Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

26.2 Block Copolymer Gels 809
scale fi llers on morphology and property development in block copolymer gels.
Electron microscopy images of gels modifi ed with colloidal silica nanoparticles
and a surface - modifi ed organoclay are provided in Figures 26.14 and 26.15 , and
indicate the extent to which the additives disperse, which is of paramount impor-
tance with regard to controllable property development. Mechanical properties are
generally found to improve when surface - modifi ed silicas [49, 78] and organoclays
[78] are incorporated into the gel matrix. Efforts to use carbon nanotube s ( CNT s)
[78] have yielded less impressive results due to challenges associated with suffi -
cient dispersion. An enhanced modulus is achieved when the additive is more
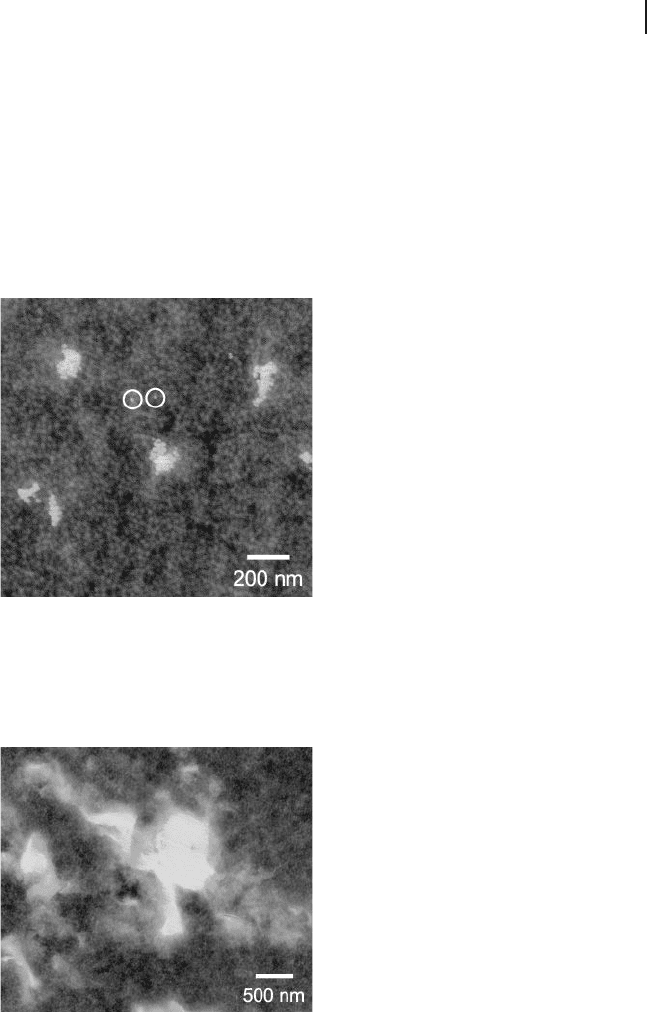
Figure 26.14 Energy - fi ltered TEM image of a
triblock copolymer organogel composed of
10 wt% SEBS copolymer and modifi ed with
3 wt% colloidal silica nanoparticles. The
styrenic micelles, stained by the vapor of
RuO
4
(aq), appear light, whereas the siliceous
nanoparticles appear bright, due to imaging
at an energy loss of 200 eV. Two neighboring
individual nanoparticles are circled.
Figure 26.15 Energy - fi ltered TEM image of the same triblock
copolymer organogel pictured in Figure 26.14 , but modifi ed
with 3 wt% organoclay. The siliceous clay platelets likewise
appear bright due to imaging under the same conditions as
those employed in the previous fi gure.

810 26 Nanostructured Organogels via Molecular Self-Assembly
compatible with the matrix (solvent and midblock), as this renders improved dis-
persion and, consequently, more effi cient stress absorption from the soft phase
[49] . Properties are observed [79] to generally improve with increasing nanofi ller
content, even at surprisingly high (60 wt%) loading levels. The addition of nano-
particles may also expand the mechanical performance and stability of block
copolymer organogels at high temperatures, especially if (i) the attractive interac-
tions between the nanoparticles and matrix are particularly strong [49] ; or (ii) the
nanoparticles themselves form a secondary, load - bearing network that remains
thermally stable [78] .
26.2.5.2 Polymeric Modifi ers
Endblock - Selective Homopolymer
In the previous section, the addition of inor-
ganic nanofi llers to a block copolymer gel resulted in a hybrid material wherein
the nanofi llers were highly dispersed to yield nearly discrete nanoscale particulates
with an ultrahigh surface - to - volume ratio. For this reason, and to avoid macro-
scopic phase separation between the nanofi llers and the gel, only very low nano-
fi ller concentrations can be considered. The addition of an endblock - selective
homopolymer to a block copolymer gel can likewise result in several different
scenarios, depending on factors such as endblock compatibility, molecular weight
disparity, and homopolymer concentration [84] . If the homopolymer is chemically
identical (hA) to the endblocks of an ABA triblock copolymer, then only the
molecular weight disparity ( α = N
hA
/ N
A
) and hA concentration constitute key
design parameters. As in solvent - free block copolymers, if α is large ( > 1), the
hA molecules will not be physically accommodated within the brush comprising
the A - rich microdomains. In this case, the brush is said to remain dry due
to the lack of penetration of homopolymer molecules [85] . This entropic penalty
favors macrophase separation between the copolymer and homopolymer
molecules even at relatively low hA concentrations. In this limit, hA - rich domains
measuring on the order of micrometers or larger coexist with the gel network,
and the accompanying mechanical properties are largely dictated by the
separating interface. The incorporation of semicrystalline syndiotactic polystyrene
( sPS ) into a SEBS gel, for example, results in the formation of discrete sPS
crystals, which appear as fi laments and sheets (cf. Figure 26.16 ) that greatly
improve the modulus due to adhesion between the crystals and the styrenic
micelles [86] .
As α becomes smaller, however, due to a reduction in N
hA
or an increase in N
A
,
the smaller hA molecules can locate within the A - rich microdomains and wet the
compatible block brush. In this limit, added hA can serve to facilitate, or even
induce, copolymer micellization because of the corresponding increase in the
population of unfavorable A – B contacts [87] , and it can likewise promote a change
in interfacial curvature and, hence, gel morphology [88, 89] . Mechanical properties
are found [90] to generally improve with increasing hA fraction up to a molecular -
weight - dependent level, beyond which macrophase separation occurs. One way to
lessen the propensity for macrophase separation and to ensure the encapsulation
of a homopolymer within the endblock - rich microdomains responsible for stabiliz-
26.2 Block Copolymer Gels 811
ing the gel network is to increase the homopolymer/endblock compatibility. In
the case of gels composed of styrenic triblock copolymers (i.e., copolymers with
PS endblocks), poly(2,6 - dimethyl - 1,4 - phenylene oxide) ( PPO ) constitutes an ideal
candidate in this regard, since χ between these two polymers is negative over all
compositions and a large temperature range [91] . Moreover, since PPO possesses
a relatively high T
g
( ∼ 210 ° C), it may be added to improve the service temperature
of styrenic SAMINs [92] . This increase has been reported [93] to be as high as
∼ 30 ° C upon the addition of 3 wt% PPO to a SEBS gel. At higher loading levels,
the mechanical properties improve substantially and morphological transitions
can be expected [91] .
Cosurfactant Although a variety of midblock - selective homopolymers can be
blended into block copolymer gels (e.g., polyolefi ns such as polypropylene [94]
added to gels with a primarily aliphatic solvent) to modify process or application
properties, such modifi cation normally results in the formation of macrophase -
separated systems consisting of homopolymer - rich and gel - rich domains that are
discrete or cocontinuous, depending on the relative concentrations. For this
reason, such multicomponent systems are not considered further here. Another
means by which to alter gel properties at the molecular level involves the addition
of an AB diblock copolymer as a cosurfactant to the ABA triblock copolymer
network. In this scenario, the AB molecules, if suffi ciently incompatible, are forced
to coreside with their ABA analogues, resulting in submicrodomain stratifi cation
[95] . Due to the presence of AB molecules, the ABA molecules are entropically
forced to form bridges (rather than re - enter to form loops) due to coronal volume
exclusion, in which case addition of an AB copolymer in small quantities can
improve mechanical properties even when c < cgc for the parent ABA solution (cf.
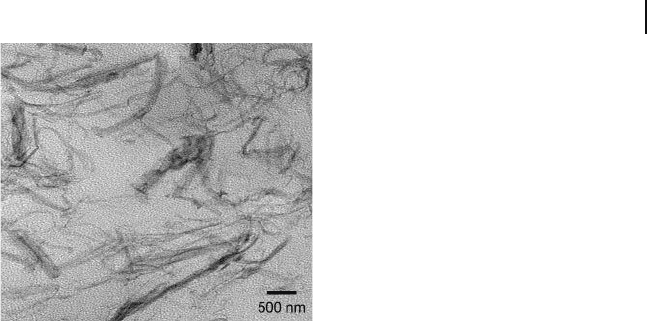
Figure 26.16 TEM image of a triblock
copolymer organogel composed of 8.5 wt%
SEBS copolymer and 1.5 wt% syndiotactic
polystyrene ( sPS ). The styrenic micelles and
crystalline sPS sheets and fi laments are both
selectively stained and appear dark. Adapted
from Ref. [86] and used with permission from
the American Chemical Society.
812 26 Nanostructured Organogels via Molecular Self-Assembly
Figure 26.17 ) [96, 97] . Complementary SAXS studies performed by Vega et al . [29]
reveal that the presence of an AB copolymer could help to prevent macrophase
separation due to a reduction in intermicellar distance, and could, in general, be
used to tune to the phase behavior of the system (cf. Figure 26.18 ).
26.2.6
Nonequilibrium Mesogels
Thermoplastic elastomer gels are normally prepared by mixing a triblock copoly-
mer and a low - volatility solvent, with or without a carrier solvent (to reduce viscos-
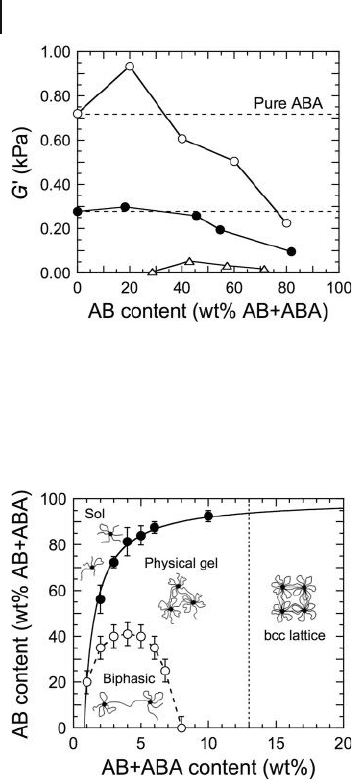
Figure 26.17 Dependence of G ′ on the
fraction of AB diblock copolymer added to
triblock copolymer organogels and solutions
varying in total copolymer concentration (in
wt%): 15 (
䊊
), 11 (
䊉
), and 7 (
䉭
). Note that a
frequency - independent modulus indicative of
a gel network is not achieved in the system
with 7 wt% copolymer until the diblock
copolymer is added. The solid lines serve to
connect the data. Adapted from Ref. [96] and
used with permission from the American
Chemical Society.
Figure 26.18 Experimental phase diagram for an ABA triblock
copolymer organogel modifi ed with an AB diblock copolymer.
The lines serve as guides for the eye. Adapted from Ref. [29]
and used with permission from John Wiley & Sons, Inc.
26.2 Block Copolymer Gels 813
ity during mixing and evaporate thereafter), at elevated temperatures and then
cooling the solution below the endblock T
g
to induce glassy crosslinks that serve
to stabilize the gel network. An alternative approach to preparing gels from the
same copolymer and solvent pair is to introduce a solvent directly into the ordered
copolymer by diffusion at temperatures below the endblock T
g
. As the solvent -
incompatible endblocks – and hence their microdomains – are glassy, they do not
dissolve as the midblocks swell. Midblock swellability depends on both the solubil-
ity of the solvent in the midblock and the extent to which the midblocks stretch
(which is entropically unfavorable), as shown schematically in Figure 26.19 . Gels
produced in this fashion from an ordered copolymer have been referred [99] to as
“ mesogels ” because they retain the characteristics of the neat copolymer mes-
ophase, since fabrication occurs under nonequilibrium conditions. King et al . [100]
have observed that, on progressive swelling, such gels derived from a copolymer
possessing the lamellar morphology retain highly swollen lamellae, as evidenced
by the TEM image shown in Figure 26.20 , even at solvent concentrations that
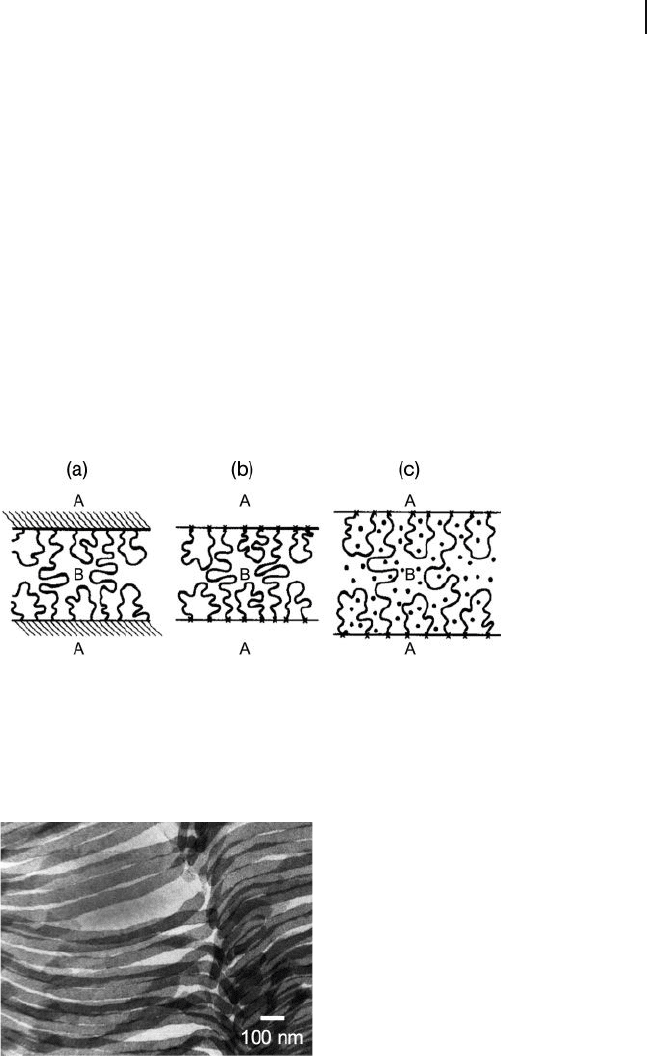
Figure 26.19 Schematic illustration of the
procedure to generate lamellar mesogels from
midblock - selective solvation of ABA triblock
copolymers. (a) Microphase ordering of the
copolymer from solvent casting or melt
processing; (b) Reduction in temperature so
that the A lamellae are rigid (i.e., glassy or
semicrystalline); (c) Diffusion of solvent into
the B lamellae to induce swelling while
retaining a layered morphology. Reproduced
with permission from Ref. [98] ; © American
Chemical Society.
Figure 26.20 Transmission electron
microscopy image of a triblock copolymer
mesogel demonstrating that the procedure
depicted in Figure 26.19 yields intact styrenic
lamellae (stained) in a solvent - swollen
midblock matrix. Reproduced with permission
from Ref. [100] ; © American Chemical
Society.

814 26 Nanostructured Organogels via Molecular Self-Assembly
would have otherwise induced morphological transformations. Li et al . [101] have
expanded earlier theoretical efforts [98, 99, 102] designed to model mesogels in
terms of swollen brushes by simulating the equilibrium swelling volume fraction
as a function of morphology, bridge fraction and midblock – solvent interaction
parameter. It is interesting to note that the extension and compression behavior
of lamellar mesogels are predicted [102] to be dissimilar. During extension, chain
elasticity dominates, whereas osmotic pressure governs compression. In general,
however, mesogels tend to exhibit improved mechanical properties (expressed in
terms of modulus [100] ) relative to their equilibrium counterparts at the same gel
composition.
26.2.7
Special Cases
26.2.7.1 Liquid Crystals
Generally speaking, liquid crystal s ( LC s) can be envisaged as anisotropic, rod - like
molecules that are capable of developing orientational and/or positional order in
the liquid state [103] . Of the three commonly encountered types of liquid crystal-
line mesophases reported (nematic, smectic and cholesteric, or twisted nematic),
the nematic, wherein the molecules align along a single direction with no posi-
tional order, constitutes the simplest [104] . Thermotropic LCs are temperature -
sensitive, and an increase in temperature causes the nematic mesophase to
disorder into an unstructured, isotropic liquid. Kornfi eld and coworkers [105]
have successfully synthesized thermoresponsive triblock copolymer gels contain-
ing a midblock - selective LC solvent. To ensure suffi cient compatibility between
the copolymer and nematic solvent (4 - cyano - 4 - n - pentylbiphenyl; known commer-
cially as 5CB) and to avoid macrophase separation, the copolymer molecule is
designed to have glassy (styrenic) endblocks and a midblock functionalized with
a nematic side group. In this case, the copolymer midblock is soluble in both
the LC and isotropic phases of 5CB. At low copolymer concentrations, the copoly-
mer endblocks are soluble in the isotropic phase, but aggregate in the nematic
phase due to endothermic mixing and a low entropy of mixing. Unlike isotropic
solvents, in which the solvent quality changes gradually with temperature, this
LC solvent undergoes an abrupt change in solvent quality at the relatively sharp
isotropic → nematic phase transition [106] . In essence, the gel exhibits an “ on – off ”
LC response at this transition temperature, thereby imparting the gel with added
functionality. At higher copolymer concentrations (20 wt%), the endblocks also
become insoluble in the isotropic phase of 5CB, in which case the gel network
remains intact even at temperatures above the nematic → isotropic transition.
Mesogels of LC triblock copolymers swollen by a nematic solvent have likewise
been investigated [107] . An interesting fi nding is that the modulus of the gel
can be reversibly changed by applying an electric fi eld. The gel can also be
sheared by applying a fi eld above a certain threshold value, thus evincing
quasi - piezoelectricity.

26.2 Block Copolymer Gels 815
26.2.7.2 Ionic Liquids
Ionic liquid s ( IL s) constitute an emerging class of functional compounds that
exhibit electrical conductivity and possess negligibly low vapor pressure, as well
as broadly tunable physical properties [108] . Lodge and coworkers [26] have fabri-
cated thermoreversible block copolymer gels by dissolving a poly( styrene - b -
ethylene oxide - b - styrene ) ( SEOS ) triblock copolymer in an IL at elevated
temperatures, and allowing the glassy endblocks to self - organize and vitrify upon
cooling. The IL used in this study remains liquid at ambient temperature. It is
interesting to note that the same type of copolymer, with a polar midblock, has
been used [109] to prepare mesogels in conjunction with poly(ethylene glycol)
( PEG ) for enhanced carbon dioxide separation. Characterization of the IL - based
copolymer gel, for which the cgc is 4 wt% at 10 ° C, reveals that the temperature
dependence of the ionic conductivity is comparable to that of the bulk ionic liquid
in the absence of the copolymer network. Similar gels have also been produced
[110] with a poly( N - isopropyl acrylamide - b - ethylene oxide - b - N - isopropyl acryla-
mide) triblock copolymer, which possesses temperature - sensitive endblocks. Gela-
tion in this system can be induced due to the lower critical solution temperature
( LCST ) behavior of the endblock in the IL solvent. Pioneering efforts such as these
are charting the course for future research in the bottom - up design of conductive
gels, especially as the copolymers and solvents can be further modifi ed to improve
both electrical and mechanical properties.
26.2.7.3 Multiblock Copolymers
Thus far, nanostructured organogels composed exclusively of ABA triblock copoly-
mers have been considered. Multiblock copolymers generically designated as
(A
n
B
n
)
m
copolymers are likewise expected to form stabilizing network structures
in an A - or B - selective solvent. Similar to triblock copolymer gels containing cosur-
factant (diblock copolymer) molecules (cf. Section 26.2.5.2 ), each microdomain in
a multiblock copolymer consists of dangling endblocks, as well as looped and
bridged midblocks, in proportions that depend only on n [111] . While the morpho-
logical and property attributes of solvent - free multiblock copolymers have received
considerable attention [112, 113] , few studies have explored the utility of well -
defi ned multiblock copolymer gels. Bansil and coworkers, for instance, have exam-
ined an ABABA pentablock copolymer in two different solvents: 1,4 - dioxane [114] ,
a slightly good solvent for A and a θ solvent for B; and n - hexane [115] , a strongly
selective solvent for the B blocks. In 1,4 - dioxane solutions, the marginally less -
soluble B blocks appear to be physically connected by swollen A blocks. An inter-
esting result is that a gel network does not develop in these solutions, even at the
highest copolymer concentrations studied, due possibly to (i) an insuffi ciently low
fraction of microphase - separated B blocks; or (ii) insuffi cient solvent selectivity.
In the presence of n - hexane, macrophase separation occurs at low copolymer
concentrations, whereas gelation is accompanied by solvent expulsion at higher
concentrations. Gindy et al . [116] have attempted to explain this result by perform-
ing Monte Carlo simulations and have proposed that, in dilute solutions, macro-
phase separation occurs when the ratio m / n exceeds a critical value (as observed

816 26 Nanostructured Organogels via Molecular Self-Assembly
experimentally in n - heptane). Gelation at higher copolymer concentrations is
attributed to the association of collapsed insoluble microdomains, similar to mul-
tiplets in ionomers. More complicated gel systems derived from randomly coupled
multiblock copolymers possessing broad block and chain polydispersities (e.g.,
polyurethanes [117] ) have likewise been generated and studied, but their nano-
structures are typically not well - defi ned, which is why they are not considered
further here.
26.2.7.4 Cosolvent Systems
Although ABA triblock copolymer gels normally consist of a single, low - volatility
solvent that is selected to be suffi ciently B - selective and A - incompatible, a mixture
of miscible solvents can certainly be employed to fi ne - tune solvent quality and
controllably alter the phase behavior and physical properties of the resultant gel
[118] . More recent studies [71] have demonstrated that this strategy can likewise
be used to adjust the time - responsive dynamic nature of such gels. The addition
of a triblock copolymer to a saturated tackifying resin, which possesses saturated
ring groups and a T
g
near or slightly above ambient temperature, yields a time -
dependent viscoelastic system that, upon uniaxial or biaxial deformation, slowly
returns to its original shape. In this case, the elastic restoring force of the copoly-
mer network is thwarted by the high viscosity of the solvent matrix. Results
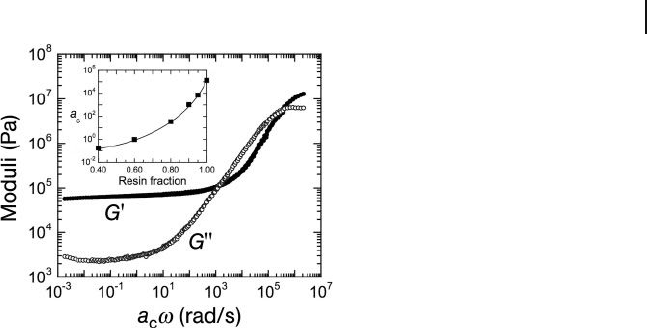
acquired from dynamic rheology confi rm that both G ′ and G ′ ′ are strong functions
of frequency, indicating that these systems are not gels according to the rheological
criteria listed earlier. The addition of a low - T
g
aliphatic oil to the system, however,
results in a composition - dependent progressive shift of the frequency spectrum
to higher frequencies. This apparent time - composition equivalence is similar in
effect to time – temperature equivalence [5] and permits the construction of a
superpositioned frequency spectrum over a broader range than could be measured
experimentally (cf. Figure 26.21 ). As frequency relates to reciprocal time, the
behavior of the cosolvent gel at very long or very short times can be accurately
assessed at ambient temperature by simply changing the cosolvent composition.
26.3
Organic Gelator Networks
Organic gelling agents, or gelators , with molecular masses of less than ∼ 2 kg mol
− 1
are referred to as low molar - mass organic gelator s ( LMOG s), and constitute a
growing class of compounds that provides fundamental insight into molecular
self - organization and practical use for applications requiring responsive materials
[1, 119, 120] . In stark contrast to solvated block copolymers that form gels by
microphase separation, LMOGs are generally classifi ed according to their molecu-
lar structure and the intermolecular interactions that promote physical gelation.
In this case, the organogel networks are stabilized via noncovalent physico -
chemical interactions such as hydrogen bonds, π – π stacking, or London dispersion
forces. As with block copolymer gels, gels produced by LMOGs are thermorevers-
26.3 Organic Gelator Networks 817
ible, in which case the load - bearing networks dissolve into the surrounding liquid
matrix upon heating above a composition - dependent dissolution temperature
( T
dis
), but reform upon cooling. Physical gels are typically generated by fi rst heating
a relatively low concentration (typically a few percent by mass) of the LMOG in an
organic solvent or low - T
g
polymeric liquid until all the components become a
solution, or sol , and then cooling the sol to below the gelation temperature ( T
gel
).
The value of T
gel
is identifi ed as the temperature at which fl ow is no longer discern-
ible over long periods [119] . It is important to recognize that T
gel
is generally lower
than T
dis
, since more thermal energy is required to break apart and dissolve the
gel network than to form it. Conversely, gel network formation may require a fi nite
degree of supercooling to initiate either (i) crystallization; (ii) precipitation; or (iii)
aggregation of the LMOG, thereby producing a gel [121] .
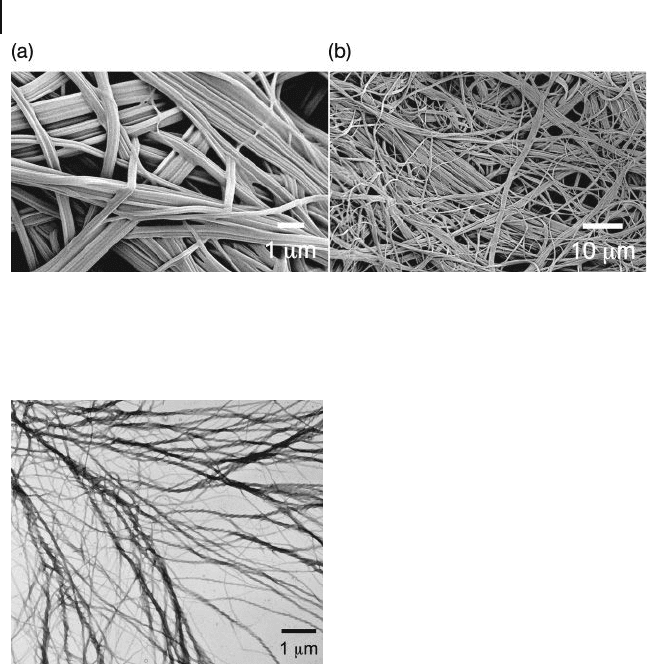
The resultant gels consist of 3 - D self - assembled fi brillar networks (SAFINs) that
are characterized by entangled nanoscale fi brils exhibiting a high surface - to -
volume ratio. These networks have been visualized by a variety of imaging methods,
including scanning electron microscopy ( SEM ), TEM and atomic force microscopy
( AFM ). Representative examples of SEM [122] and TEM [123] images of SAFINs
are provided in Figures 26.22 and 26.23 , respectively, and demonstrate that the
fi brils can range in size from nanometers to micrometers in width, and from
micrometers to millimeters in length. Due to the large solid – liquid interfacial area,
the matrix solvent is effectively entrapped by capillary forces within the network.
At the macroscopic level, the total volume of solvent is immobilized, resulting in
a solid - like material [124] . Because the networks do not consist of long, elastic
chains (as in block copolymer gels), LMOG - based gels tend to be exquisitely shear -
sensitive, and their networks readily break apart during steady or large - amplitude
Figure 26.21 Frequency spectra shown as
master curves for G ′ and G ′ ′ measured from
triblock copolymer organogels composed of
25 wt% copolymer and a cosolvent (mineral
oil/tackifying resin) that varies in
composition, thereby demonstrating that a
ternary SAMIN system exhibits time –
composition equivalence. The composition -
dependent shift factor ( a
c
), determined using
a reference composition of 60 wt% tackifying
resin, is included as a function of resin
fraction in the inset.
818 26 Nanostructured Organogels via Molecular Self-Assembly
Figure 26.22 Scanning electron microscopy
images collected at (a) high and (b) reduced
magnifi cation from a polycatenar organogel
consisting of 3 wt% gelator, illustrating the
morphology representative of SAFIN
organogels. Reproduced with permission from
Ref. [122] ; © American Chemical Society.
Figure 26.23 Transmission electron
microscopy image acquired from a
1 - acetonitrile organogel consisting of
0.07 wt% gelator, confi rming the existence of
nanofi brils (measuring 40 – 70 nm in diameter),
some of which exhibit helical twist with a
pitch of ∼ 150 nm. To improve contrast, the
nanofi brils have been selectively stained.
Adapted from Ref. [123] and used with
permission from John Wiley & Sons, Inc.
oscillatory shear, but reform upon cessation of shear. The kinetics of network
healing depend on the chemistry (and interaction mechanism) of the LMOG, the
concentration of the LMOG, and the quality of the solvent matrix. As gels produced
with LMOGs depend on specifi c intermolecular interactions, this section is divided
into three types of interactions that LMOGs require to promote physical gelation,
namely hydrogen bonding, π – π stacking, and London dispersion forces.
26.3.1
Hydrogen Bonding
Hydrogen bonding is the attractive force that exists between an electronegative
atom and a hydrogen attached to another electronegative atom, thereby imparting
the hydrogen with a partial positive charge. The electronegative atom must possess
