupied by the colloidal particle with its center-of-
mass at the origin (as indicated by the subscript “0”), and
χ
d
(r)isthechar-
acteristic function for the dye on and within the core of the colloidal particle

672 Gerhard N¨agele, Jan K. G. Dhont, and Gerhard Meier
(χ
d
(r) = 1 when there is a dye molecule at r, and = 0 otherwise). The ex-
ponential with the position coordinate originates from the transformation to
the spatial integration with respect to a coordinate system where the center-
of-mass is chosen as the origin (similar as has been done in Sect. 16.2, dealing
with the principles of light scattering). The integral in (16.173) depends only
on the way dye molecules are distributed over its surface and possibly inside
its core. The volume V
0
is time dependent for colloidal particles where the
dye distribution is non-spherically symmetric, through the orientation of the
colloidal particle. Possible rotational contributions to the correlation function
C(t) originate from this integral. Such orientational contributions become im-
portant when the largest linear dimension of the fluorescent macromolecule
are of the order of or larger than the dimensions of the confocal volume. In
that case, a mere rotation of the macromolecule can give rise to a change of
the fluorescent intensity. Translational contributions originate from the time
dependence of the position coordinate r
c
. From (16.171), (16.172) we have
c
d
(q,t) c
d
(q
,t=0)∼exp{iq · r
c
(t)} exp{iq
· r
c
(t =0)} . (16.174)
Since, for a homogeneous system, the probability density function P of
{r
c
(t), r
c
(t =0)} depends only on the difference r
c
(t) − r
c
(t = 0), it fol-
lows that (with R = r
c
+ r
c
and ∆r = r
c
− r
c
)
c
d
(q,t) c
d
(q
,t=0)
∼
dr
c
dr
c
P (r
c
− r
c
,t)exp{iq · r
c
} exp{iq
· r
c
}
∼
dR exp{i
1
2
(q + q
) · R}
×
d∆r P (∆r,t)exp{i
1
2
(q − q
) · ∆r}
. (16.175)
Since
dR exp{ i
1
2
(q + q
) · R}∼δ(q + q
) , (16.176)
where δ is the Dirac delta distribution, it follows from (16.175), that
c
d
(q,t) c
d
(q
,t=0)∼c
d
(q,t) c
d
(−q,t=0)δ(q + q
) . (16.177)
This equation expresses translational invariance of a homogeneous system.
Substitution into (16.171) thus leads to
C
f
(t) ∼
dq I
2
0
(q) B
f
(q,t) B
f
(q,t=0)exp{iq · (r
c
(t) − r
c
(t =0))} ,
(16.178)
where “#” stands for complex conjugation. Note that, when the core of the
colloidal particle is homogeneously labelled, the ensemble average in (16.178)

16 Diffusion in Colloidal and Polymeric Systems 673
for the fluorescence autocorrelation function is nothing but the electric field
autocorrelation function in (16.20) that one would measure in a dynamic
light scattering experiment, provided that the scattering power is homoge-
neously distributed over the core of the particles. The fluorescence correlation
function is a weighted q-average of the light scattering correlation function.
When the confocal volume is large compared to the linear dimensions of the
colloidal particles, the rotational contributions arising from the fluorescence
amplitudes B
f
in (16.178) do not contribute to the time dependence of the
fluorescence correlation function (in fact, numerical calculations for stiff rods
show that this is already the case when σ
1
≥ L/2, with L the length of the
rods). Hence, for any practical application we have
C
f
(t) ∼
dq I
2
0
(q) exp{iq · (r
c
(t) − r
c
(t =0))} . (16.179)
Since the fluorescent intensity only changes when the colloidal particle is
displaced over a distance larger or comparable to dimensions of the confocal
volume, which is in turn larger than the linear dimensions of the colloidal par-
ticles, the correlation function in the above equation is the long-time limiting
form as given in (16.165). Using that the Fourier transform of the Gaussian
profile in (16.169) is given by
I
0
(q) ∼ exp{−(q
2
x
+ q
2
y
)σ
2
1
/2} exp{−q
2
z
σ
2
2
/2} , (16.180)
where q
j
is the j-component of q, it is thus found that
C
f
(t) ∼
dq
x
exp{−q
2
x
!
D
l
s
t + σ
2
1
"
}×
dq
y
exp{−q
2
y
!
D
l
s
t + σ
2
1
"
}
×
dq
z
exp{−q
2
z
!
D
l
s
t + σ
2
2
"
}∼
1+
D
l
s
t
σ
2
1
−1
1+
D
l
s
t
σ
2
2
−1/2
. (16.181)
The fluorescent intensity autocorrelation function thus decays algebraically
with time, with the two time constants σ
2
1,2
/D
l
s
. The geometrical constants
σ
1,2
can be determined from a measurement of a dilute system where D
l
s
is equal to the Stokes-Einstein diffusion coefficient, that can be obtained
independently from dynamic light scattering.
When the size of the particles is not small in comparison with the confocal
volume, the q-dependence of the fluorescence amplitudes in (16.178) comes
into play. For spherical colloidal particles, in which the fluorescent dye is
radially symmetrically distributed, the amplitudes are independent of time,
so that (16.178) simplifies to
C
f
(t) ∼
dq
!
I
eff
0
(q)
"
2
exp{iq · (r
c
(0) − r
c
(t =0))} ,
where

674 Gerhard N¨agele, Jan K. G. Dhont, and Gerhard Meier
I
eff
0
(q)=I
0
(q) |B(q)|
is the Fourier transform of the intensity complying with an “effective confocal
volume”. For homogeneously labelled spheres, | B(q) | can be represented
quite accurately by a Gaussian ∼ exp{−0.11 q
2
a
2
}, up to wavevectors where
B(q) is small enough that it does not contribute anymore to the integral over
q. Here, a is the radius of the spherical colloidal particle. We thus obtain the
“effective size” of the confocal volume as
σ
eff
1,2
=
*
σ
2
1,2
+0.22 a
2
.
When the particles are large, the fluorescent intensity is non-zero already
when the outer part of a particle enters the confocal volume. At that moment
the center of the colloid is still outside the confocal volume. This leads to an
increase of the apparent confocal volume, as quantified for homogeneously
labelled spheres by the present expression for σ
eff
1,2
.
Experimental correlation functions show some features that we have not
discussed above. First of all, in the various steps taken in the above analysis,
prefactors are always omitted. It turns out, by including all prefactors, that
the proportionality constant in (16.181) is equal to 1/[the average number
of fluorescent colloidal particles in the confocal volume]. This reflects the
relative decrease of number fluctuations in a given volume with increase of
volume. Secondly, there may be free dye molecules, not attached to colloidal
particles, in the solvent. When this is the case, there is a second, additive
contribution of the form (16.181), except that the time constants are much
smaller as compared to those for the colloidal particles. Free dye is therefore
seen only at very small times. Thirdly, dye molecules that are excited in
long-lived triplet states do not contribute to the fluorescent intensity and
therefore temporarily reduce the number of fluorescent dye molecules. Triplet
state excitation can thus be regarded as “reversible bleaching” (contrary to
the irreversible bleaching in the FRAP experiments described earlier). The
relative amplitude of such contributions depends on the sort of dye that is
used, as well as the concentration of dye on the surface and/or inside the core
of a colloidal particle.
A recent overview of FCS can be found in [44]. An extensive literature list
can be found on the FCS website of Zeiss. The above analysis is a simplified
version of a more elaborate analysis in [45].
As far as we are aware, there are no systematic FCS investigations pub-
lished on macromolecular systems. An example of a fluorescence correlation
function on fd-virus (a semi-flexible rod of length 880 nm and width of about
6 nm, with a persistence length of 2200 nm), labelled with TARAM , is given
in Fig. 16.20 [46]. At very small times a triplet contribution is found, at in-
termediate times a decay due to free dye and at longer times decay due to
diffusion of the fd-virus. The curve is fitted with a sum of three terms: a
single exponential function to account for the triplet contribution, and a sum
of two contributions of the form as in (16.181) (one for the free dye and one
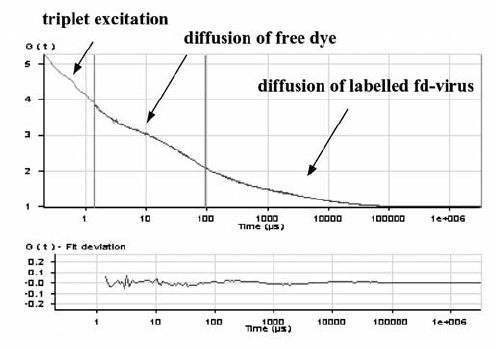
16 Diffusion in Colloidal and Polymeric Systems 675
Fig. 16.20. A fluorescence correlation function for fd-virus, labelled with TARAM.
At small times a triplet contribution is clearly present, at intermediate times the
correlation function decays due to the presence of free dye molecules, and at larger
times due to diffusion of fd-virus. The lower part in the figure gives the residues
after a least-square fit as described in the main text.
for the fd-virus). Clearly, the conditions should be optimized, in particular
to minimize the triple contribution.
16.5 Theoretical and Experimental Results on Diffusion
of Colloidal Spheres and Polymers
For times t τ
B
, which is the regime of most DLS experiments, the veloci-
ties of the colloidal spheres have relaxed to equilibrium, so that only the slow
relaxation of the particle positions and orientations is probed. Therefore, the
dynamics of interacting spheres is entirely described on this coarse-grained
level in terms of a many-particle pdf, P (r
N
,
ˆ
u,t), in the configuration space
of positional and orientational degrees of freedom. The pdf depends thus,
in principle, on the center-of-mass position vectors r
N
=(r
1
, ··· , r
N
)and
orientation vectors
ˆ
u
N
=(
ˆ
u
1
, ··· ,
ˆ
u
N
)ofallN spheres in the suspension
(scattering volume) V
s
. The equation of motion for P (r
N
,
ˆ
u
N
,t)isagener-
alization of the one-particle diffusion equation (16.45) to interacting parti-
cle systems. This many-particle diffusion equation is known among colloid
scientists as the generalized Smoluchowski equation (GSE). In the polymer
science community, it is better known as the Kirkwood-Riseman-Zimm equa-
tion [13, 47].
The description of the configurational evolution by means of the GSE is
founded on the separation of time scales between the fast fluctuating parti-

676 Gerhard N¨agele, Jan K. G. Dhont, and Gerhard Meier
cle velocities, and the slow configurational changes. Such a description can-
not be applied to polymer blends since the relaxation of the matrix, which
plays the role of the solvent, is comparable to those of the other compo-
nents. To explore the polymer dynamics in the diffusion limit (or Markovian
limit) of small wavenumbers and long times, we resort therefore to the semi-
phenomenological dynamic RPA. Using the RPA, we will study the interdif-
fusion process in a ternary homopolymer mixture.
16.5.1 Colloidal Spheres
Consider N identical colloidal spheres in a quiescent and unbound Newtonian
solvent. The evolution for the pdf of the particle positions alone
P (r
N
,t)=
dr
N
P (r
N
,
ˆ
u
N
,t) (16.182)
is described, for t τ
B
, by the translational GSE
∂
∂t
P (r
N
,t)=
<
O(r
N
)P (r
N
,t) , (16.183)
where
<
O(r
N
)=
N
i,j=1
∇
i
· D
ij
(r
N
) · [∇
j
− βF
j
] (16.184)
is the Smoluchowski differential operator. Here, F
j
= −∇
j
U(r
N
) denotes
the force that all other N − 1 spheres exert on sphere j, through potential
interactions U(r
N
). It is assumed here that the potential energy U (r
N
)isin-
dependent of orientation so that the particles exert no torques on each other.
Otherwise, the translational motion would be coupled to the rotational one.
The solvent appears in (16.183) only through the time-independent transla-
tional diffusivity tensors D
ij
(r
N
), which describe the solvent-mediated HI
between the spheres. The tensors D
ij
(r
N
) relate the hydrodynamic forces
{F
H
j
}, exerted on the surfaces of the spheres by the surrounding fluid to the
resulting drift velocity v
i
of a sphere i in form of a generalized Stokes’ law
v
i
= −
1
k
B
T
N
j=1
D
ij
(r
N
) · F
H
j
. (16.185)
The propagation of hydrodynamic disturbances (via sound and diffusion of
shear waves) appears to be infinitely fast for times t τ
η
. Therefore, the
HI between the spheres act quasi instantaneously on these time scales and
D
ij
(r
N
) can be determined, in principle, from solving the stationary lin-
earized Navier-Stokes equation describing the creeping flow in an incompress-
ible fluid, augmented by stick boundary conditions on the sphere surfaces.
Yet, actual analytical calculations of the D
ij
(r
N
) are very difficult and have

16 Diffusion in Colloidal and Polymeric Systems 677
been fully achieved only on the pairwise-additive level, mainly in form of
inverse distance expansions. Hereby one disregards the influence of other
spheres on the HI between a given pair of spheres, an approximation which is
valid only for large interparticle distances. Note for spheres that the D
ij
r
N
)
depend only on the positions. For non-spherical particles, HI couple position
and orientation variables. If we ignore HI, D
ij
= δ
ij
D
0
1 and (16.185) reduces
to the standard Stokes’ law for isolated single spheres.
In equilibrium, (∂/∂t)P = 0, and the GSE is satisfied by the equilibrium
pdf
P
eq
(r
N
)=exp{−βU(r
N
)}/
dr
N
exp{−βU(r
N
)} (16.186)
independent of the D
ij
(r
N
). This shows that the HI are dynamic forces with
no effect on static equilibrium properties.
Using the GSE, one can express equilibrium time-correlation functions
like S
c
(q, t)andS
s
(q, t)as
S
A
(q, t)=ρ
A
(−q)
exp{
<
O
B
t}ρ
A
(q)
, (16.187)
with A = a, c and microscopic densities ρ
c
(q)=ρ(q) (see (16.24)) and
ρ
s
(q)=exp{iq · r
1
}.Here
<
O
B
(r
N
)=
N
i,j=1
∇
i
+
1
k
B
T
F
i
· D
ij
(r
N
) ·∇
j
(16.188)
is the adjoint (or backward) Smoluchowski operator, and
··· =
dr
N
P
eq
(r
N
)(···) (16.189)
is the equilibrium ensemble average. Note that
<
O
B
operates only on ρ
A
,and
not on P
eq
.
In the following subsection, we describe theoretical methods based on the
GSE. From a theoretical point of view, it is appropriate to treat short-time
diffusion and long-time diffusion separately, since the former is needed as an
input to the latter one.
Short-Time Diffusion
In general, S
A
(q, t) cannot be calculated exactly from (16.187), owing to the
complicated form of the operator
<
O
B
for interacting particles. However, for
short times t τ
I
, S
A
(q, t) can be expressed in a series of cumulants, that is
678 Gerhard N¨agele, Jan K. G. Dhont, and Gerhard Meier
S
A
(q, t)=S
A
(q)exp
∞
l=1
(−t)
l
Γ
(l)
A
(q)
l!
(16.190)
= S
A
(q)exp
−Γ
(1)
A
(q)t +
1
2
Γ
(2)
A
(q)t
2
+ ···
with S
A
(q)=S
A
(q, t). The higher-order cumulants, Γ
(l)
A
, with l =2, 3, ···,
measure the deviation of S
A
(q, t) from a single-exponential decay. Cumulant
analysis is a customary tool to analyze DLS data at short times, whereby
mainly the first and second cumulants have been determined. One can al-
ternatively expand S
A
(q, t) in a time Taylor series, resulting in the so-called
moment expansion
S
A
(q, t)=
∞
n=0
t
n
n!
S
(n)
A
(q)=S
A
(q)+tS
(1)
a
(q)+
1
2
t
2
S
(2)
A
(q)+... , (16.191)
with moments
S
(n)
A
(q)=
1
N
ρ
A
(−q)(
<
O
B
)
n
ρ
A
(q). (16.192)
In deriving (16.192), we have expanded the time evolution operator exp{
<
O
B
}
in (16.187) in powers of t. From a small-t expansion of (16.191), it follows for
the two leading cumulants that
Γ
(1)
A
(q)=−
S
(2)
A
(q)
S
A
(q)
(16.193)
Γ
(2)
A
(q)=
S
(2)
A
(q)
S
A
(q)
−
S
(1)
A
(q)
S
A
(q)
2
.
In specializing to A = c, the first cumulant of the collective dynamic structure
factor follows as
Γ
(1)
c
(q)=q
2
D
s
c
(q)=q
2
D
0
H(q)
S
c
(q)
. (16.194)
Here, D
s
c
(q) is the apparent short-time collective diffusion coefficient already
defined in (16.98), and H(q)isgivenby
H(q)=
1
ND
0
N
l,j=1
ˆ
q · D
lj
(r
N
) ·
ˆ
q exp{iq · [r
l
− r
j
]}. (16.195)
The function H(q) ≥ 0 contains, through the diffusion tensors D
lj
, the in-
fluence of HI on the short-time collective diffusion. For this reason, H(q)
is known as the hydrodynamic function. Without HI, H(q) ≡ 1, so that
D
s
c
(q)=D
0
/S
c
(q)inthiscase.Anyq-dependence of H(q)isthusanindicator

16 Diffusion in Colloidal and Polymeric Systems 679
for the non-negligible influence of HI. Comparison with the phenomenological
(16.108) shows that
lim
q→0
H(q)=
U
s
U
0
. (16.196)
Hence, the long-wavelength limit of the hydrodynamic function is equal to
the relative (short-time) sedimentation velocity in a homogeneous suspension.
According to (16.195), H(q) is indeed a short-time equilibrium average.
Next, the first cumulant for the self-dynamic structure factor is deter-
mined as (see (16.192))
Γ
(1)
s
(q)=q
2
D
s
s
, (16.197)
with the microscopic expression
D
s
s
=
ˆ
q · D
11
(r
N
) ·
ˆ
q (16.198)
for the translational short-time self-diffusion coefficient. Without HI, D
s
s
=
D
0
since at short times the Brownian motion of a sphere is not influenced by
direct forces.
For large q q
m
, strong oscillations in the exponential factors in (16.195)
cancel each other for l = j,andH(q) becomes therefore equal to the reduced
short-time self-diffusion coefficient, i.e.
H(q q
m
) ≈
D
s
s
D
0
. (16.199)
Likewise, D
s
c
(q q
m
) ≈ D
s
s
since S
c
(q q
m
) = 1. Thus, it is in principle
possible to determine short-time self-diffusion properties from DLS experi-
ments performed at long wavenumbers without a need for contrast variation.
Index matching is needed, however, to determine the MSD at longer times.
Moments up to the third order have been calculated for S
A
(q, t). The
expression for the second moment of S
c
(q, t)readswithoutHI
S
(2)
c
(q)=(q
2
D
0
)
2
1+
βρ
0
q
2
dr g(r)(1− cos(q · r)) (
ˆ
q ·∇)
2
u(r)
.
(16.200)
It is given in terms of the pair distribution function g(r), and derivatives of
the pair potential, u(r), between two spheres. Hereby it is assumed that the
total potential energy is pairwise additive, that is U(r
N
)=
i<j
u(|r
i
−r
j
|).
The second cumulant, S
(2)
s
(q), of S
s
(q, t) is given by (16.200) with the cos(q·r)
term omitted, since
S
c
(q q
m
,t)=S
s
(q, t) (16.201)
due to smallness of the distinct part, S
d
(q, t), of S
c
(q, t)forq q
m
(cf.
(16.28)).
Little is known about the higher-order moments. With HI, even the sec-
ond moment becomes quite complicated, invoking now up to four-particle
