Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


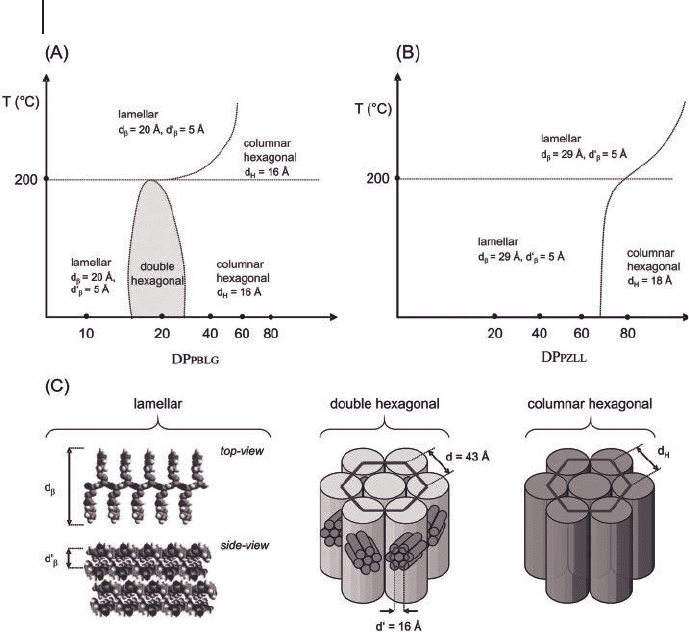
27.3 Solid-state Structures of Polypeptide-based Block Copolymers 849
due to the fact that for such short peptide block lengths, a substantial fraction of
the peptide blocks adopts a β - strand secondary structure. Self - assembly of these
diblock copolymers in a β - sheet type fashion results in the lamellar structures
observed by SAXS. For PS
10
- b - PBLGlu
20
a peculiar and until then unprecedented
structure was found. This structure that consisted of hexagonally packed diblock
copolymer molecules, which are organized in a hexagonal superlattice, has been
referred to as the double hexagonal or hexagonal - in - hexagonal morphology. Apart
from several unconventional solid - state nanoscale structures, another factor that
distinguishes the phase diagrams in Figure 27.10 from those of most conventional,
conformationally isotropic block copolymers is the infl uence of temperature. For
a number of diblock copolymers, increasing the temperature above 200 ° C results
in a change from a hexagonal - in - hexagonal (PS
10
- b - PBLGlu
20
) or hexagonal (PS
10
-
b - PBLGlu
40
, PS
10
- b - PZLLys
80
) to a lamellar morphology. FT - IR spectroscopy experi-
ments suggested that these morphological transitions are induced by an increase
in the fraction of peptide blocks that have a β - strand conformation.
Figure 27.9 SFM height images of a fi lm of PS
52
- b - PBLGlu
104
obtained by spin - coating from a 5 mg mL
− 1
THF solution and
subsequent exposure to saturated THF vapor for 3.5 (A), 22.5
(B) and 42 h (C) (reprinted from [72] with permission of The
American Chemical Society) .
850 27 Self-assembly of Linear Polypeptide-based Block Copolymers
27.3.1.3 Polyether - based Diblock Copolymers
PEG – polypeptide block copolymers are of particular interest, from both a struc-
tural and a functional point of view. Unlike the hybrid block copolymers discussed
in the previous paragraphs, which were based on amorphous synthetic polymers,
PEG is a semi - crystalline polymer. In addition to microphase separation and the
tendency of the peptide blocks towards aggregation, crystallization of PEG intro-
duces an additional factor that can infl uence the structure formation of these
hybrid block copolymers. Ma and coworkers [75] have investigated the solid - state
structure and properties of three PEG - b - PAla copolymers that were prepared from
a PEG macroinitiator with M
n
= 2 kg mol
− 1
. The diblock copolymers contained 39.8,
49.6 and 65.5 mol - % alanine. From FT - IR spectra and DSC measurements, these
workers proposed a microphase - separated bulk structure.
AB diblock and ABA triblock copolymers composed of PEG as the A block and
random coil segments of poly( d , l - valine - co - d , l - leucine) as the B block(s) were
Figure 27.10 Phase diagrams describing the
solid - state nanoscale structure of (A)
PS - b - PBLGlu and (B) PS - b - PZLLys diblock
copolymers; (C) illustration of the lamellar,
double hexagonal and hexagonal
morphologies found for the low molecular
weight hybrid block copolymers (reprinted
from [73, 74] with permission of The
American Chemical Society) .

27.3 Solid-state Structures of Polypeptide-based Block Copolymers 851
investigated by Cho and coworkers [76] . DSC experiments revealed PEG crystal-
lization and showed that the PEG melting temperature was decreased compared
with that of the PEG homopolymer. TEM micrographs suggested a larnellar micro-
phase - separated structure for one of the triblock copolymer samples.
27.3.1.4 Polyester - based Diblock Copolymers
One of the fi rst studies focusing on the solid - state properties of polypeptide – pol-
yester synthetic hybrid block copolymers was reported by J é r ô me and coworkers
[77] . DSC experiments on a poly( ε - caprolactone)
50
- block - poly( γ - benzyl l - glutamate)
40
-
(PCL
50
- b - PBLGlu
40
) diblock copolymer revealed two endotherms. The fi rst endo-
therm was found at 60 ° C and is due to the melting of the PCL. The second
endotherm, which was located at 110 ° C, was, mistakenly, interpreted as the
melting transition of PBLGlu. This transition, however, is not a melting transition,
but instead refl ects the conformational transition of the PBLGlu helix from a 7/2
to an 18/5 helical structure. Although no further structural investigations were
carried out, the observation of two separate endotherms occurring at temperatures
identical to the transitions found for the respective homopolymers was a fi rst
indication for the existence of a microphase - separated structure. Similar results
were reported by Chen and coworkers [78] who investigated the thermal properties
of a series of PCL - b - PBLGlu copolymers composed of PCL blocks containing 13 – 51
repeat peptide segment units with and 22 – 52 amino acid repeat units.
Caillol et al. [79] have studied the solid - state structure and properties of a series
of PLL - b - PBLGlu [PLL = poly( l - lactide)] copolymers. The PLL block in these copoly-
mers contained 10 – 40 repeat units and the peptide segments were composed of
20 – 100 repeat units. DSC thermograms of the block copolymers revealed three
transitions corresponding to the T
g
of PLL ( ≈ 50 ° C), the 7/2 to 18/5 helix transition
of PBLGlu ( ≈ 100 ° C) and the melting temperature of PLL ( ≈ 160 ° C), respectively.
This observation was already providing a fi rst hint towards a microphase - separated
bulk morphology. SAXS experiments, which were performed at 100 ° C, indicated
the existence of hexagonally ordered assemblies of α - helical PBLGlu chains. With
decreasing glutamate content, the peaks corresponding to this hexagonal organiza-
tion decreased in intensity and another scattering peak appeared, which was
ascribed to a lamellar assembly of PBLGlu chains with a β - strand secondary struc-
ture. Increasing the temperature to 200 ° C not only resulted in melting of PLL, but
also led to a decrease in intensity of the diffraction peaks corresponding to the
hexagonally ordered α - helical PBLGlu segments and an increase in the fraction of
PBLGlu segments that are ordered in a lamellar β - strand fashion.
27.3.1.5 Diblock Copolypeptides
A step forward in the design of hierarchically ordered structures with biofunction-
ality has been the subject of recent reports on the synthesis of block copolymers
based on polypeptides. In the fi rst such report [80] , organo - nickel initiators rather
than amines were used to avoid the unwanted α - amino acid N - carboxyanhydrides
(NCA) side reactions, which had, for more than 50 years, hampered the formation
of well - defi ned copolypeptides. This approach gave rise to various peptidic - based
852 27 Self-assembly of Linear Polypeptide-based Block Copolymers
block copolymers that have mainly been studied in solution (see previous section).
A second approach addressed the side reaction problem directly by using amines
in combination with high - vacuum techniques, to ensure the necessary conditions
for the living polymerization of NCAs: PBLGlu - b - PGly (PGly = polyglycine) were
prepared for the fi rst time with this methodology [81] .
Despite these important synthetic efforts, the solid - state morphology of purely
peptidic block copolymers is largely unexplored. Hadjichristidis and coworkers
[81] recently investigated the self - assembly of a series of narrow polydispersity
PBLGlu - b - PGly diblock copolymers within the composition range 0.67 < f
BLGlu
< 0.97
and the temperature range 303 < T < 433 K. SAXS and WAXS,
13
C NMR and DSC
were used for the structure investigation coupled with dielectric spectroscopy for
both the peptide secondary structure and the associated dynamics. These tech-
niques not only provided insight into the nanophase morphology but also gave
information about the type and persistence of peptide secondary structures. Par-
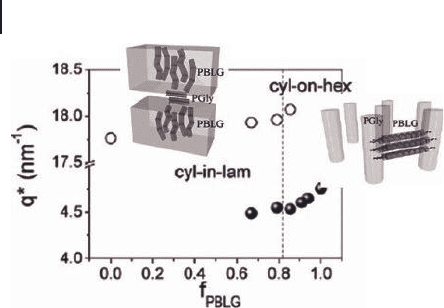
ticular evidence has been found for hexagonal - in - lamellar and cylinder - on - hexag-
onal nanostructures (Figure 27.11 ). The thermodynamic confi nement of the
blocks within the nanodomains and the disparity in their packing effi ciency results
in multiple chain folding of the PGly secondary structure that effectively stabilizes
a lamellar morphology for high f
BLGlu
. Nanoscale confi nement proved to be impor-
tant in controlling the persistence length of secondary peptide motifs.
27.3.2
Triblock Copolymers
27.3.2.1 Polydiene - based Triblock Copolymers
Whereas Gallot and coworkers have mainly studied the solid - state organization
of PB - based diblock copolymers, Nakajima et al. concentrated on ABA - type hybrid
block copolymers containing PB as the B component ( “ once - broken rods ” ). In a
Figure 27.11 Dependence of the WAXS peak
positions on the PBLGlu (PBLG) volume
fraction, corresponding to the distance
between PBLGlu α - helices (fi lled circles) and
PGly β - sheets (open circles). The interhelix
(inter - sheet) distance increases (decreases)
with increasing polypeptide volume fraction.
The vertical line separates the two
nanodomain morphologies.

27.3 Solid-state Structures of Polypeptide-based Block Copolymers 853
fi rst series of publications, the structure and properties of PBLGlu - b - PB - b - PBLGlu
triblock copolymers containing 7.5 – 32.5 mol - % (= 3.0 – 14.3 vol - %) PB were inves-
tigated [82 – 84] . Infrared spectroscopy and WAXS experiments on fi lms of the
triblock copolymers indicated that the PBLGlu blocks were predominantly α -
helical. From the WAXS experiments, it was concluded that the PBLGlu blocks
assembled into different structures, depending on the type of solvent that was
used to cast the fi lms. In benzene cast fi lms, the peptide helices were relatively
poorly ordered, similar to the so - called form A morphology of PBLGlu [85] . In
contrast, the PBLGlu segments in fi lms cast from CHCl
3
were well ordered and
contained paracrystalline and mesomorphic regions. Based on TEM, a cylindrical
microstructure was proposed for a triblock copolymer containing 8 vol - % PB.
Electron micrographs for other samples were not reported, but based on volume
fraction considerations it was predicted that triblock copolymers containing 12
and 14 vol - % PB would form either cylindrical or lamellar superstructures [83] .
Interestingly, copolymers having the same composition but polypeptide segments
made of either enantiomerically pure or racemic γ - benzyl glutamate exhibited not
only different secondary structures ( α - helix or random coil, respectively; FTIR
and WAXS) but also different superstructures (TEM). A cylindrical or lamellar
morphology was proposed in the fi rst case and a more spherical superstructure
in the second [86] .
Further support for the microphase - separated structure of the PBLGlu - b - PB - b -
PBLGlu triblock copolymers was obtained from dynamic mechanical spectroscopy
and water permeability experiments [84] . The temperature dependence of the
dynamic modulus and the loss modulus could be explained well by assuming a
microphase - separated structure. Furthermore, the hydraulic permeability of water
through membranes prepared from the copolymers was approximately three
orders of magnitude larger compared with a pure PBLGlu membrane. The hydrau-
lic water permeability was found to increase with increasing PB content in the
block copolymers. This was explained in terms of microphase - separated structure
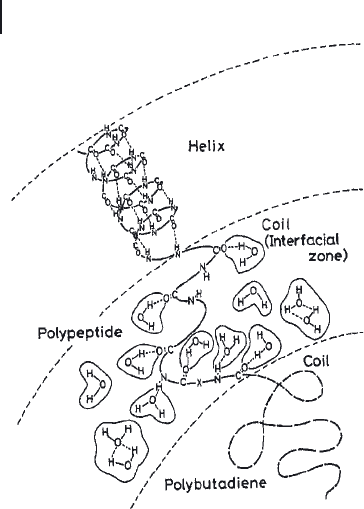
and the presence of an interfacial zone that separates the ordered domains formed
by the α - helical PBLGlu chains from the unordered PB phase (Figure 27.12 ). The
interfacial zone consists of amino acid residues that are located close to the
N - terminus of the peptide block and in the vicinity of the PB segment. The amino
acid residues in the interfacial zone do not form regular secondary structures. As
the amide groups of the peptide chains in the interfacial zone are not involved in
intramolecular hydrogen bonding, they are able to bind water molecules. Conse-
quently, increasing the interfacial zone, e. g., by increasing the PB content, leads
to an increase in the water permeability.
The bulk and surface structure of solvent - cast fi lms from a series of PBLGlu - b -
PB - b - PBLGlu triblock copolymers with much higher PB contents (50 – 80 mol - %)
than the samples discussed above have been described by Gallot and coworkers
[87] . The organization of these copolymers was compared with that of three other
triblock copolymers with approximately the same PB content ( ≈ 50 mol - %) but
which were composed of poly( N
ε
- trifl uoroacetyl l - lysine) (PTLLys), poly( N
5
- hydrox-
yethyl l - glutamine) (PHLGln) or polysarcosine (PSar) as the peptide block. For any
854 27 Self-assembly of Linear Polypeptide-based Block Copolymers
sample investigated, X - ray scattering experiments indicated a hexagonal - in - lamel-
lar bulk morphology. X - ray photoelectron spectroscopy (XPS) measurements
revealed that for the triblock copolymers with hydrophobic peptide blocks, i. e.,
PBLGlu or PTLLys, the surface composition was identical with that in the bulk of
the sample. In contrast, the surfaces of fi lms prepared from the triblock copoly-
mers with the more hydrophilic peptide segments, i.e., PHLGln or PSar, were PB
enriched. Furthermore, the XPS data suggested that the lamellar superstructures
formed by the triblock copolymers were perpendicular to the air – polymer
interface.
In addition, the solid - state organization and properties of PZLLys - b - PB - b - PZL-
Lys triblock copolymers have been investigated. Nakajima and coworkers [88, 89]
have studied copolymers composed of a central PB block with M
n
= 3.6 kg mol
− 1
and PB contents ranging from 12 to 52 mol - %. WAXS patterns obtained from
solution - cast triblock copolymer fi lms were in agreement with the α - helical sec-
ondary structure of the peptide blocks. The bulk microphase - separated structure
of the fi ve different block copolymer samples could be successfully characterized
by means of TEM. For the samples with the largest PB volume fraction (56 and
65 vol - %), a lamellar superstructure was found. However, the electron micro-
graphs suggested cylindrical and spherical microphase - separated structures for
triblock copolymers with smaller PB volume fractions.
Figure 27.12 Hydrogen - bonded water and water clusters in an
interfacial zone formed by unordered peptide chains that
separate the PB domains from the helical PBLGlu phase in
fi lms of ABA triblock copolymers.

27.3 Solid-state Structures of Polypeptide-based Block Copolymers 855
Other polybutadiene - based ABA type triblock copolymers that have been inves-
tigated include PMLGlu - b - PB - b - PMLGlu and PMGlu - b - PB - b - PMGlu [PMGlu = poly
( γ - methyl d , l - glutamate)] [86, 90] . Infrared spectroscopy experiments on solvent-
cast fi lms indicated that the incorporation of 50% of the d - isomer disrupts the
α - helical secondary structure and induces a random coil conformation in signifi -
cant portions of the peptide blocks. From the infrared spectra and WAXS experi-
ments, it was estimated that the helix content of a PMGlu homopolypeptide was
about 60% of that of the corresponding PMLGlu. TEM images of OsO
4
stained
samples provided evidence for the microphase - separated solid - state structure.
Interestingly, different morphologies were observed when comparing the images
of PMLGlu - b - PB - b - PMLGlu and PMGlu - b - PB - b - PMGlu samples with the same PB
content ( ≈ 30 mol - %). A cylindrical morphology was proposed for the fi rst and a
spherical structure for the second [86] (see above). The difference in morphology
was ascribed to the less regular secondary structure of the peptide block in the
case of the d , l - triblock copolymer, which prevents a highly ordered organization
of the peptide domains and facilitates the formation of spherical PB domains. The
ATRIR spectra further showed that adsorption of bovine serum albumine (BSA)
and bovine fi brinogen (BF) did not lead to denaturation. From these observations,
these workers concluded that the surfaces of the PMGlu - b - PB - b - PMGlu mem-
branes interact only weakly or reversibly with these plasma proteins and it was
predicted that this may also lead to a good overall biocompatibility.
The solid - state structure and properties of PELGlu - b - PB - b - PELGlu [PELGlu =
poly( γ - ethyl l - glutamate)] triblock copolymers containing 31.5 – 94.5 mol - % (= 17 –
88 vol - %) PELGlu have been studied using the same techniques as described
above for the other triblock copolymers [91, 92] . The secondary structure of the
PELGlu blocks was found to be predominantly α - helical and the helix content
in the triblock copolymers decreased from 95 to 60% upon decreasing the
peptide content from 95 to 61%. Interestingly, the WAXS data suggested that
the PELGlu helices were packed in a pseudohexagonal, i.e., monoclinic, arrange-
ment instead of the hexagonal structure observed for most of the other inves-
tigated peptide – synthetic hybrid block copolymers. TEM experiments on OsO
4
stained fi lms indicated a microphase - separated structure. Based on the electron
micrographs, a spherical microphase - separated structure was proposed for the
copolymer containing 17 vol - % PB, while cylindrical and lamellar morphologies
were suggested for triblock copolymers containing 28 and 44 vol - %, respectively,
68 and 88 vol - % PB. The biocompatibility of the PELGlu - b - PB - b - PELGlu triblock
copolymers was assessed by coating the samples onto a polyester mesh fi ber
cloth, which was subsequently subcutaneously implanted in mongrel dogs for
four weeks. It was found that the foreign body reaction and degradation of the
PELGlu - b - PB - b - PELGlu samples were less pronounced as compared with
PMLGlu - b - PB - b - PMLGlu, PBLGlu - b - PB - b - PBLGlu and PZLLys - b - PB - b - PZLLys
triblock copolymers.
The bulk nanoscale structure of a series of PBLGlu - b - PI - b - PBLGlu copolymers
containing 37.4 – 81.1 mol - % PBLGlu was studied by means of infrared spectros-
copy, WAXS, dynamic mechanical analysis and electron microscopy [93] . Based

856 27 Self-assembly of Linear Polypeptide-based Block Copolymers
on the electron micrographs, a cylindrical morphology was proposed for triblock
copolymers containing 74.6 and 81.1 mol - % PBLGlu. Water permeability measure-
ments also supported the microphase - separated bulk morphology [94] . Further
insight into the bulk morphology of the PBLGlu - b - PI - b - PBLGlu triblock copoly-
mers was obtained from pulsed proton NMR experiments [95] . The NMR signals
of the block copolymers were composed of three components with different spin –
spin relaxation times ( T
2
). The three different T
2
values were attributed to the
microphaseseparated structure, which consists of three regions (the ordered
helical peptide domains, the unordered interfacial peptide region and the rubbery
PI phase) with different molecular mobility. The spin – lattice relaxation times ( T
1
)
that were obtained provided insight into the domain sizes, which were in good
agreement with the results from TEM. The surface structure of CHCl
3
- cast fi lms
was studied by XPS and contact angle measurements [96] . It was found that the
chemical composition of the microphase - separated fi lms at the surface was differ-
ent from that in the bulk. The PI content at the fi lm surface was higher than that
in the bulk. Water contact angle measurements indicated that the block copolymer
fi lms were wetted easier than the respective homopolymers for the same reasons
as the previous samples.
Treatment of a PBLGlu - b - PI - b - PBLGlu fi lm with a mixture of 3 - amino - 1 - propa-
nol and 1,8 - octamethylenediamine led to the formation of hydrophilic, cross -
linked PHLGln - b - PI - b - PHLGln membranes being obtained [97] . The swelling ratio
of these membranes in pseudoextracellular fl uid (PECF) was found to decrease
with increasing PI content and increasing cross - link density. Tensile tests in PECF
revealed that the triblock copolymer membranes had a larger Young ’ s modulus,
increased tensile strength and elongation at breaking compared with membranes
prepared from PBLGlu homopolymer. Enzymatic degradation experiments using
papain showed that the triblock copolymer fi lms were more resistant towards
degradation than the corresponding homopolypeptide membranes. The half - times
for sample degradation increased with decreasing peptide content, which was in
agreement with the swelling behavior of the membranes.
27.3.2.2 Polystyrene - based Triblock Copolymers
Tanaka and coworkers studied ABA type triblock copolymers composed of a
central PS block fl anked by two polypeptide segments (PBLGlu, PZLLys or PSar)
[98] . TEM of a CHCl
3
- cast fi lm of PBLGlu
25
- b - PS
165
- b - PBLGlu
25
that was stained
with phosphotungstic acid revealed a lamellar phase separated structure. In con-
trast, no microphase separation was observed in a fi lm of PSar
73
- b - PS
421
- b -
PSar
73
. These workers proposed that the different block copolymer morphologies
could be related to the different secondary structure of the peptide block; while
the PBLGlu segments are predominantly helical, the PSar may not form any
regular secondary structure. Fibrinogen adsorption on the block copolymer
fi lms was studied with ATR - IR spectroscopy and compared with that on the cor-
responding homopolymer fi lms [98] . It was found that fi brinogen adsorption on
PS and PSar homopolymer fi lms and on PSar - b - PS - b - PSar triblock copolymer
fi lms led to denaturation of the protein. In contrast, protein adsorption on the

27.3 Solid-state Structures of Polypeptide-based Block Copolymers 857
microphase - separated PBLGlu - b - PS - b - PBLGlu surfaces was reported to stabilize
the protein ’ s secondary structure. Blood clotting tests suggested that thrombus
formation was retarded compared with the respective homopolymers.
Samyn and coworkers [99] extended the investigations of ABA triblock copoly-
mers and studied the solid - state organization of three different PBLGlu - b - PS - b -
PBLGlu copolymers containing 34, 55 and 92 wt - % PBLGlu. TEM micrographs of
ultramicrotomed and RuO
4
stained specimens and SAXS experiments indicated
a lamellar morphology for the copolymers with 34 and 55 wt - % PBLGlu. The
sample containing 92 wt - % PBLGlu did not form a lamellar structure. WAXS pat-
terns yielded d - spacings refl ecting the intermolecular distance between neighbor-
ing peptide α - helices. Ion permeability measurements on dioxane - cast fi lms
indicated that the bulk morphology infl uences the membrane properties [100] . The
membranes prepared from the lamellae forming 34 and 55 wt - % PBLGlu contain-
ing triblock copolymers showed cation selectivity. In contrast, the membrane
prepared from the triblock copolymer containing 92 wt - % PBLGlu did not show
such selectivity. It was proposed that uptake of cations into the triblock copolymer
membranes was facilitated by the interactions between the cations and the ester
functions in the block copolymers. The difference in selectivity was explained in
terms of the interfacial zone (as discussed earlier), which separates the PS and
PBLGlu domains only in the fi lms generated by the former two triblock
copolymers.
27.3.2.3 Polysiloxane - based Triblock Copolymers
Imanishi and coworkers [101] have studied the structure, antithrombogenicity and
oxygen permeability of ABA triblock copolymers composed of poly(dimethylsiloxane)
(PDMS) as the B block and PBLGlu, PBGlu [poly( γ - benzyl d , l - glutamate)], PZLLys
or PSar as the A block. Several series of triblock copolymers were prepared using
bifunctional PDMS macroinitiators and targeting various peptide block lengths.
TEM images of DMF - cast fi lms provided evidence for a microphaseseparated
morphology for PZLLys
49
- b - PDMS
400
- b - PZLLys
49
and PZLLys
91,160
- PDMS
256
- PZL-
Lys
91,160
. The images revealed a spherical morphology composed of PDMS islands
in a PZLLys matrix. The formation of these spherical domains was attributed to
the solvent that was used for sample preparation. While DMF is a good solvent
for PZLLys, it is a poor solvent for PDMS. In a separate publication, the same
workers also described non - spherical microphase - separated structures [102] . In
CH
2
Cl
2
- cast fi lms of a triblock copolymer with a very high PDMS content (PBLGlu
48
-
b - PDMS
508
- b - PBLGlu
48
, 83 mol - % PDMS) more extended, rod - like PBLGlu aggre-
gates in a matrix of PDMS were observed. The TEM experiments also provided
insight into the effects of peptide secondary structure and the nature of the casting
solvent on the thin fi lm morphology [101] .
Thin fi lms of PBLGlu
42
- b - PDMS
148
- b - PBLGlu
42
prepared from DMF showed a
spherical morphology. Changing the solvent from DMF (a good solvent for
PBLGlu) to CH
2
Cl
2
(a fairly non - selective solvent) resulted in coarsening of the
microphase - separated structures. PBGlu
42
- b - PDMS
148
- b - PBGlu
42
fi lms prepared
from CH
2
Cl
2
also showed a microphase - separated structure in which spherical

858 27 Self-assembly of Linear Polypeptide-based Block Copolymers
PDMS domains were embedded in a PBGlu matrix. The dimensions of the spheri-
cal domains, however, were much smaller than those observed by TEM. These
different morphologies refl ect the infl uence of the peptide secondary structure on
the block copolymer self - assembly; PBLGlu
42
adopts an α - helical conformation
and PBGlu
42
a random coil conformation. Studies on adsorption/denaturation of
proteins and oxygen permeation measurements from these triblock copolymers
also tend to describe the relationship between the fi lm morphology and these
properties. In addition, a detailed study of the gas permeation properties of
PBLGlu - b - PDMS - b - PBLGlu fi lms cast from CH
2
Cl
2
and DMF solution with PDMS
contents ranging from 46 to 83 mol - % has been reported [103] and revealed that
the oxygen permeability of the triblock copolymer fi lms in water was found to
increase exponentially with increasing PDMS content, in agreement with a micro-
phase - separated morphology of the membranes. Similar results were reported by
Kugo et al. , who studied oxygen and nitrogen transport across PBLGlu - b - PDMS -
b - PBLGlu triblock copolymers containing 63 – 81 mol - % PBLGlu [104] .
27.3.2.4 Polyether - based Triblock Copolymers
Inoue and coworkers [105, 106] studied the adhesion behavior of rat lymphocytes
on solvent - cast fi lms of PBLGlu - b - PEG - b - PBLGlu triblock copolymers. The triblock
copolymers were prepared from α , ω - bis - amino functionalized PEG macroinitia-
tors with molecular weights of 1.0 and 4.0 kg mol
− 1
and had PEG contents varying
from 11 to 33 wt - %. Rat lymphocyte adhesivity was found to decrease with increas-
ing PEG content. At the same PEG content, the adhesivity of the triblock copoly-
mers based on the macroinitiator with a molecular weight of 4 kg mol
− 1
was lower
than that of samples based on the macroinitiator with 1 kg mol
− 1
. In addition to
overall lymphocyte adhesivity, these workers also studied the adhesion of specifi c
subpopulations: B - cells and T - cells. All triblock copolymers showed a preference
towards B - cells. These experiments, however, revealed that the observed differ-
ences in cell adhesion behavior were neither due to differences in the conforma-
tion of the peptide blocks, nor could they be attributed to differences in surface
hydrophilicity. It was therefore proposed that the observed effects were caused by
differences in the higher order surface structures, i.e., in terms of the microphase -
separated morphology and/or PEG crystallinity.
Kugo et al. [107] studied the solid - state conformation of the peptide segment of
a series of PBLGlu - b - PEG - b - PBLGlu copolymers containing a PEG segment with
a molecular weight of 4 kg mol
− 1
and 36 – 86 mol - % PBLGlu. FT - IR spectroscopy
experiments on CHCl
3
- cast fi lms revealed that the PBLGlu blocks, which had
degrees of polymerization of 25 – 276, had an α - helical secondary structure. The
helix content of the triblock copolymer containing PBLGlu
276
blocks was found to
be similar to that of the PBLGlu homopolymer. Swelling the triblock copolymer
fi lms with water resulted in a decrease in helix content, as indicated by the CD
spectra. This decrease in helicity was attributed to competition of water clusters
to form hydrogen bonds with the peptide backbone. The effect was even more
pronounced when pseudo - extracellular fl uid was used instead of water.
