Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


27.2 Solution Self-assembly of Polypeptide-based Block Copolymers 839
length of the PLLys segment. A conclusive explanation of this unexpected aggrega-
tion behavior could not be given.
Core - forming Polypeptides Aggregates with an insoluble polypeptide core have
been prepared with block or random copolymers having linear or branched archi-
tecture. Most studies focused on aqueous systems (designated for use as drug
carriers in biomedical applications), and the only inverse systems investigated to
date are PB - b - PLGlu in dilute tetrahydrofuran (THF) and CH
2
C1
2
solution and
PS - b - PZLLys in CCl
4
.
Harada and Kataoka [38 – 40] were the fi rst to investigate the formation of polyion
complex (PIC) micelles in an aqueous milieu from a pair of oppositely charged
linear polypeptide block copolymers, namely of PEG
113
- b - PAsp
18,78
polyanions and
PEG
113
- b - PLLys
18,78
polycations [PEG = poly(ethylene glycol)]. Complexation studies
were carried out at pH 7.29, where both block copolymers had the same degree
of ionization ( α = 0.967) and were thus double - hydrophilic in nature and did not
form aggregates in water. Mixing of the copolymers at a 1 : 1 ratio of amino acid
residues resulted in the formation of stable and monodispersed spherical core -
shell assemblies of 30 nm in diameter (DLS). Another interesting feature con-
nected with PIC micelles is that of “ chain - length recognition ” [40] . PIC micelles
are exclusively formed by matched pairs of chains with the same block lengths of
polyanions and polycations, even in mixtures with different block lengths. The key
determinants in this recognition process are considered to be the strict phase
separation between the PIC core and the PEO corona, requiring regular alignment
of the molecular junctions at the core – corona interface, and the charge stoichiom-
etry (neutralization).
Yonese and coworkers [41] studied the aggregation behavior of PEG
113
- b -
PMLGlu
20,50
and lactose - modifi ed PEG
75
- b - PMLGlu
32
in water. As shown by DLS,
the copolymers formed large aggregates with a hydrodynamic radius of R
h
≈ 250 nm.
Contrary to what was claimed by these workers, it seems more likely that these
aggregates were vesicles rather than spherical micelles. Key in the aggregation
behavior might be the association of α - helical PMLGlu segments, as evidenced by
CD spectroscopy, promoting the formation of plane bi - layers which then close into
vesicles [15] . Further systematic studies on this system and detailed analysis of
structures are lacking.
Closely related to this system are PEO
272
- b - PBLGlu
38 – 418
and PNIPAAm
203
- b -
PBLGlu
39 – 123
[PEO = poly(ethylene oxide), PNIPAAm = poly( N - isopropylacryla-
mide)] described by Cho and coworkers [42, 43] . The aqueous polymer solutions,
prepared by the dialysis of organic solutions against water, contained large spheri-
cal aggregates ( R
h
≈ 250 nm) with a broad size distribution (DLS). Although the
size suggested a vesicular structure of the aggregates, aggregation numbers
( Z < 100, method of determination not specifi ed) were far below the values of
several thousands typically being reported for polymer vesicles [18] . It is also worth
noting that the PNIPAAm chains exhibit LCST (lower critical solution tempera-
ture) behavior. However, raising the temperature to the LCST ( ≈ 34 ° C) had no
serious impact on the size of the aggregates.

840 27 Self-assembly of Linear Polypeptide-based Block Copolymers
Dong and coworkers described symmetric triblock copolymers with a glyco
methacrylate middle block and two outer poly( l - alanine) (PLAla) or PBLGlu blocks
[44, 45] . The aggregates formed in dilute aqueous solution were spherical in shape
and were 200 – 700 nm in diameter (TEM). TEM further revealed a compact struc-
ture of the aggregates as with multi - lamellar vesicles. The dimensions of the
particles, however, were found to decrease with increasing concentration of the
copolymer.
Naka et al. [46] studied the aggregation behavior of poly(acetyliminoethylene) -
block - poly( l - phenylalanine), PAEI
41
- b - PLPhe
4,8
, in a 0.05 M phosphate buffer at pH
7. Aggregates were observed despite the very low number of hydrophobic l - phe-
nylalanine units, and the size of which was in the order of R
h
= 425 nm (DLS).
The seemingly high tendency of these polymers to form aggregates was attributed
to the establishment of hydrogen bridges between the amino acid units, as shown
by IR spectroscopy, in addition to hydrophobic interactions. Visualization of the
aggregates with TEM strongly suggested the presence of coacervates or large clus-
ters of small micelles but no vesicles.
The existence of vesicles could be demonstrated for PS
258
- b - PZLLys
57
in dilute
CCl
4
solution (Losik and Schlaad [47] ). Scanning electron microscopy (SEM)
showed collapsed hollow spheres of about 300 – 600 nm in diameter, indicative of
vesicles, and also sheet - like structures, supposedly bi - layers that are not yet closed
to vesicles (Figure 27.2 A) [15] . The preference for a lamellar structure might be,
as in the previous examples, attributed to a stiffening of the core by the 2D - arrange-
ment of crystallizable PZLLys α - helices. PS
258
- b - PZLLys
109
, on the other hand, was
found to form large compact fi brils being hundreds of nanometers in diameter
and several tens of microns in length (Figure 27.2 B); these aggregates might be
cylindrical multi - lamellar vesicles. However, the processes involved in the forma-
tion of these structures are not yet known.
Likewise, Lecommandoux and coworkers [31] found vesicles for PB
48
- b - PLGlu
20
in THF and in CH
2
Cl
2
solution ( R
h
= 106 – 108 nm, DLS/SLS). The formation of
Figure 27.2 SEM images of the aggregates formed by
(A) PS
258
- b - PZLLys
57
and (B) PS
258
- b - PZLLys
109
in dilute CCl
4
solution; specimens were prepared by shock - freezing a
0.2 wt - % polymer solution with liquid nitrogen and
subsequent freeze - drying [47] .

27.2 Solution Self-assembly of Polypeptide-based Block Copolymers 841
vesicles rather than micelles was attributed to the α - helical rod - like secondary
structure of the insoluble PLGlu that forms a planar interface.
27.2.1.2 Block Copolypeptides
Besides the polypeptide hybrid block copolymers described earlier, a few purely
peptide - based amphiphiles and block/random copolymers (copolypeptides) exist.
In the latter case, both the core and corona of aggregates consist of a polypeptide.
All of the studies reported so far have dealt with aggregation in aqueous media.
Doi and coworkers [48, 49] observed the spontaneous formation of aggregates
of PMLGlu
10
with a phosphate (P) head group in water. Immediately after sonica-
tion, the freshly prepared solution contained globular assemblies (diameter: 50 –
100 nm, TEM), which after 1 h transformed into fi brous aggregates, promoted by
intermolecular hydrogen bonding between peptide chains. After one day, these
fi brils assembled into a twisted ribbon - like aggregate (TEM). As its thickness
was ≈ 4 nm, which is close to the contour length of PMLGlu
10
- P in a β - sheet con-
formation (CD and FT - IR spectroscopy), these workers concluded that the forma-
tion of the ribbon was driven by a stacking of anti - parallel β - sheets via hydrophobic
interactions (see above) [22] .
Lecommandoux and coworkers [50] showed that zwitterionic PLGlu
15
- b - PLLys
15
in water can self - assemble into unilamellar vesicles with a hydrodynamic radius
of greater than 100 nm (Figure 27.3 ). A change in the pH from 3 to 12 induced
an inversion of the structure of the membrane (NMR) and was accompanied by
an increase in the size of vesicles from 110 to 175 nm (DLS).
Non - ionic block copolypeptides made of l - leucine and ethylene glycol modifi ed
l - lysine residues, PLLeu
10 – 75
- b - PELLys
60 – 200
, were described by Deming and cowork-
ers [51] . The copolymers adopted a rod - like conformation, due to the strong ten-
dency of both segments to form ct - helices, as confi rmed by CD spectroscopy. The
self - assembled structures observed in aqueous solutions included (sub - ) microm-
eter vesicles, sheet - like membranes and irregular aggregates. Here again, it was
shown that the vesicle formation is related to the systematic presence of the
polypeptide in a rod - like conformation in the hydrophobic part of the membrane,
inducing a low interfacial curvature and as a result a hollow structure.
Figure 27.3 Schematic representation of the self - assembly of
zwitterionic PLGlu
15
- b - PLLys
15
in water into unilamellar
vesicles [50] .

842 27 Self-assembly of Linear Polypeptide-based Block Copolymers
Meyrueix and coworkers [52] performed the selective precipitation of PLLeu
180
-
b - PLGlu
l80
and obtained nanoparticles, which could be purifi ed and further sus-
pended in water or in a 0.15 M phosphate saline buffer at pH 7.4. The colloidal
dispersions were stable, due to the electrosteric stabilization of the particles by
poly(sodium l - glutamate) brushes, containing spherical or cylindrical micelles,
besides the large hexagonally - shaped platelets with a diameter of about 200 nm
(TEM). Different shapes of particles were due to the heterogeneity of copolymer
chains with respect to chemical composition (NMR): glutamate - rich chains formed
micelles and leucine - rich ones formed platelets. CD spectroscopy and X - ray dif-
fraction suggested that the core of platelets consisted of crystalline, helical PLLeu
segments, and the structural driving force was thus related to the formation of
leucine zippers in a three - dimensional array.
27.2.2
Polypeptide - based Hydrogels
Protein - based hydrogels are used for many applications, ranging from food and
cosmetic thickeners to support matrices for drug delivery and tissue replacement.
These materials are usually prepared using proteins extracted from natural
resources, which can give rise to inconsistent properties unsuitable for medical
applications.
Recently, Deming and coworkers [53 – 56] designed and synthesized diblock
copolypeptide amphiphiles containing charged and hydrophobic segments. It was
found and demonstrated that gelation depends not only on the amphiphilic nature
of the polypeptides, but also on chain conformation, meaning α - helix, β - strand or
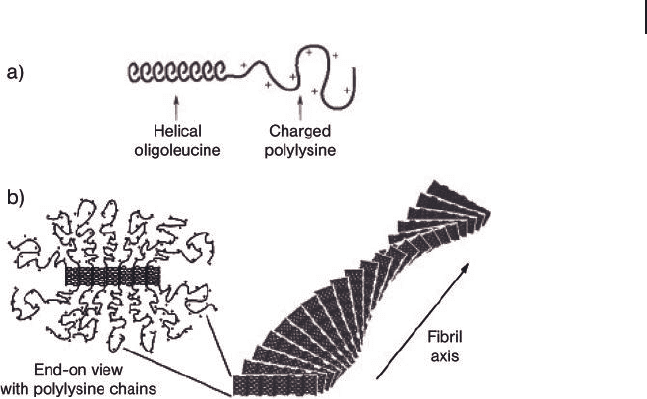
random coil. Specifi c rheological measurements were performed to evidence the
self - assembly process responsible for gelation [55] : the rod - like helical secondary
structure of enantiomerically pure PLLeu blocks is instrumental for gelation at
polypeptide concentrations as low as 0.25 wt - %. The hydrophilic polyelectrolyte
segments have stretched coil confi gurations and stabilize the twisted fi brillar
assemblies by forming a corona around the hydrophobic cores (Figure 27.4 ).
Interestingly, these hydrogels can retain their mechanical strength up to tem-
peratures of about 90 ° C and recover rapidly after stress. This new mode of assem-
bly was found to give rise to polypeptide hydrogels with a unique combination of
properties, such as heat stability and injectability, making them attractive for
applications in foods, personal care products and medicine. In this context, their
potential application as tissue engineering scaffolds has been recently studied [57] .
27.2.3
Organic/Inorganic Hybrid Structures
Recently, polypeptide - based copolymers have also been used for the stabiliza-
tion or synthesis of inorganic species. As a fi rst example, Stucky and coworkers
[58] used block copolypeptides to direct the self - assembly of silica into
spherical and columnar morphologies at room temperature and neutral pH.
27.2 Solution Self-assembly of Polypeptide-based Block Copolymers 843
Stucky, Deming and colleagues [59] also designed a double - hydrophilic block
copolypeptide poly{ N
ε
- 2[2 - (2 - methoxyethoxy)ethoxy]acetyl l - lysine}
100
- b -
poly(sodium l - aspartate)
30
(PELLys - b - PNaLAsp) that can direct the crystallization
of calcium carbonate into microspheres. They incorporated PLAsp in the diblock
because domains of anionic aspartate residues are known to nucleate calcium
carbonate crystallization. This effect is believed to be caused by matching
interactions between aspartate and the atomic spacing of certain crystal faces
in the growing mineral.
Also worth mentioning is the application of polypeptide - based copolymers in
the production of magnetic nanocomposite materials. Lecommandoux et al. [60]
obtained stable dispersions of super - paramagnetic micelles and vesicles by com-
bining an aqueous solution of PB
48
- b - PLGlu
56 – 145
with a ferrofl uid consisting of
maghemite ( γ - Fe
2
O
3
) nanoparticles. Incorporation of one mass equivalent of
ferrofl uid into the hydrophobic core of aggregates did not alter their morphol-
ogy, as deduced from SLS and SANS data, but caused a substantial increase
in the outer diameter by a factor of 6 (DLS). Interestingly, the hybrid vesicles
underwent deformation under a magnetic fi eld, as shown by 2D - SANS experi-
ments. Held and coworkers [61] earlier reported that monodisperse, highly crys-
talline maghemite nanoparticles in organic solvents could be transferred into
an aqueous medium using tetramethylammonium hydroxide stabilized at neutral
pH. Combination of the aqueous maghemite solution with PELLys
100
- b -
PAsp
30
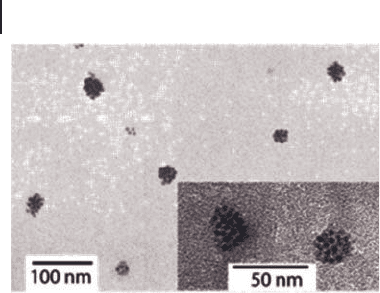
led to the formation of uniform clusters comprising approximately 20
nanoparticles (Figure 27.5 ).
Figure 27.4 Drawings showing (a)
representation of a block copolypeptide chain
and (b) proposed packing of block
copolypeptide amphiphiles into twisted
fi brillar tapes, with helices packed
perpendicular to the fi bril axes. Polylysine
chains were omitted from the fi bril drawing
for clarity (reprinted from [55] with permission
of The American Chemical Society) .
844 27 Self-assembly of Linear Polypeptide-based Block Copolymers
27.3
Solid - state Structures of Polypeptide - based Block Copolymers
27.3.1
Diblock Copolymers
27.3.1.1 Polydiene - based Diblock Copolymers
One of the fi rst reports on the nanoscale solid - state structure of peptide – synthetic
hybrid block copolymers was published by Gallot and coworkers [62] in 1976. In
this publication, the solid - state structure of a series of PB - b - PBLGlu and PB - b -
PHLGln diblock copolymers was investigated using a combination of techniques,
including infrared and CD spectroscopy, X - ray scattering and TEM. The block
copolymers covered a broad composition range with peptide contents ranging
from 19 to 75%. Interestingly, SAXS revealed a well - ordered lamellar superstruc-
ture characterized by up to four higher - order Bragg spacings for all of the inves-
tigated samples. The lamellar superstructure was confi rmed by electron microscopy
experiments, which were carried out on OsO
4
- stained specimens. The intersheet
spacings determined from the electron micrographs were in good agreement with
the diffraction data. Wide - angle X - ray scattering (WAXS) experiments indicated
that the α - helical peptide blocks were assembled in a hexagonal array. For a
number of block copolymer samples it was found that the calculated length of
the peptide helix was larger than the thickness of the polypeptide layer. To accom-
modate this difference, it was proposed that the peptide helices were folded in
the peptide layer. The lamellar structure consists of plane, parallel equidistant
sheets. Each sheet is obtained by superposition of two layers: (1) the PB chains
in a more or less random coil conformation and (2) the α - helical polypeptide
blocks in a hexagonal array of folded chains. The hexagonal - in - lamellar structure
was also found for PB - b - PZLLys and PB - b - PLLys block copolymers by the same
Figure 27.5 TEM images of clusters of maghemite
nanoparticles deposited from dispersions in water in the
presence of PELLys
100
- b - PAsp
30
(reprinted from [61] with
permission of The American Chemical Society) .
27.3 Solid-state Structures of Polypeptide-based Block Copolymers 845
workers [63 – 66] . In the case of the PB - PLLys block copolymers, no periodic
arrangement of the PLLys chains in the peptide layer was found. This is due to
the fact that the polypeptide segments in these block copolymers are not exclu-
sively α - helical but are composed roughly of 50% random coil, 35% α - helix and
15% β - strand domains [63] .
More recently, the solid - state nanoscale structure of PI - b - PZLLys diblock copoly-
mers was reported [34] . Diblock copolymers composed of a P1 block with a
number - average degree of polymerization of 49 and a PZLLys block containing
61 – 178 amino acid residues were investigated with dynamic mechanical analysis
and X - ray scattering. For the PI
49
- b - PZLLys
35
, PI
49
- b - PZLLys
61
and PI
49
- b - PZLLys
92
,
the X - ray scattering data were in agreement with a hexagonal - in - lamellar morphol-
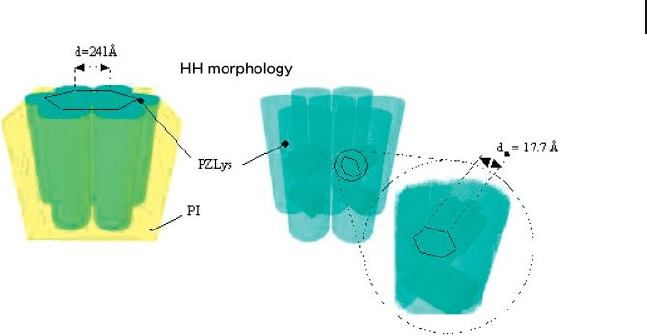
ogy. Interestingly, for PI
49
- b - PZLLys
92
the lamellar spacing was found to decrease
when the samples were prepared from dioxane instead of THF/ N , N - dimethylfor-
mamide (DMF) and suggested folding of the peptide helices. For PI
49
- b - PZLLys
123
and PI
49
- b - PZLLys
178
a hexagonal - in - hexagonal structure was found. This morphol-
ogy is illustrated in Figure 27.6 . This structure is unprecedented for polydiene -
based peptide hybrid block copolymers, but has also been found for low molecular
weight PS - b - PBLGlu copolymers [73] .
27.3.1.2 Polystyrene - based Diblock Copolymers
In an early study, Gallot and coworkers [64] reported on the bulk nanoscale struc-
ture of PS - b - PZLLys diblock copolymers, which were based on a PS block with a
number - average molecular weight of M
n
= 37 kg mol
− 1
and had peptide contents
ranging from 18 to 80 mol - %. X - ray scattering patterns of dry samples that had
been evaporated from dioxane showed two sets of signals, characteristic of a
hexagonal - in - lamellar superstructure. At very low angles, Bragg spacings charac-
teristic of a layered superstructure were found, whereas at somewhat larger angles,
Figure 27.6 Schematic model representation of the hexagonal
in hexagonal (HH) morphology obtained for Pl
49
- b - PZLLys
178
copolymer cast from dioxane solution.
846 27 Self-assembly of Linear Polypeptide-based Block Copolymers
there was a second set of refl ections pointing towards a hexagonal arrangement
of peptide helices. For several samples, the calculated length of the peptide helix
was larger than the peptide layer thickness as determined from the X - ray data. In
these cases, it was proposed that the helical PZLLys chains were folded in the
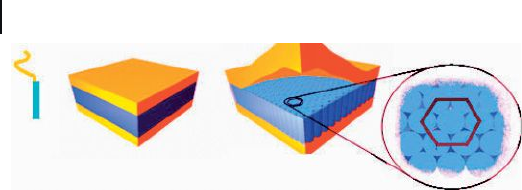
peptide layer. Thus, the bulk nanoscale structure of the PS - b - PZLLys copolymers
can be described in terms of the same hexagonal - in - lamellar model as was also
proposed for the PB - based block copolymer described earlier (Figure 27.7 ).
Removal of the of the side - chain protective groups of the peptide segment
resulted in PS - b - PLLys diblock copolymers [63] . These copolymers were not water
soluble, but formed mesomorphic gels at water contents of less than 50%. The
X - ray scattering patterns indicated a lamellar superstructure, both in the gel state
and the dry samples. In contrast to the side - chain protected block copolymers, no
evidence for a periodic arrangement of the peptide chains was found. This is not
too surprising considering that IR spectra indicated that roughly 50% of the
peptide blocks have a random coil conformation, 35% an α - helical secondary
structure and 15% a β - strand conformation.
Along the same lines, Douy and Gallot [66] also studied the bulk nanoscale
organization of PS - b - PBLGlu. For block copolymers composed of a PS block with
M
n
= 25 kg mol
− 1
and containing 31 – 94 mol - % peptide, the same hexagonal - in -
lamellar morphology as described above for the PS - b - PZLLys was found. The
biocompatibility of PS - b - PBLGlu copolymers has been discussed in two publica-
tions [67, 68] . Mori et al. [68] studied diblock copolymers composed of a PS block
with a number - average degree of polymerization of 87 and PBLGlu segments with
number - average degrees of polymerization of 23, 52 or 83. Thrombus formation
was assessed by exposing fi lms of the diblock copolymers and the corresponding
homopolypeptides to fresh canine blood. It was found that thrombus formation
on the diblock copolymer fi lms was reduced compared with the corresponding
homopolymers. For the block copolymers, thrombus formation decreased with
decreasing PBLGlu block length. Also, adsorption of plasma proteins such as
bovine serum albumine, bovine γ - globulin and bovine plasma fi brinogen was
reduced on the block copolymers compared with PS homopolymer.
The characterization of the solid - state nanoscale organization of PS - polypeptide
hybrid block copolymers has recently been refi ned in a series of publications by
Figure 27.7 Schematic model representation of the hexagonal -
in - lamellar (HL) morphology obtained for polypeptide - based
rod – coil diblock copolymers cast from solutions.

27.3 Solid-state Structures of Polypeptide-based Block Copolymers 847
Schlaad and coworkers [69 – 71] . In a fi rst report, three PS - b - PZLLys diblock copoly-
mers with peptide volume fractions of 0.48, 0.74 and 0.82 were investigated [69] .
SAXS patterns recorded from DMF cast fi lms confi rmed the hexagonal - in - lamellar
morphology published earlier by Gallot and coworkers [64] . In their paper, Schlaad
and coworkers went a step further and analyzed their SAXS data using the
interface - distribution concept and the curvature - interface formalism. These evalu-
ation techniques suggested that the bulk nanoscale structure of the PS - b - PZLLys
diblock copolymers does not consist of plain but of undulated lamellae. The
concept of the interface - distribution function and the curvature - interface formal-
ism were also applied to compare the solid - state structures of two virtually identical
PS based diblock copolymers; PS
52
- b - PZLLys
111
( φ
peptide
= 0.82) and PS
S2
- b - PBLGlu
104
( φ
peptide
= 0.79) [70] . Analysis of the SAXS data obtained on DMF - cast fi lms indi-
cated a hexagonal - in - undulated (or zigzag) lamellar morphology for both block
copolymers. However, the X - ray data also revealed two striking differences
between the samples. The fi rst difference concerns the thickness of the layers,
which are a factor of three smaller for PS
52
- b - PBLGlu
104
as compared with PS
52
-
b - PZLLys
111
. Whereas the PZLLys helices are fully stretched, the PBLGlu helices
are folded twice in the layers. As peptide folding increases the area per chain at
the PS - PBLGlu interface, the thickness of the PS layers also has to decrease in
order to cover the increased interfacial area. The second difference concerns the
packing of the peptide helices. For the PZLLys - based diblock copolymer it was
estimated that about 180 peptide helices form an ordered domain. The level of
ordering, however, was considerably lower for the peptide blocks of PS
52
- b -
PBLGlu
104
and only ≈ 80 helices were estimated to form a single hexagonally
ordered domain.
In addition, the infl uence of the polydispersity of the polypeptide block on the
solid - state morphology of PS - b - PZLLys diblock copolymers has also been studied
[71] . To this end, a series of fi ve diblock copolymers was prepared from an identical
ω - amino - polystyrene macroinitiator ( P
n
= 52; polydispersity index, PDI = 1.03).
The peptide content in these diblock copolymers varied between 0.43 and 0.68 and
the PDI ranged from 1.03 to 1.64. Evaluation of the SAXS data with the interface -
distribution function and the curvature - interface formalism confi rmed, as expected,
the hexagonal - in - undulated (or zigzag) lamellar solid - state morphology. Fractiona-
tion of the peptide helices according to their length leads (locally) to the formation
of an almost plane, parallel lamellar interface, which is disrupted by kinks (undula-
tions). The curvature at the PS - PZLLys interface, however, was found to be strongly
dependent on the chain length distribution of the peptide block. Block copolymers
with the smallest molecular weight distribution produced lamellar structures with
the least curvature. Increasing the chain length distribution of the peptide block
(block copolymers with PDI ≈ 1.25) leads to larger fl uctuations in the thickness of
the PZLLys layers, which increases the number of kinks and the curvature at the
lamellar interface. At even larger polydispersities (PDI ≈ 1.64), however, the
number of kinks decreases again. With increasing polydispersity of the peptide
block, the thickness fl uctuations become larger and larger, as does the interfacial
area. At a certain point, at suffi ciently high polydispersity, the system tries to

848 27 Self-assembly of Linear Polypeptide-based Block Copolymers
compensate for the increased interfacial tension and minimizes the number of
kinks (Figure 27.8 ).
Ludwigs et al. [72] used SFM to investigate the formation of hierarchical struc-
tures of PS
52
- b - PBLGlu
104
in thin fi lms. Thin fi lms with a thickness of ≈ 4 and 40 nm
were prepared by spin - coating of dilute polymer solutions on silicon substrates
and were subsequently annealed in saturated THF vapor to achieve a controlled
crystallization of the α - helical PBLGlu. On the smallest length - scale, the structure
was found to be built of short ribbons or lamellae of interdigitated polymer chains.
PBLGlu helices were fully stretched in thin fi lms, in contrast to what has been
observed in the 3D organized bulk mesophase (see above). Depending on the time
of solvent annealing, different ordered structures on the micrometer length - scale
could be observed (Figure 27.9 ).
The examples discussed so far have all involved relatively high molecular weight
diblock copolymers. In these cases, the molecular weight of the polypeptide block
is usually suffi ciently high so that it forms a stable α - helix and the common
hexagonal - in - lamellar morphology is found. The situation changes, however,
when the molecular weight of the block copolymers is signifi cantly decreased. The
infl uence of molecular weight on the solid - state organization of polystyrene - based
peptide – synthetic hybrid block copolymers has been studied for a series of low -
molecular weight PS - b - PBLGlu and PS - b - PZLLys [73, 74] . These diblock copoly-
mers consisted of a short PS block with P
n
≈ 10, a polypeptide block containing
≈ 10 to 80 amino acid repeat units and were characterized by means of variable
temperature FT - IR spectroscopy and X - ray scattering. These experiments allowed
the construction of “ phase diagrams ” , which are shown in Figure 27.10 . The phase
diagrams reveal a number of interesting features. At temperatures below 200 ° C
and for suffi ciently long polypeptide blocks, a hexagonal arrangement of the
diblock copolymers was found, analogous to the hexagonal - in - lamellar morphol-
ogy of the high - molecular weight analogues. Upon decreasing the length of the
peptide block, however, several novel solid - state structures were discovered. For
very short peptide block lengths (PS
10
- b - PBLGlu
10
, PS
10
- b - PZLLys
20
, PS
10
- b - PZL-
Lys
40
and PS
10
- b - PZLLys
60
) a lamellar supramolecular structure was found. This is
Figure 27.8 Schematic representation of the
disordered zigzag lamellar morphology
formed by polypeptide - based diblock
copolymers with low (A), moderate (B) and
high polydispersity (C) with respect to the
length of helices. Polypeptide helices are
represented as cylinders, and polyvinyl sheets
are depicted in black (reprinted from [71] with
permission of The American Chemical
Society) .
