Gottstein G., Shvindlerman L.S. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications
Подождите немного. Документ загружается.

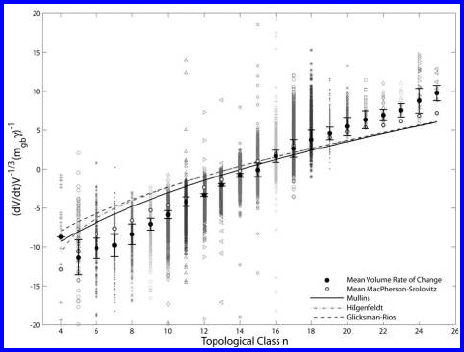
4.7 Grain Growth in 3D Systems 425
FIGURE 4.68 (SEE COLOR INSERT FOLLOWING PAGE 424)
Volume rate of change as a function of the topological class; the lines represent
the analytical approaches of Mullins, Rios, and Glicksman, and Hilgenfeldt et
al. The normalized volume rate of change was calculated directly from the
change of grain volume with time [554].
and computer simulations signifies that there is no constant growth rate for
a given topological class. Hence, grains with the same number of triple junc-
tions may exhibit different growth rates depending on the difference between
the mean width and the length of the triple junctions. Since this is the case,
none of the approaches that formulate the growth rate as a function of the
topological class (4.99, 4.102, 4.103) can be entirely valid for single crystals,
since they can yield a correct result only under very specific conditions, i.e. if,
at a certain time, there are grains fulfilling the relations. In order to test the
predictive power of these approaches, the volume rate of change as a function
of the topological class is plotted for these relations in Fig. 4.68 and compared
to simulation results ([554]). It is evident that each topological class can have
various growth rates in accordance with the Cahn-MacPherson-Srolovitz rela-
tions (4.104, 4.108). The mean normalized volume rate of change
4
˙
VV
−1/3
5
for each topological class is represented as a solid black circle, whereas open
circles represent the mean according to the Cahn-MacPherson-Srolovitz rela-
tion that was calculated from the mean L(D), e(D), and volume for each topo-
logical class, i.e.
4
˙
VV
−1/3
5
=2πm
gb
γ (L(D)
n
− 1/6 a(D)
n
) · <V >
−1/3
n
.
If only the mean values for each topological class are compared, all approaches
show very good agreement with the simulation results. This has also been
pointed out by Wakai [555], Weygand [556] and in phase-field simulations by
Kim [557]. The mean value determined by the Cahn-MacPherson-Srolovitz
© 2010 by Taylor and Francis Group, LLC

426 4 Thermodynamics and Kinetics of Connected Grain Boundaries
relation renders also a good agreement; however, it must be stressed that
this relation is not meant to be used as a general descriptor of the average
4
˙
VV
−1/3
5
.
The main property which distinguishes the observed analytical geometrical
approaches is that all of them, except the Cahn-MacPherson-Srolovitz rela-
tion, do not include the metrics of the grain.
The theories of Mullins, Glicksman and Rios and Hilgenfeldt et al. are con-
structed by analogy with the Von Neumann-Mullins approach, they are purely
topological. That is why the major weakness of the approaches mentioned
are not quantitative discrepancies in computer simulations. All of these ap-
proaches give asymptotically a reasonable rate of the grain volume evolution,
but in the absence of metrics. This is the reason why it has been impossi-
ble in the frame work of these approaches to consider problems connected
to grain growth in systems with finite mobility of grain boundary junctions.
The influence of the metrics comes particularly into play when it is impossible
to assume that grain boundary junctions have an infinite mobility. Such an
attempt was undertaken in [542].
When deriving Eq. (4.104) Cahn, MacPherson and Srolovitz assumed that
the dihedral angles at triple lines attain their equilibrium value of 120
◦
.Con-
sequently, the angle between triple lines at quadruple junctions must reach
the tetrahedral angle of ∼ 109.47
◦
[542]. Since a finite mobility of the junc-
tions influences the value of these angles, one might expect that the Cahn-
MacPherson-Srolovitz relation will not be able to predict adequately the vol-
ume rate of change of a grain in the case of a finite mobility of grain boundary
junctions. This principal problem was studied in [542, 554] for different triple
junction and quadruple junction mobility. In all cases a tangible deviation
from the values predicted by Eq. (4.104) was observed. However, for a finite
quadruple junction mobility the effect is much smaller than for a finite triple
junction mobility.
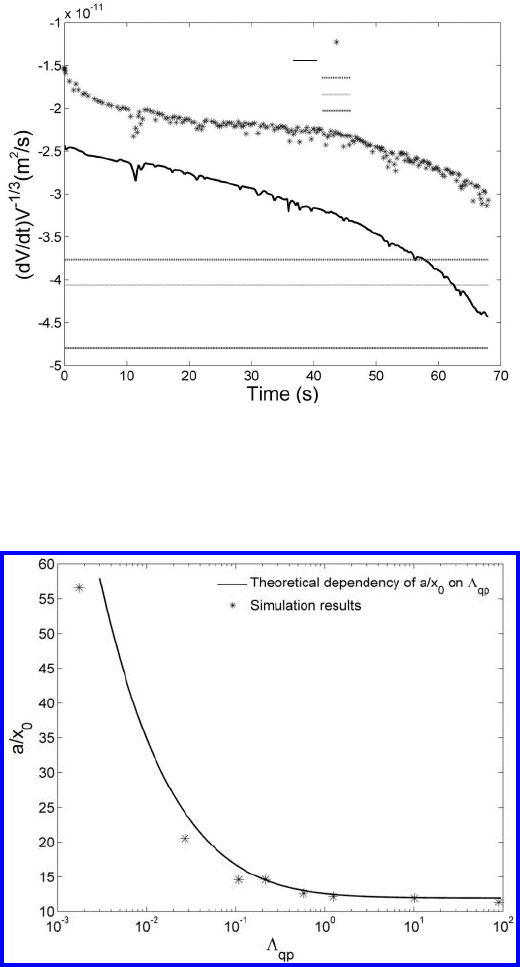
In Fig. 4.69 the results of computer simulation and different analytical
approaches are presented. Under the action of a finite mobility of triple lines
(Λ
tj
=0.2), the different analytical approaches predict values of the volume
change rate which depart greatly from the simulation results both in a quan-
titative and qualitative sense. For a better agreement the diverse approaches
need to consider the change of curvature that comes with a boundary junction
of finite mobility. One major effect of a finite quadruple junction mobility is
a change in the curvature of the adjoining triple lines, which implicitly alters
also their length. The total length of the triple lines d(D) for the configuration
shown in Fig. 4.61 is given by the sum of all triple line lengths a and d.The
length of the triple lines for a hexagonal cross section can be easily calculated
as (Figs. 4.37 and 4.61) [427, 441]
a =2 =
2x
0
ln sinΘ
ln
1+cosΘ
sinΘ
(4.115)
© 2010 by Taylor and Francis Group, LLC
4.7 Grain Growth in 3D Systems 427
Glicksman-Rios
simulation Λ
tj
= 0.2
Hilgenfeldt
Mullins
Cahn-MacPherson-Srolovitz
Glicksman-Rios
simulation Λ
tj
= 0.2
Hilgenfeldt
Mullins
Cahn-MacPherson-Srolovitz
FIGURE 4.69
A comparison of the different approaches with simulation results for the case
of a finite triple junction mobility reveals a strong discrepancy [542, 554].
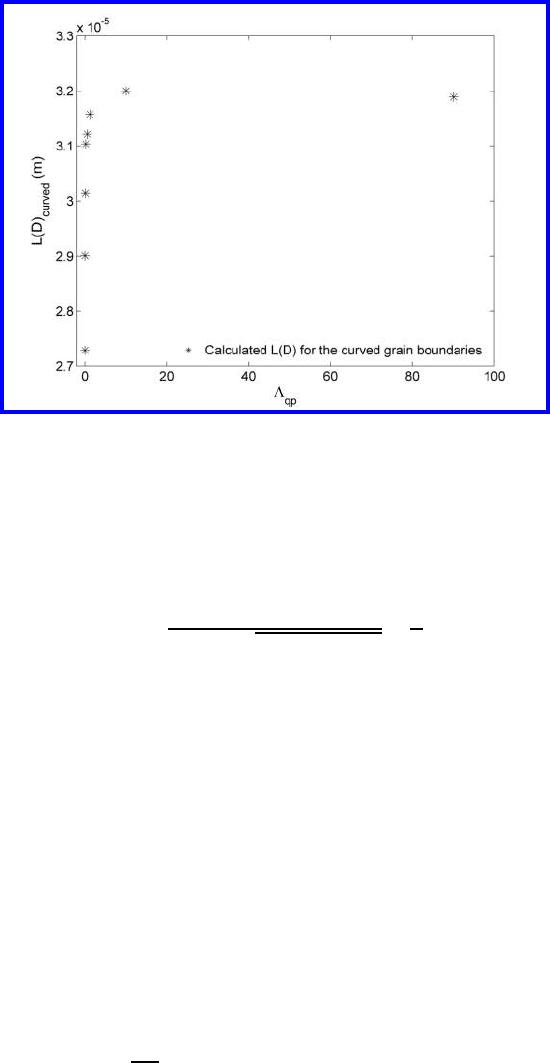
FIGURE 4.70
Dependency of the triple line length on the parameter Λ
qp
[542].
© 2010 by Taylor and Francis Group, LLC
428 4 Thermodynamics and Kinetics of Connected Grain Boundaries
FIGURE 4.71
A comparison of the different approaches and with the simulation results for
the case of a finite triple mobility reveals a strong discrepancy [542].
where Θ can be expressed as a function of the criterion Λ
qp
Θ
∼
=
2Λ
qp
3Λ
qp
+
!
Λ
qp
(9Λ
qp
+2)
+
π
2
(4.116)
On the other hand the length d can be represented as
d = d
0
− 2v
b
t (4.117)
where d
0
is the length of the longitudinal triple line at t =0,v
b
is the velocity
of the front and rear faces of the grain.
The simulation gives us the possibility of extracting the dependency of the
parameters a and L(D)onΛ
qp
(Figs. 4.70 and 4.71). One can see that L(D)
increases rapidly for rather small Λ
qp
to a value of L(D), which corresponds
to the curvature of the grain boundary for Λ
qp
→∞. The dependence of the
parameters L(D)ande(D) on a finite quadruple junction mobility questions,
strictly speaking, the applicability of the Cahn-MacPherson-Srolovitz relation
to grain growth in real 3D systems. However, it can be expected that, similar
to the generalized Von Neumann-Mullins relation [427, 441, 442] there is a
generalized Cahn-MacPherson-Srolovitz relation
dV
dt
= f (L [Λ
qp
, Λ
tj
] e [Λ
qp
, Λ
tj
]) (4.118)
© 2010 by Taylor and Francis Group, LLC

4.8 Kinetics of Grain Growth Inhibited by Vacancy Generation 429
4.8 Kinetics of Grain Growth Inhibited by Vacancy
Generation
Even for a crystal of a pure element it is thermodynamically profitable to con-
tain “intrinsic impurities” — the vacancies. It it a rather elaborate problem
to account for the effect of vacancies on the processes in solids. Even if we
restrict ourselves to processes where the vacancies are generated as a product
of a kinetics process the list is long enough. Vacancy production by moving
jogs on dislocations during plastic deformation, by a progressing solid/liquid
interface in solidification, or, generally, in every first-order phase transition,
which includes the crystalline phase, shrinking voids in sintering, or, finally,
during grain growth due to the different density of grain boundary and the
ideal crystal [20], [455]–[457] (grain boundary excess free volume — see Chap-
ter 1). As for the latter example of vacancy production, which is the subject of
this paragraph, the point is that the excess of grain boundary free volume lib-
erated by the reduction of total grain boundary area can be considered a flux
of vacancies into the bulk of a sample, a cause of elastic stresses or a source of
plastic deformation. However, in principle, the approach which considers the
elimination of volume excess by vacancies is most versatile. In fact, the way
to reduce the excess through elastic stresses is a deadlock. The relaxation of
elastic stresses is a long-term and slow process. That is why in a short time
the reduction of grain boundary area will result in a strong increase of free
energy. Moreover, as mentioned above, the relaxation of elastic stresses occurs
by a directed flux of vacancies. The inhibiting effect of vacancies on the very
process in which they are generated will be considered from a thermodynam-
ics viewpoint [11, 12], [458]–[460].
The excess free volume a system has to get rid of in such processes can be
released as vacancies which have to be accommodated by the crystal bulk. A
vacancy can be understood as an “impurity atom” which is dissolved in a crys-
tal, and the distinctive property of which (compared to ordinary impurities)
is that a vacancy is born from vacuum and disappears in vacuum, i.e. there is
no conservation rule. Other than that the properties of vacancies are similar
to the usual impurities: creating a solution even in absolutely pure crystals,
vacancies decrease the free energy; if the vacancy concentration exceeds the
solubility limit — the equilibrium concentration of vacancies c
eq
— vacancies
try to leave the crystal or to precipitate as second-phase particles, i.e. voids.
The kinetics aspect of vacancy influence on grain growth was considered in
[198, 199]. In [11, 12], [458]–[460] it was shown that in the course of grain
growth a self-drag of grain boundaries can be observed, caused by the libra-
tion of excess free volume in terms of vacancy emission.
In the most general terms, the Gibbs free energy G of a system with vacan-
cies can be written as
G = G
non-vac
+ G
vac
(4.119)
© 2010 by Taylor and Francis Group, LLC

430 4 Thermodynamics and Kinetics of Connected Grain Boundaries
where G
non-vac
is the non-vacancy part of the Gibbs free energy and G
vac
is
the contribution due to vacancies.
Obviously small deviations of the vacancy concentration c from its equilib-
rium value c
eq
will increase the last term in Eq. (4.119), irrespective of the
sign of the difference c − c
eq
. As shown in [459]
d
2
G
vac
dc
2
eq
=
NkT
c
eq
1+exp
−H
f
v
kT
∼
=
NkT
c
eq
(4.120)
Here n and N are the number of vacancies and the number of atomic sites per
unit volume, respectively, and H
f
v
is the vacancy formation enthalpy. Then,
taking into account that at equilibrium
dG
vac
dc
= 0, we arrive at
G = G
non-vac
+ G
vac
(c
eq
)+
1
2
NkT
c
eq
(c − c
eq
)
2
(4.121)
Higher order terms in c−c
eq
have been neglected. It is noted that this compact
formula for the vacancy part of the free energy embraces both the vacancy
formation enthalpy term and the entropy contribution,
The formulation given in [459] makes it possible to look at the kinetic
processes involving vacancies from a viewpoint of their thermodynamic feasi-
bility. Obviously, a kinetic process will be promoted thermodynamically if the
derivative of G with respect to time t, G, is negative, i.e.
˙
G =
˙
G
non-vac
+
NkT
c
eq
(c − c
eq
)˙c<0 (4.122)
The time derivative of the vacancy concentration ˙c can be written as
˙c =˙c
+
− ˙c
−
(4.123)
representing a competition between the vacancy production (˙c
+
)andtheva-
cancy removal (˙c
−
) rates. While the latter quantity can be expressed in a
generic form
˙c
−
=
D
v
d
2
(c − c
sink
) (4.124)
where D
v
is the vacancy diffusivity, d is the sink spacing and c
sink
the vacancy
concentration at a sink, the vacancy production rate depends on the particular
mechanism of vacancy generation and is related to the rate of variation of
the first, “non-vacancy,” term of the free energy
˙
G. In what follows, kinetic
processes which are significantly affected by this coupling with their “by-
product,” i.e. the vacancies produced, will be investigated.
As a “by-product” of this process, vacancies are released into the crystal
bulk. Indeed, the density of a grain boundary is generally lower than that of
the bulk (see Chapter 1).
The excess free volume released during the reduction of the grain boundary
area has to be accommodated by the bulk. This assumption is supported by
© 2010 by Taylor and Francis Group, LLC
4.8 Kinetics of Grain Growth Inhibited by Vacancy Generation 431
recent computer simulations of grain boundary motion [457]. The supply of
vacancies by moving grain boundaries may produce a vacancy supersaturation
in the bulk, raising the Gibbs free energy and producing a thermodynamic
force on the boundary. As can be expected intuitively, particularly by analogy
with the Le Chatelier principle, this thermodynamic force will oppose grain
bounday migration. Under certain conditions considered in [11, 458, 459], this
effect may be strong enough to temporarily suppress grain growth altogether.
In the approach proposed uninhibited grain growth was considered to occur
only during a limited time t
∗
(Eq. (4.125)). After that time, on reaching the
condition when the time derivative of the free energy of the system becomes
positive, “locking” of grain growth occurs
t ≥ t
∗
=
1
24
·
γc
eq
R
2
NkT(V
ex
)
2
V
(4.125)
Under the assumption that t
∗
τ = d
2
/D
v
the expression for the effective
velocity of grain growth V
eff
can be written as
V
eff
=
t
∗
τ
V =
1
24
·
γD
SD
NkTZ(V
ex
)
R
d
2
(4.126)
Here d and D
v
are the average spacings between vacancy sinks and the va-
cancy diffusivity, correspondingly. V is the “unperturbed” rate of grain growth
driven by the boundary energy; γ, δ, β and m are grain boundary charac-
teristics: free energy per unit area, thickness, the relative excess free volume,
and mobility, respectively;
¯
R is the average grain size (radius), D
SD
is the
bulk self-diffusion coefficient, N is the number of atoms per unit volume and
Z is the coodination number; kT has its usual meaning. Clearly it is more
correct to express the product βδ as a grain boundary excess free volume
V
ex
m
3
/m
2
.Theratio
d
2
D
v
determines the characteristic vacancy annihila-
tion time. Smoothing the discontinuous solution by replacing V
eff
with the
time derivative of the average grain size d
¯
R/dt and solving Eq. (4.125) yields
the time law for the grain growth model of [11, 459]
1
¯
R
0
−
1
¯
R
=
1
24
·
γD
SD
t
NkTZ(V
ex
)
2
d
2
(4.127)
where
¯
R
0
is the initial grain size.
The model outlined above accounts for the inhibiting effect of vacancies on
grain growth, but, of course, it is not more than an approximation of the real
continuous process of grain growth. Guided by the principle that “natura non
facit saltus,” the authors of [12, 460] suggested a description of grain growth
as a continuous process.
An equation expressing the balance of energy associated with an increment
of grain size dR within a time increment dt can be written as
−
d
d
¯
R
3γ
2
¯
R
d
¯
R =
3
2
·
1
¯
R
d
¯
R/dt
2
m
b
dt +
NkT
c
eq
(c − c
eq
)
6V
ex
¯
R
2
d
¯
R (4.128)
© 2010 by Taylor and Francis Group, LLC

432 4 Thermodynamics and Kinetics of Connected Grain Boundaries
Eq. (4.128) expressed the energy balance: the energy release associated with
the reduction of the GB area (the left-hand side of Eq. (4.128)) is distributed
between the dissipation due to the drag forces (first term on the right-hand
side of (4.128) and the vacancy sub-system — second term on the right-hand
side of Eq. (4.128)).
˙c =
6V
ex
¯
R
2
·
d
¯
R
dt
−
D
v
d
2
(c − c
eq
) (4.129)
Eqs. (4.128) and (4.129) render a full description of the evolution of the grain
system in terms of the average grain radius and the vacancy concentration.
In a dimensionless form they can be rewritten as
ξ
dξ
dt
+ AΨC −A = 0 (4.130)
dC
dt
=
p
ξ
2
dξ
dt
− C (4.131)
Here ξ =
¯
R/d, C = c − c
eq
, A = m
b
γ/D
v
,Ψ=
4NkT(V
ex
)
c
eq
γ
, p =6V
ex
/d;the
time t is now non-dimensional and is measured in units of d
2
/D
v
.
Numerical solutions of the above set of equations for a broad range of pa-
rameters have shown that for sufficiently small initial grain size ξ
0
= R
0
/d
the grain growth uninhibited by vacancies is preceded by an incubation time
during which the growth rate is substantially reduced, the time dependence of
the grain size exhibiting a plateau-like behavior (Fig. 4.72). For large values
of ξ
0
no incubation time is observed. The incubation time is defined as the
time at which the grain growth rate is a maximum. This time corresponds to
the termination of the plateau-like behavior and a transition to uninhibited,
parabolic grain growth (Fig. 4.72). During the incubation time the vacancy
concentration stays approximately constant (Fig. 4.72). Numerical results also
show that the non-dimensional incubation time is inversely proportional to ξ
0
and can be represented by the following formula
τ
incubation
=
P Ψ
ξ
0
(4.132)
or in dimensional form
t
incubation
=24
NkT(V
ex
d)
2
γ
¯
R
0
D
SD
(4.133)
where D
SD
is the bulk self-diffusion coefficient.
For times larger than the incubation time a common parabolic grain growth
law follows, while within the incubation time an approximate analytical solu-
tion of the set of Eqs. (4.132) and (4.133) reads
C =1/Ψ (4.134)
© 2010 by Taylor and Francis Group, LLC
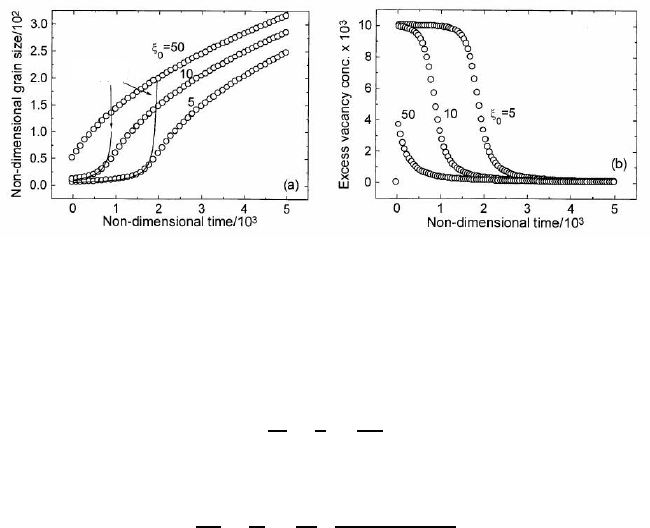
4.8 Kinetics of Grain Growth Inhibited by Vacancy Generation 433
Eq. (4.139)Eq. (4.139)
FIGURE 4.72
Dependence of non-dimensional grain size (a) and of excess vacancy concen-
tration (b) on the non-dimensional time calculated from Eqs. (4.130), (4.131)
for the following values of parameters: A =10, Ψ = 100, and p = 100 [12].
1
ξ
0
−
1
ξ
=
1
Pξ
(4.135)
or rewritten in dimensional form
1
¯
R
0
−
1
¯
R
=
1
24
·
γD
SD
t
NkT(V
ex
)
2
d
2
(4.136)
This demonstrates that the approximation [11, 458] based on the intermittent
locking-unlocking scheme with smoothing, provides a quantitatively adequate
representation of continuous grain growth in the regime where the inhibiting
effect of grain growth-induced vacancies is operative. Indeed, Fig. 4.72 demon-
strates an excellent match between the ξ(t) dependencies over the incubation
period given by Eqs. (4.135) and (4.136).
The condition for the occurrence of the vacancy-induced stabilization
against grain growth can be extracted from a comparison of the inhibited grain
growth with the common, parabolic grain growth kinetics. In fact, parabolic
growth occurs if the vacancy effect can be neglected, i.e. if the vacancy con-
centration is sufficiently close to its equilibrium value. From Eq. (4.130) it
then follows
ξ
2
− ξ
2
0
=2At (4.137)
Obviously, the solution of the set of Eqs. (4.130) and (4.131) coincides with the
parabolic law given by Eq. (4.137) for a time tending to zero and also for times
much larger than the incubation time, when C tends to zero, cf. Fig. 4.72. A
real inhibition of grain growth means that the rate of growth in the plateau
region, described by Eq. (4.135) is much smaller than that corresponding to
“free” uninhibited growth described by Eq. (4.137). This condition reads
ξ (ApΨ)
1/3
(4.138)
© 2010 by Taylor and Francis Group, LLC

434 4 Thermodynamics and Kinetics of Connected Grain Boundaries
or in the dimensional form
¯
R
0
¯
R
∗
c
=
24NkTZ(V
ex
)
2
d
2
m
b
D
SD
1/3
(4.139)
One can see that this condition is not very material sensitive, particularly due
to the fact that the ratio of the grain boundary mobility and the coefficient
of bulk self-diffusion is not strongly material dependent, and also due to the
power of 1/3. One possible source of structural dependence is the sink spacing
d, for example if it is related to the inverse of the square root of the dislocation
density that may bring about some variability of the quantity on the right-
hand side of inequality (Eq. (4.139)).
One particular case needs to be considered separately, though. It is the case
when the initial grain size is so small that no other vacancy sinks but the grain
boundaries themselves are available, so that d is to be identified with the grain
size
¯
R. Eq. (4.136) then changes to
dC
dt
=
1
ξ
2
6
dξ
dt
− C
(4.140)
Eq. (4.135) remains unchanged. However, the meaning of the non-dimensional
grain size and the non-dimensional time are different now: ξ =
¯
R(V
ex
), and
time is measured in units of (V
ex
)
2
/D
v
.
Solving the set of Eqs. (4.135) and (4.140) numerically, one can see that the
temporal behavior of the grain size in this case is different from the behavior in
the case of constant sink spacing d. After a short initial period of time t
trans
the case of vacancy-inhibited growth slows down considerably (Fig. 4.73).
After that grain growth effectively stays inhibited for very long times. The
growth rate is thus always lower than the respective rate of usual parabolic
growth (Fig. 4.73). Again, using the fact that after the transient period t
trans
the vacancy concentration is sustained at an approximately constant level and
that the grain size does not change significantly, we arrive at an approximate
solution of the set of Eqs. (4.135) and (4.140) which reads
¯
R −
¯
R
0
=
1
24
·
γD
SD
t
NkTZ(V
ex
)
2
(4.141)
From Fig. 4.73 one can see that Eq. (4.140) indeed provides a good analytical
approximation for sufficiently short times, which nevertheless should be longer
than t
trans
.
The stability condition of nanocrystalline material against grain growth can
be expressed by the inequality
¯
R
0
¯
R
c
=24NkTZ(V
ex
)
2
m
b
D
SD
(4.142)
© 2010 by Taylor and Francis Group, LLC
