Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

3 Diffusion Studies of Solids by Quasielastic Neutron Scattering 141
0,0 0,5 1,0 1,5 2,0
0,0
0,2
0,4
0,6
0,8
1,0
0,0 0,5 1,0 1,5 2,0
0,0
0,2
0,4
0,6
0,8
1,0
2.18 Å
1.54 Å
1.45 Å
1.00 Å
Rb
3
H(SeO
4
)
2
T=500 K
NEAT
λ
0
=6.2 Å
(
∆t)
H
= 0.4x10
-10
s
EISF
Q [ Å
-1
]
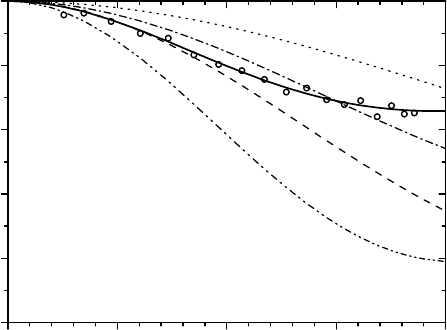
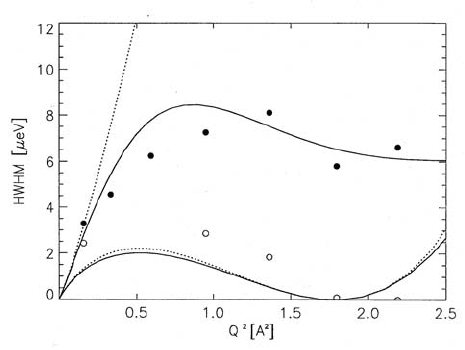
Fig. 3.28. Theoretical EISF curves for several examples of simple two-site and
three-site proton jump models (dashed, dotted and dashed-dotted lines) are com-
pared to the “apparent” EISF (open circles) obtained at medium energy resolution
with the time-of-flight spectrometer NEAT (after [112]). The simple models are
not able to reproduce the experimentally observed data, whereas the successful
trigonal-asymmetric hydrogen bond (TAHB) model (solid line; see text) gives good
agreement [122].
The time-dependent site occupation probabilities for the 4 sites are de-
noted by W
j
(t). Their time-averages,
W
∞
j
(t) = lim
t→∞
W
j
(t), (3.67)
are used to introduce an order parameter η,
η =2W
∞
1
− 1, (3.68)
where full order (η = 1) means that there is no proton exchange with the ex-
ternal sites. The incoherent scattering function of the model is given by [122]:
S
inc
(Q,ω)=EISF(Q) ·δ(ω)+QISF
2
(Q) ·L
2
(H
2
,ω)+QISF
3
(Q) ·L
3
(H
3
,ω)
(3.69)
Here the elastic and quasielastic incoherent structure factors, EISF and
QISF
j
, depend on the jump vectors and on η. The two Lorentzians are given
by:
L
j
(H
j
,ω)=H
j
/[(H
j
)
2
+ ω
2
]/π (3.70)
H
2
=(3/τ
1
+1/τ
2
)=6/(1 − η)/τ
1
; (3.71)
142 Tasso Springer and Ruep E. Lechner
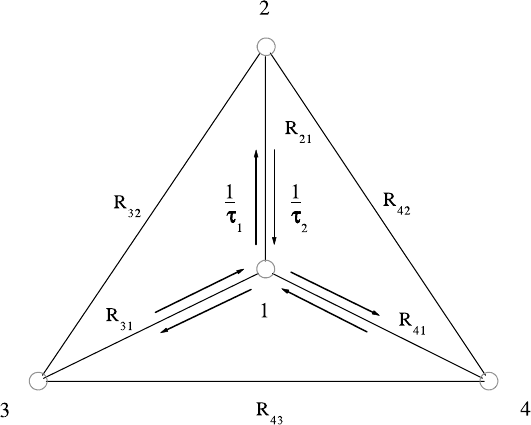
Fig. 3.29. Local site arrangement corresponding to the trigonal-asymmetric hy-
drogen bond model. This model is compatible with the trigonal symmetry of
Rb
3
H(SeO
4
)
2
and with the measured EISF. The central site 1, with the average
proton residence time τ
1
, is connected to the three external sites, with residence
time τ
2
,bythejumpvectorsR
21
, R
31
and R
41
. The EISF analysis (Fig. 3.28)
suggests that most of the proton probability density is located in site 1. This is,
because the two proton exchange rates, 1/τ
1
and 1/τ
2
, are different (after [122]).
H
3
=1/τ
2
=3(1+η)/(1 − η)/τ
1
. (3.72)
A comparison of this model to the QINS spectra obtained at 500 K gave η
= 0.746 and R
21
=1.785
˚
A. The asymmetry represented by this value of η
is appreciable. Accordingly, the occupancy of site 1 is at least 5 times larger
than the sum of the occupation probabilities of the 3 external sites. From
this it also follows, that – at the temperature of the experiment – the average
lifetime of the dimer is about 20 times shorter than that of the monomers, a
consequence of the asymmetry of the potential. The meaning of these obser-
vations has been discussed in more detail in [112] and [122]. To summarize,
the important aspect of the obtained η-value is the result that the proton
essentially remains bonded to the original selenate top oxygen, i.e. to one
selenate group, for a period of the order of at least 10
−10
s. The value of the
jump distance R
21
may, however, be taken as an indication that the proton is
also exploring - on the 10
−11
s to 10
−10
s time scale - a path into the hydrogen
bridge, although with rather low probability. These observations have been
interpreted as a consequence of the presence of an intracrystalline chemical
equilibrium reaction: alternation between the association of the monomers
[HSeO
4
]
1−
and [SeO
4
]
2−
, resulting in the dimer [H(SeO
4
)
2
]
3−
(H-bond for-
3 Diffusion Studies of Solids by Quasielastic Neutron Scattering 143
mation), and the dissociation of the latter into the two monomers (H-bond
breaking) [112]. Reaction rates and potential barriers for association and dis-
sociation processes have been determined, as well as temperature-dependent
proton exchange rates between neighbouring selenate ions.
It may be concluded, that the dynamical disorder existing in the high-
temperature phase of Rb
3
H(SeO
4
)
2
can be described by the above-mentioned
chemical equilibrium reaction. The two distinct transient chemical states play
different specific roles in the mechanism of protonic conductivity. While in the
monomer state the protonated monomer via reorientation makes the proton
available for three different elongated (and therefore weak) hydrogen bonds,
the dimer state corresponding to the creation of a strong (but short-lived)
H-bond, with the proton close to the center of the bond, provides the oppor-
tunity for proton transfer across the hydrogen bridge.
3.11 Two-Dimensional Diffusion
In view of the important role played by low-dimensional ionic conduction
and diffusion in solid-state physics, electrochemistry and even in biology, we
present several results concerning this problem. These have been obtained,
for instance, in studies of layered structures exhibiting quasi two-dimensional
proton conduction and diffusion, such as CsOX · X
2
O, M
3
X(AO
4
)
2
,and
Zr(XBO
4
)
2
· X
2
O, (where M = NH
4
,Rb,Cs;A=S,Se;B=P,As;X
=H,D).
Obviously, the concept of low-dimensionality should not be understood in
the sense of strictly planar motions, since there will be local deviations from
this idealized picture. The essential condition is, that the diffusing protons
stay within the conducting layers of the crystal at least during the observa-
tion time defined by the energy resolution of the experiment (see Sect. 3.2,
(3.21), and the related discussion). This also implies that the mean square
displacement parallel to the layer, the proton has acquired during this time,
is much larger than perpendicular to it.
In previous paragraphs, we have already mentioned the cases of the lay-
ered structures Ba
2
NH [88] (see Sect. 3.8), CsOH · H
2
O [97] (see Sect. 3.9)
and Rb
3
H(SeO
4
)
2
[122] (see Sect. 3.10). All these structures are pseudo-
hexagonal or trigonal. The distances between neighbouring (transient) sites
of mobile ions found in crystallographic studies are much shorter within the
layer planes than in directions parallel to the c-axes being perpendicular to
these planes. This leads to the assumption, that ion diffusion and ionic con-
ductivity are likely to be confined to two dimensions. Such assumptions have
been made occasionally, in the absence of single crystals which would al-
low the direct determination of the dimensionality by a QENS experiment.
For CsOH · H
2
O, this assumption has been confirmed by spin-lattice relax-
ation time (T
1
) measurements revealing a logarithmic frequency dependence
of the relaxation rate T
−1
1
[43], typical for two-dimensional diffusion. It is

144 Tasso Springer and Ruep E. Lechner
known from theory [123–125], that the corresponding behaviour of T
−1
1
as a
function of ln ν
0
is linear, whereas for three-dimensional diffusion T
−1
1
is inde-
pendent of the nuclear magnetic resonance frequency ν
0
at high frequencies
(for details see Sect. 9.2 in Chap. 9). Spin-lattice relaxation also led to the ex-
perimental confirmation of 2D-diffusion of Li ions between graphite layers in
Li-graphite intercalation compounds via the observed logarithmic magnetic-
field dependence of the diffusion-induced component of T
−1
1
[126]. The same
authors also employed quasielastic neutron scattering in order to determine
the mean residence time and the jump distance in the diffusion plane of Li
ions from the oscillatory Q-dependent behaviour of the measured quasielas-
tic linewidth. In the case of Rb
3
H(SeO
4
)
2
, the two-dimensional character of
proton diffusion was revealed by conductivity measurements on single crys-
tals [114,115]. Zr(HPO
4
)
2
,initsβ-phase, is another two-dimensional proton
conductor with a proton site lattice similar to that of Rb
3
H(SeO
4
)
2
[127].
The proton diffusion mechanism in this crystal comprises – in addition to the
two-dimensional long-range transport – a very fast localized diffusive motion
related to librations of protonated phosphate tetrahedra [121].
The incoherent neutron scattering function in the low-Q limit for long-
range translational diffusion (TD) was already considered in Sect. 3.3 (see
(3.31) to (3.33). Accordingly, in three dimensions, when the diffusive motion
is isotropic, with a diffusion coefficient D
3D
,wehaveforthisfunction
S
3D
TD
(Q,ω)=
1
π
D
3D
Q
2
(D
3D
Q
2
)
2
+ ω
2
, (3.73)
Certain analytic results [128] concerning the anisotropic case, where the diffu-
sion process is restricted to the planes of a layered structure, will be discussed
in the following. For a single-crystalline sample we have then [129]
S
2D
TD
(Q,ω)=
1
π
D
2D
(Q sin θ)
2
(D
2D
(Q sin θ)
2
)
2
+ ω
2
, (3.74)
where D
2D
is the coefficient of self-diffusion in two dimensions, Q sin θ the
component of the the scattering vector in the diffusion plane, and θ the angle
between Q and the normal to this plane. If single-crystals are not available,
one has to resort to polycrystalline samples requiring orientational averaging
of the above expression. It is known, that the resulting integral over all orien-
tations exhibits a logarithmic singularity at zero energy transfer [129]. This
is caused by the fact that diffusion planes which are perpendicular (or close
to perpendicular) to the scattering vector Q contribute elastic (or almost
elastic) scattering to the QINS function. Experimentally, such a singularity
cannot easily be distinguished from truly elastic scattering, and the numeri-
cal convolution with a resolution function is rather tedious. The question is
to what extent the characteristic feature of low-dimensionality represented
by the above-mentioned singularity can still be exploited when the observed

3 Diffusion Studies of Solids by Quasielastic Neutron Scattering 145
scattering function is resolution-broadened. Evidently the logarithmic sin-
gularity is cancelled by finite resolution. It has been shown that for resolu-
tion functions which have the shape of a Lorentzian (with HWHM equal to
H) or of a sum of Lorentzians (which is typically the case in BSC experi-
ments) the orientationally averaged resolution-broadened QINS function for
2D-diffusion,
S
2D
TD
(Q,ω)
resol
orient
=
1
2π
2π
0
dΦ
1
2
π
0
sin θdθL(θ, ω) (3.75)
where
L(θ, ω)=
1
π
D
2D
(Q sin θ)
2
+ H
[D
2D
(Q sin θ)
2
+ H]
2
+ ω
2
(3.76)
can be written in closed form [128]. The explicit expression is lengthy
and will not be given here. Instead, Fig. 3.30 shows the result of numer-
ical calculations of this function for a two-dimensional diffusion coefficient
D
2D
with D
2D
Q
2
=7.5 µeV and for four different values of the resolution
∆(ω)=H. The curves are shown as full lines with circles. For compari-
son, the Lorentzian- shaped three-dimensional diffusion scattering functions
are also shown (simple full lines). Here D
3D
has been set equal to 2D
2D
/3,
because this leads to identical linewidths (and lineshapes) for two- and three-
dimensional diffusion in the low-resolution limit. The resolution functions are
shown together with the scattering functions, but on a reduced scale. Al-
though a difference between the lineshapes for different dimensionality starts
to be visible, when D
2D
Q
2
∼ H (see Fig. 3.30b), it is seen that H must be
about 10 times smaller than D
2D
Q
2
in order to permit a clear distinction of
2D- and 3D-diffusion by the shapes of the respective scattering functions. The
expression given above is valid only at small Q, where Lorentzian linewidths
(for single-crystals) exhibit the D
2D
Q
2
-behaviour and are therefore very small
and difficult to be measured. At large Q, i.e. outside of the D
2D
Q
2
-regime,
where much larger and correspondingly more easily measurable linewidths are
observed, lineshapes very similar to expression (3.75) are expected. But un-
fortunately, in this Q-region the shape typical for low-dimensionality cannot
be observed in its pure form, because of the simultaneous presence of addi-
tional quasielastic components due to fast localized diffusive motions usually
connected with the diffusion mechanism. These are probably the reasons, why
the lineshape predicted for polycrystalline samples by (3.75) has so far not
been observed experimentally.
The study of the anisotropy of translational diffusion in single-crystalline
material is straight-forward. As an example we discuss the transport of wa-
ter molecules on the surface of a membrane with a two-dimensional crys-
talline structure [130, 131]. In biological membranes (cf. Chap 12), proton
diffusion connected with conduction of protons provides an important mech-
anism of energy transduction in living organisms. The purple membrane
146 Tasso Springer and Ruep E. Lechner
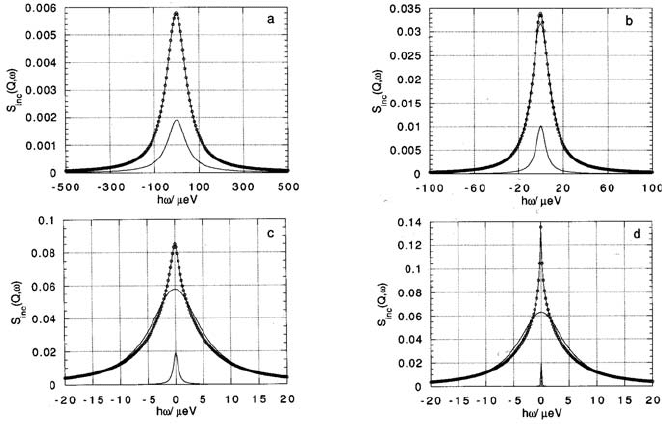
Fig. 3.30. Assuming a two-dimensional diffusion coefficient D
2D
,withD
2D
Q
2
=
7.5 µeV, the resolution-broadened scattering function (3.75) has been calculated for
four different energy resolutions H (HWHM): (a) 50 µeV; (b) 5 µeV; (c) 0.5 µeV;
(d) 0.05 µeV. The corresponding curves are shown as full lines with circles. For
comparison the Lorentzian-shaped three-dimensional diffusion scattering functions
for the same “effective” diffusion coefficient are also shown (simple full lines). The
resolution functions are given on a reduced scale (after [128]).
(PM) of Halobacterium salinarum, for instance, contains the protein bacteri-
orhodopsin (BR) which becomes a one-dimensional pulsed proton conductor
when activated by light. This is a light-driven proton pump generating an
electrochemical gradient across the membrane, which is employed by the bac-
terium as an energy source. After having been pumped from the cell-interior
to the membrane surface, the protons are transported by a mechanism of sur-
face conduction towards other proteins located within the same membrane:
The light-generated electrochemical potential across the cell membrane is
utilised by the halobacteria to furnish the driving force for ATP synthesis
by energizing the rotation of the turbine-like machinery in ATP synthase.
Water molecules near the surface are known to be relevant for this biological
function, since they have been shown to assist the proton conductivity [132].
Hydration water, its interaction with the surface of biological macromolecules
and macromolecular complexes, and its diffusion generally play an important
role in structure, dynamics and function of biological systems [133, 134]. It
is therefore of considerable interest to study the proton diffusion mechanism
within and close to the hydration layers of membranes. Similar to the case
of bulk water, it is expected that during the diffusion process protons are
3 Diffusion Studies of Solids by Quasielastic Neutron Scattering 147
exchanged between water molecules acting either as acceptors (forming for
instance (H
3
O)
+
) or as donors (producing (OH)
−
ions). A “solid-like” Grot-
thuß feature (see Sect. 3.9) is added to the diffusion in the liquid water phase
by the presence of fixed protonation sites on the surface of purple mem-
brane. It is worthwhile to note, that these sites are arranged in space in a
perfectly regular manner, since the bacteria use the most efficient packing of
BR: trimers of BR molecules embedded in a lipid bilayer matrix are aligned
in a two-dimensional hexagonal single-crystalline structure [135]. However,
since the pH-value is not very different from 7, the concentration of charged
particlesisverylow.Theprotonsspendmostofthetimeaspartofdif-
fusing neutral water molecules with only rare events of exchange between
different “vehicles” (see Sect. 3.9). Therefore, for the purpose of analyzing
neutron scattering experiments, the whole mechanism of diffusion may to a
good approximation be classified as that of molecular diffusion.
The crystalline order of PM has been exploited in QINS [130] and PFG-
NMR [131] measurements in a study of the anisotropy of proton diffusion
relative to the membrane surface. For this purpose about 20.000 layers of
purple membranes were stacked approximately parallel to each other (mosaic
spread: about 12
◦
FWHM) at defined relative humidities (r.h.). The mem-
brane stacks had been produced, starting from an aqueous suspension of
membrane pieces, by alignment of the membranes through evaporation of
water, using aluminium foils as a substrate. At 100 % r.h., with water layer
thicknesses of about 10
˚
A between neighbouring membranes, the protons of
water molecules were found to participate in a process of two-dimensional
long-range translational diffusion parallel to the membrane plane, with a self-
diffusion coefficient D
s
=4.4 · 10
−6
cm
2
s
−1
at room temperature, i.e. about
five times smaller than the known bulk value of water. At the same time, they
are also participating in a fast localized diffusive motion which can - at least
partially - be asigned to the rotation of water molecules. This was observed to
be about six times slower than in bulk water. These motions are sufficiently
fast to produce quasielastic broadenings clearly seen with an elastic energy
resolution of 16 µeV FWHM (see Fig. 3.31). Finally, a translational diffusion
jump distance of 4.1
˚
A was derived from the Q-dependent behaviour of the
quasielastic linewidth at large scattering angles (see Fig. 3.32). This distance
is three times larger than the corresponding quantity of bulk water.
The relative slowness of the diffusion process may partly be due to
the restricted space available within the hydration layers. It might also be
caused, together with the large value of the jump distance, by the presence of
fixed protonation sites on the membrane surface. These might have nearest-
neighbour distances similar to those of neighbouring lipid head groups, which
are of this order of magnitude. At these protonation sites, hydrogen bonds
are expected to be formed, with lifetimes exceeding those of bonds which
exist between neighbouring H
2
O molecules in bulk water.
148 Tasso Springer and Ruep E. Lechner
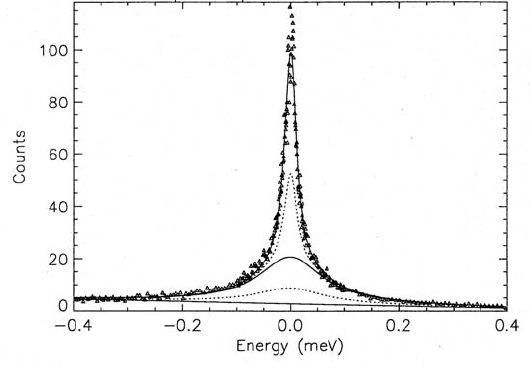
Fig. 3.31. Fit of a model for two-dimensional translational diffusion of water to
QINS spectra (data points shown as triangles) of hydrated purple membrane stacks
oriented with an angle α = 135
◦
with respect to the incident neutron beam. These
data were taken with the inverted time-of- flight technique (IRIS, see Sect. 3.4) at
the scattering angle ϕ =90.4
◦
. The spectra were not corrected for multiple scat-
tering (MSC), but MSC was taken into account in the fitted theoretical model:
The solid lines represent (from top to bottom) the fit result for the total scatter-
ing function including all terms, the sum of all rotation-broadened components of
the scattering function (including the backgound B), and B. The dotted lines are
the two MSC contributions: a rotation-broadened MSC component sitting on the
background line, and a MSC component without rotation broadening on top of the
rotation-broadened single-scattering contribution; after [131].
An attempt to relate the measured proton diffusion coefficient D
s
to the
protonic conductivity σ (see Sect. 3.8) is of interest for elucidating the trans-
port mechanism [91]. The surface conductivity is known from dielectric mea-
surements [136]. By extrapolation to the hydration level of H =0.28 em-
ployed in [130, 131] (H is given in units of g of water per g of PM), a value
of σ =2.15 · 10
−8
Ω
−1
cm
−1
was obtained. From this the charge diffusion co-
efficient D
σ
is found in the region 4.4 ·10
−6
cm
2
s
−1
≤ D
σ
≤ 58 ·10
−6
cm
2
s
−1
,
if the (unknown) concentration of the positive charge carriers is varied from
pH = 5.9 to pH = 7.0, where the lower limit would correspond to D
σ
= D
s
.
The Haven ratio, H
R
= D
s
/D
σ
, contains information on the proton transport
mechanism (see Sect. 3.8 and Chap. 1). Whereas H
R
= 1 would correspond to
the unrealistic case of a pure vehicle mechanism, the upper limit (H
R
=1/13)
probably is equally unrealistic, because the PM surface is known to be neg-
atively charged, which means that pH < 7.0 near the membrane surface.
An intermediate ratio D
s
/D
σ
appears to be the reality. It corresponds to a
mixed mechanism, where protons are transported by molecular ion diffusion
3 Diffusion Studies of Solids by Quasielastic Neutron Scattering 149
Fig. 3.32. Translational diffusion linewidths (exhibited by spectra as shown in
Fig. 3.31) of water on hydrated purple membrane, plotted as a function of Q
2
for
two sample orientations: α =45
◦
,opencircles;α = 135
◦
, full circles. The experi-
mental values are compared with the theoretical width corresponding to an isotropic
approximation of the Chudley-Elliott jump-diffusion model in two dimensions. Note
that for α = 135
◦
,theQ vector is exactly parallel to the membrane plane, when
the scattering angle is ϕ =90
◦
,whereasQ is perpendicular to the membrane for
the same scattering angle, when α =45
◦
. Therefore the linewidth curve for the
latter case approaches zero near Q
2
=1.784
˚
A
−2
, in agreement with the values
observed experimentally. This means that diffusion parallel to the membrane plane
has clearly been seen in this experiment, whereas in the direction perpendicular
to it the diffusive motion is too slow to be observable with the QINS technique.
Dotted lines: behaviour of the width according to the D
s
Q
2
-law, if this was valid
in the whole region of Q
2
shown in the figure (after [131]).
and exchanged from time to time between diffusing particles which encounter
each other.
3.12 Coherent Quasielastic Scattering
The diffusion of individual particles treated in the preceding experimental
sections is investigated by measuring S
inc
(Q, ω), i.e. by scattering without
the interference of waves that have been scattered by different atoms. Now
we deal with aspects of collective motions which reflect correlations between
different diffusing particles. Information on these correlations is obtained from
the coherent dynamic structure factor S
coh
(Q,ω). It yields more details on
the scattering system; however, the theoretical interpretation is a priori more
complex; so far the number of experimental studies on this matter has re-
mained comparatively small.
150 Tasso Springer and Ruep E. Lechner
The theoretical derivation of the coherent scattering function for transla-
tional diffusion requires the introduction of the mutual interaction between
different particles. A very simple description is the “hard core” or exclusion
potential: The particles diffuse over a lattice of cells or sites which are acces-
sible or blocked. This leads to a modified Chudley-Elliott formula [137] and
the resulting coherent width is given by
Γ
coh
(c)=Γ
CE
(Q,c→ 0) . (3.77)
which we call Γ
0
(Q), and where c is the average site occupancy. In this
case the width is equal to the result for incoherent scattering on the dilute
system, Γ
CE
, as derived before (see (3.30)). This coincidence is due to the
fact that the thermodynamic coefficient in the chemical diffusion coefficient,
∂(µ/k
B
T )/∂ ln c (proportional to 1/(1 − c)) just cancels the blocking factor
in the single particle diffusion coefficient, (1 − c).
Instead of the hard core model, a general potential of the mutual in-
teraction is introduced by the method of linear response in the mean-field
approximation [138] which leads to the result
S
coh
(Q,ω)=
c(1 − c)Γ
0
(Q)/π
Γ
2
coh
(Q)+ω
2
(3.78)
with
Γ
coh
= Γ
0
(Q)
1+
c(1 − c)V (Q)
kT
. (3.79)
Γ
0
(Q) is again the value for the dilute case of incoherent scattering, c → 0.
V (Q) is the Fourier transform in space of the atomic interaction potential.
With the Clapp-Moss relation [139] the unknown potential can be directly ex-
pressed by the static structure factor for the short-range order of the diffusing
particles (corrected for contributions from the host lattice distortion)
S
coh
(Q)=
c(1 − c)
1+
c(1−c)V (Q)
kT
. (3.80)
This finally leads to
S
coh
(Q,ω)=S
coh
(Q)
Γ
coh
/π
Γ
2
coh
+ ω
2
(3.81)
with the coherent line width
Γ
coh
(Q)=
c(1 − c)Γ
0
(Q)
S
coh
(Q)
. (3.82)
For very small Q, S
coh
(Q)
−1
is proportional to the thermodynamic factor
∂(µ/k
B
T )/∂ ln c, the derivative of the chemical potential with respect to the
