Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


10.2 Preparations 335
onto supports [37 – 39] , and/or by grafting the nanoparticles onto the support [40] .
Supported metal nanocatalysts can also be fabricated lithographically, using
electron beam lithography [41, 42] .
10.2.1
Silver Nanocatalysts
By using the above - described methods, silver materials with zero - , one - , or two -
dimensional nanostructures, including monodisperse nanoparticles, nanowires,
nanodisks, nanoprisms, nanoplates, and nanocubes, have each been prepared and
are recognized as having great potential for applications in optics, catalysis, and
other fi elds [43 – 47] .
10.2.2
Copper Nanocatalysts
Copper nanoparticles have been synthesized and characterized by different
methods. Notably, chemical reduction, pulsed laser ablation, radiolytic reduction,
and the reduction of copper ions using supercritical fl uids have been developed
to synthesize spherical and different - shaped nanoparticles [48 – 50] . Stability and
reactivity are the two important factors that impede the use and development of
metal clusters. In contrast to noble metals, such as Ag and Au, pure metallic
copper particles usually cannot be obtained via the reduction of simple copper
salts (e.g., copper chloride or copper sulfate) in aqueous solution, because the
reduction tends to stop at the Cu
2
O stage due to the presence of a large number
of oxygenous water molecules. However, this problem can be overcome by the
addition of other reagents carrying functional groups that can form complexes
with copper ions, or by using soluble surfactants as capping agents to prepare
copper particles in aqueous solution. Although zero - valent copper forms initially
in the solvent, ultimately it can be transformed relatively easily into oxides, in
solvents with high dipole moments and under ambient conditions. The use of
reverse micelles as microreactors and protecting shells has also helped to over-
come some of these complications. Likewise, electrolytic techniques have been
used to synthesize a variety of transition metal colloids of either decahedral or
isohedral shape, by controlling the electrode potential. The extreme air - sensitivity
of copper nanoparticles requires that care be taken during such preparation in
order to avoid oxidation.
10.2.3
Gold Nanocatalysts
Beyond preparing nanoscale materials in the form of colloids, considerable effort
has been expended in the preparation of supported heterogeneous catalysts, and
in particular the nature of the support and the process to immobilize an active
metal on the support (mostly metal oxides and active carbon). The use of gold

336 10 Oxidation Catalysis by Nanoscale Gold, Silver, and Copper
nanocatalysts is cited as an example here when introducing different methods for
preparing nanocatalysts.
Due to the lower melting point of gold, and its poor affi nity for metal oxides, it
is diffi cult to prepare stable gold catalysts that are well dispersed on metal oxides.
The typical impregnation methods that are widely used to prepare supported Pd
or Pt catalysts are ineffective in the case of gold, because the presence of chloride
ions can cause a signifi cant enhancement in the coagulation of gold particles
during the calcination of HAuCl
4
.
Haruta has summarized the methods used to prepare supported gold catalysts
(Table 10.1 ), and categorized them into four groups [51] :
• The fi rst group includes coprecipitation [54] , amorphous alloying [55] , and
cosputtering [56] . These procedures generally consist of two steps: (i) the
preparation of well - mixed gold/metal oxide precursors; and (ii) transformation
of the gold precursor into gold particles, normally by calcinations in air above
550 K. Well - mixed precursors and high - temperature calcination are equally
Table 10.1 Preparation techniques for nanoparticulate gold catalysts [51] .
Categories Preparation techniques Support materials Reference(s)
Preparation of mixed
precursors of Au and
the metal component
of supports
Coprecipitation
(hydroxides or
carbonates) (CP)
Be(OH)
2
, TiO
2
,
Mn
2
O
3
, Fe
2
O
3
, Co
3
O
4
,
NiO, ZnO, In
2
O
3
,
SnO
2
[52 – 54]
Amorphous alloy
(metals) (AA)
ZrO
2
[55]
Cosputtering (oxides) in
the presence of O
2
(CS)
Co
3
O
4
[56]
Strong interaction of
Au precursors with
support materials
Deposition – precipitation
(HAuCl
4
in aqueous
solution) (DP)
Mg(OH)
2
, Al
2
O
3
,
TiO
2
, Fe
2
O
3
, Co
3
O
4
,
NiO, ZnO, ZrO
2
,
CeO
2
, Ti - SiO
2
[57]
Liquid - phase grafting
(organogold complex in
organic solvents) (LG)
TiO
2
, MnOx, Fe
2
O
3
[58, 59]
Gas - phase grafting
(organogold complex)
(GG)
All types, including
SiO
2
, Al
2
O
3
- SiO
2
, and
activated carbon
[60, 61]
Mixing colloidal Au
with support materials
Colloid mixing (CM) TiO
2
, activated carbon [13]
Model catalysts using
single crystal supports
Vacuum deposition (at
low temperature) (VD)
Defects are the sites
for deposition, MgO,
SiO
2
, TiO
2
[62 – 64]

10.3 Selective Oxidation of Carbon Monoxide (CO) 337
important to ensure a strong contact between the Au particles and the crystalline
metal oxides.
• The strategy for the second group is based on the concept of depositing or
adsorbing Au compounds onto metal oxide surfaces. Among the three methods
referred to here, deposition – precipitation ( DP ) is widely used to produce active
Au catalysts. By controlling the pH and concentration of the HAuCl
4
solution,
the deposition of Au(OH)
3
can be controlled on the surfaces of the support metal
oxides so as to prevent precipitation in the liquid phase. Aggregation of the gold
nanoparticles, induced by chloride ions, can be prevented by washing the gold
compound before drying, and this represents one of the main reasons for the
high activity of these catalysts. The primary limitation here is that DP can only
be applied to metal oxides with an isoelectric point > 5. Although previously it
was shown that Au(OH)
3
could not be deposited on SiO
2
and active carbon,
recent studies have found that this constraint may be overcome by correct
surface modifi cation [65] .
• In the third group, the procedure involves the direct immobilization of Au
colloids on modifi ed metal oxide surfaces. In theory, this method could be
applied to all metal oxides, and the catalysts prepared would normally have a
good gold particle size distribution. However, there is often a relatively poor
contact between the gold particles and the support.
• In the fourth group, vacuum deposition is considered to be an important method
for preparing model catalysts that play a critical role when studying reaction
mechanisms, and especially the active sites of the supported gold catalysts. Au
anion clusters can be deposited with homogeneous dispersion at relatively low
temperatures [62, 64] on single crystals of MgO and TiO
2
(rutile). Surface defects
or specifi c surface cages have been suggested as possible sites for stabilizing the
Au clusters [62, 63] .
10.3
Selective Oxidation of Carbon Monoxide ( CO )
10.3.1
Gold Catalysts
During the 1980s, Haruta et al . [66] found that gold nanoparticles, when supported
on α - Fe
2
O
3
, were highly active in the oxidation of CO, and especially at very
low temperatures, although this surprisingly high activity was not replicated
by other metals (Figure 10.1 ). In a later series of investigations conducted by the
same group [52] , Au/TiO
2
was found to be an equally effective catalyst, and this
in turn led to extensive studies of gold nanocatalysts supported on a variety of
metal oxides.
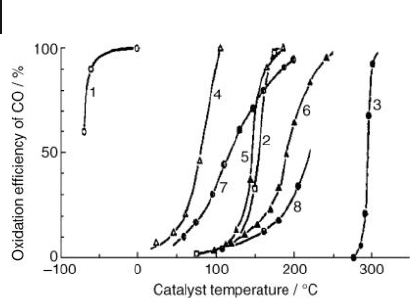
338 10 Oxidation Catalysis by Nanoscale Gold, Silver, and Copper
Figure 10.1 CO conversion over various
catalysts as a function of temperature. Curve
1, Au/ α - Fe
2
O
3
(Au/Fe = 1/19, coprecipitation,
400 ° C); Curve 2, 0.5 wt% Pd/ γ - Al
2
O
3
(impregnation, 300 ° C); Curve 3, fi ne Au
powder; Curve 4, Co
3
O
4
(carbonate, 400 ° C);
Curve 5, NiO (hydrate, 200 ° C); Curve 6,
α - Fe
2
O
3
(hydrate, 400 ° C); Curve 7, 5 wt%
Au/ α - Fe
2
O
3
(impregnation, 200 ° C); Curve 8,
5 wt% Au/ γ - Al
2
O
3
(impregnation, 200 ° C).
Reproduced with permission from Ref. [66] ;
© 1987, Chemical Society of Japan, Tokyo.
Owing to the possible applications of polymer electrolyte fuel cells to automo-
biles and also to residential electricity - heat delivery systems, the low - temperature
water - gas - shift reaction continues to attract renewed interest. When compared to
commercial catalysts that are based on Ni or Cu and operated at 900 K or 600 K,
respectively, supported Au catalysts appear to have a clear operational advantage
in that they function at temperatures as low as 473 K [67] . During the course of
investigating the hydrogenation of CO
2
over supported Au catalysts, it was found
that Au/TiO
2
was selective towards the formation of CO, in that the reverse water -
gas - shift reaction could be conducted at a temperature as low as 473 K [67] . Later,
Au/TiO
2
was confi rmed also to be active for the water - gas - shift reaction [68] .
The oxidation of CO is a typical reaction for which Au catalysts are extraordinar-
ily active at room temperature, and indeed are much more active than other noble
metal catalysts at temperatures below 400 K. One focal point of recent studies has
been the elaboration of the mechanism for CO oxidation [69, 70] . Although the
available data on this topic are vast – and occasionally contradictory – several pieces
of information were identifi ed that were critical to developing an understanding
of the mechanism. For example, active catalysts always contain metallic Au parti-
cles which produce a CO absorption band at 2112 cm
− 1
, whereas oxidic Au species
that produce a CO absorption band at 2151 cm
− 1
are not responsible for steady -
state, high catalytic activity [71] . However, as the smooth surfaces of metallic Au
do not adsorb CO at room temperature [72] , this indicates that CO is adsorbed
only on steps, edges, and corner sites. As a consequence, the smaller metallic Au
particles are preferable [73] .
A theoretical calculation [74] has been used to explain why the smooth surface
of Au is noble in the dissociative adsorption of hydrogen. However, when Au is
deposited as nanoparticles on metal oxides by means of coprecipitation and DP
techniques, it exhibits a surprisingly high catalytic activity for CO oxidation at a
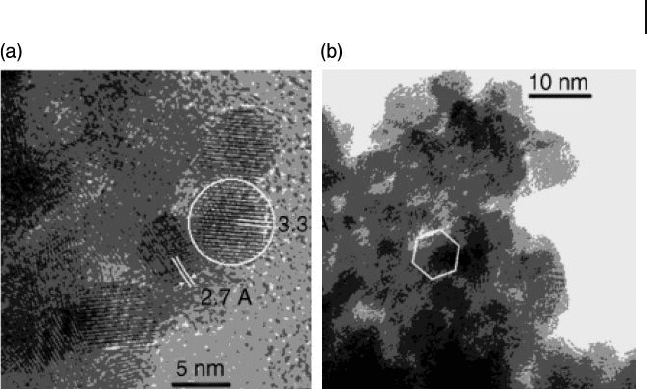
10.3 Selective Oxidation of Carbon Monoxide (CO) 339
Figure 10.2 High - resolution transmission
electron microscopy images of the 2.8% Au/
CeO
2
sample. (a) The white lines correspond
to the (202) Ce
6
O11 (3.3 Å ) and the (200)
CeO
2
(2.7 Å ) lattice spacing; (b) A hexagonal
faceted (111) Au crystal is indicated.
Reproduced with permission from Ref. [75] ;
© 2005, WILEY - VCH Verlag GmbH & Co.
KGaA, Weinheim.
temperature as low as 200 K [12, 52] . During the 1990s, this fi nding led to many
research groups conducting extensive investigations into the catalysis of Au.
One remarkable study among many was conducted by Corma and coworkers [75] ,
who showed that gold nanoparticles supported on nanocrystalline CeO
2
, in conjunc-
tion with the DP method, proved to be a very active catalyst for CO oxidation (Figure
10.2 ). Indeed, the catalysts were found to be an order of magnitude more active for
CO oxidation than comparable catalysts prepared using a non - nanocrystalline
support. The Au/CeO
2
catalyst also showed excellent selectivity for CO oxidation in
the presence of H
2
at 60 ° C (close to the operating temperature of fuel cell), where
the selectivity of normal Au active catalysts would be negatively affected [76] .
Most gold catalysts which have been reported as active for CO oxidation were
prepared using the DP method, which provides catalysts with a strong interaction
between the gold and the metal oxide matrix. However, a major drawback of this
method is that it cannot be used to deposit gold at metal oxides with an isoelectric
point ( IEP ) < 5, such as SiO
2
. Subsequently, Sheng Dai and coworkers [65] success-
fully deposited gold at the surface of mesoporous SiO
2
by using the DP method
following a sol – gel surface modifi cation with TiO
2
. The results showed the gold
nanoparticles (0.8 – 1.0 nm) in the mesopores to be highly active in terms of CO
oxidation (Figure 10.3 ).
Today, whilst it is widely recognized that these supported gold nanocatalysts
show a high activity in the low - temperature oxidation of CO, many questions
remain unanswered concerning the relatively simple reaction of CO oxidation. The
most notable of these are “ What is the reaction mechanism? ” , and “ What is the
nature of the active site? ”
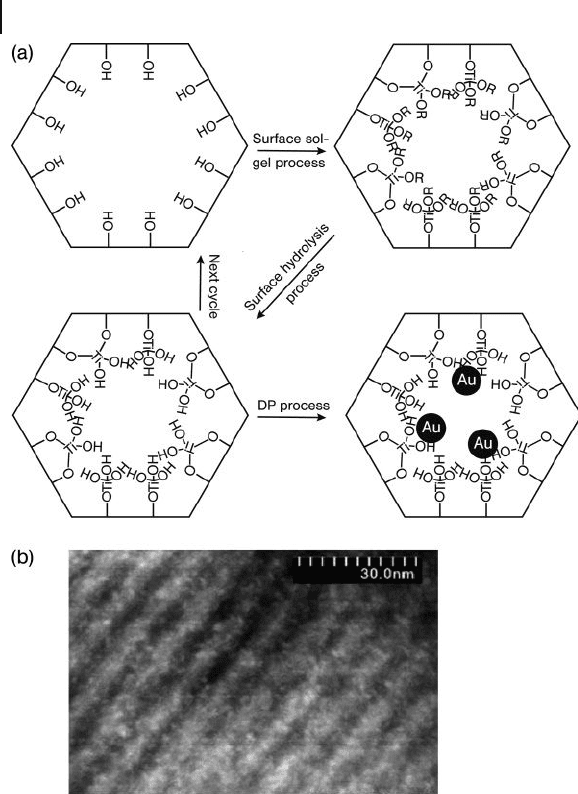
340 10 Oxidation Catalysis by Nanoscale Gold, Silver, and Copper
Figure 10.3 (a) Scheme of preparation of
gold/mesoporous material catalysts; (b)
Z - contrast TEM image of ultrasmall gold
nanoparticles on ordered mesoporous
materials. The bright spots (0.8 – 1.0 nm)
correspond to gold nanoparticles. Reproduced
with permission from Ref. [65] ; © 2004, ACS
Publications, Washington.
A substantial proportion of the research into CO oxidation catalyzed by sup-
ported gold has been motivated by the goal of identifying those catalyst properties
which affect the activity. In this respect, two early classes of observation were
important in determining the approaches used recently to investigate supported
gold catalysts: (i) that various preparation routes lead to catalysts with different
activities [52, 77] ; and (ii) that catalysts consisting of gold supported on reducible
metal oxides (e.g., Fe
2
O
3
, CeO
2
, TiO
2
) are typically more active than those
10.3 Selective Oxidation of Carbon Monoxide (CO) 341
supported on nonreducible metal oxides (e.g., γ - Al
2
O
3
, MgO, SiO
2
). Such observa-
tions led to a wide acceptance of the inferences that the preparation method
infl uenced activity [12] , and that the support played a role in the catalysis.
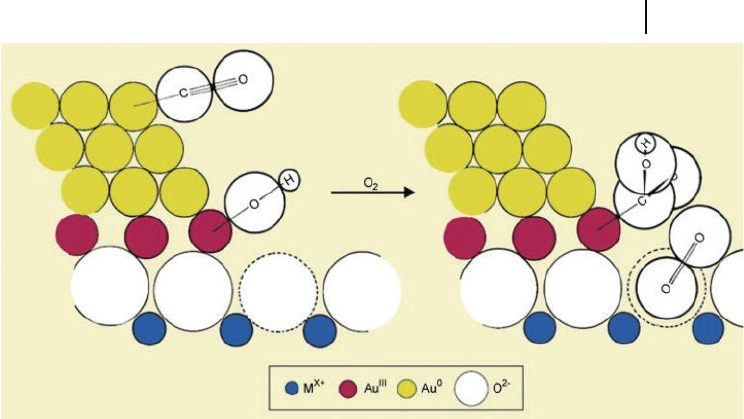
Bond and Thompson [77] , in their review of the literature which extended to
the year 2000, and in an attempt to reconcile some apparently contradictory
hypotheses, proposed a mechanism for CO oxidation that was catalyzed by
supported gold (Scheme 10.1 ). The proposed active site consisted of nanoparticles
incorporating both zero - valent and cationic gold, with the latter positioned at
the metal – support interface. The suggestion by Bond and Thompson of the pres-
ence of cationic gold was based on observations by various authors of ν CO infrared
(IR) bands that were characteristic of CO bonded to cationic gold. However,
evidence was lacking not only of any such species in working catalysts, but also
of the suggestion that cationic gold was a “ glue ” which held the nanoclusters
to the support.
In 2002, Haruta [78] presented a review of the literature and proposed, on
the basis of measurements of the kinetics of CO oxidation catalyzed by supported
gold, that there were three temperature regions, each with different kinetics and
activation energies of the CO oxidation reaction. Haruta suggested that, at tem-
peratures below 200 K, the reaction catalyzed by Au/TiO
2
took place at the surfaces
of small gold nanoparticles dispersed on the support, but at temperatures above
300 K the reaction occurred at gold atoms at the perimeter sites of the supported
gold nanoparticles.
Scheme 10.1 Schematic representation of an active site and
possible reaction mechanism for CO oxidation catalyzed by
supported gold. Reproduced with permission from Ref. [77] ;
© 2000, World Gold Council, London.

342 10 Oxidation Catalysis by Nanoscale Gold, Silver, and Copper
10.3.2
Silver Catalysts
Silver catalysts also have a relatively high activity for the selective oxidation of
CO at low temperatures. A silver catalyst was deactivated remarkably following
pretreatment in H
2
at high temperatures, but could be reactivated by treatment in
oxygen at similarly high temperatures. Interestingly, these changes in activities
were mostly reversible. The structures of the silver particles were seen to experi-
ence massive changes during the course of various pretreatments, and the exist-
ence of subsurface oxygen resulting from an oxygen treatment at high temperatures
was shown to be crucial for high selectivity and activity in CO selective oxidation
[79, 80] . As CO oxidation is generally claimed to be a structure - sensitive reaction,
restructuring of the silver particles is likely to exert an infl uence on the activity of
the catalyst. Yang and Aoyama [81, 82] studied the thermal stability of uniform
silver clusters supported on oxidized silicon or aluminum surfaces in both oxidiz-
ing and reducing atmospheres, and found the thermal stability of the silver
clusters to be signifi cantly lowered under oxidizing conditions. Moreover, heating
above 350 ° C under oxidizing condition could induce a migration of the
silver clusters.
Size selectivity in catalysis was reported for propylene partial oxidation and low -
temperature CO - oxidation, with Ag nanoparticles of < 5 nm diameter being shown
to have equal activity as the Au nanoparticles. In contrast, for ethylene epoxidation
only those Ag particles > 30 nm could catalyze the reaction [83] . Recently, much
attention has been focused on the use of spherical or undetermined - shape nano-
particles for catalyzing reactions. Very few studies have been undertaken in which
catalysis was conducted with nanoparticles of known shapes [84] , for example,
using truncated octahedral Pt nanoparticles to catalyze the electron - transfer reac-
tion, and cubic Pt nanoparticles in the decomposition of the oxalate capping agent.
The formation of different oxygen species, depending on the Ag particle size sput-
tered on the highly ordered pyrolytic graphite ( HOPG ) surface, resulted in a vari-
ation in catalytic activity of CO oxidation using oxygen under ( ultra - high vacuum )
UHV conditions revealed CO oxidation to be sensitive towards the size of particle
[85] . The oxygen uptake of a smaller Ag nanoparticle was seen to be signifi cantly
higher than that of a larger particle and a bulk - like Ag which enhanced the reactiv-
ity of CO oxidation.
10.3.3
Gold – Silver Alloy Catalysts
The recent progress in polymer electrolyte membrane fuel cells has particularly
motivated the search for a highly effi cient catalyst for CO selective oxidation at low
temperatures. Thus, combinations of metals in the forms of alloys, core – shell and
“ decorated ” surfaces (Pt, Pd, Rh, Ru, Au, Ag, Cu, Co, Fe, In, Ga) with different
supports, such as zeolite, Al
2
O
3
, SiO
2
, and activated carbon, have produced active
catalysts for the CO reaction. One such alternative catalyst, namely gold – silver
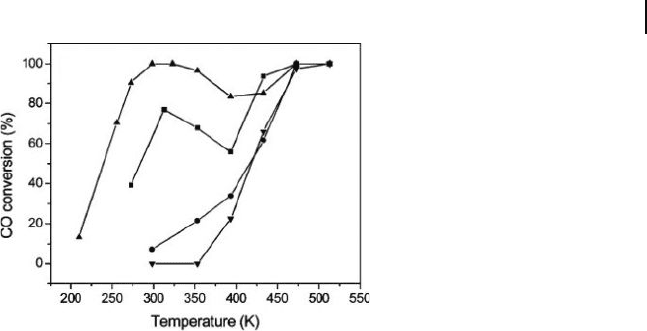
10.3 Selective Oxidation of Carbon Monoxide (CO) 343
Figure 10.4 CO conversion over reaction temperatures at
various molar ratios of Au : Ag. 䉱 , ratio 3 : 1;
䊏
, ratio 1 : 1;
䊉
,
ratio 1 : 0;
䉲
, ratio 0 : 1. Reproduced with permission from
Ref. [88] ; © 2005, ACS Publications, Washington.
alloy nanoparticles deposited on MCM - 41, demonstrated an exceptionally high
catalytic activity which was comparable to the most active catalysts (Figure 10.4 )
such as Au/TiO
2
and Au/Fe
2
O
3
[86, 87] . The alloying of Au and Ag showed a strong
synergistic effect in promoting the low - temperature oxidation of CO [88] . The alloy
catalyst activation was shown to depend on the composition (the Ag ratio was
crucial), the aluminum content in the support, and the pretreatment conditions.
10.3.4
Copper Catalysts
Copper nanoparticles are also active for the selective oxidation of CO. The majority
of studies with copper particles have been performed with fi nely dispersed copper
on various supports, and have demonstrated high catalytic activities for CO oxida-
tion. Likewise, CuO mixed with ZnO or with CeO
2
have also shown promise as
catalysts. The results of a recent density functional theory ( DFT ) study showed that
gold and copper had a lower barrier for CO oxidation than for H
2
oxidation.
Ceria has a promoting effect on the activity of the Au/Al
2
O
3
catalyst in CO oxida-
tion [89] . The addition of Li
2
O and/or CeOx to copper, silver, and gold catalysts of
3 nm size on γ - Al
2
O
3
for the preferential oxidation of CO in a hydrogen atmosphere
[90] , have shown signifi cant changes in the conversion. The nanoscale metal par-
ticles or metal complexes in polymer matrices show quite interesting chemical
and catalytic reactivity towards a variety of small gas molecules under relatively
mild conditions that differ from those of the corresponding free transition metal
complexes, or from those in inorganic oxide - supported systems. The incorporation
of copper nanoparticles into cellulose acetate, and the subsequent oxidation of
small gas molecules (e.g., CO, H
2
, D
2
, O
2
, NO, and olefi ns) over a temperature
range of 25 to 160 ° C, has also been examined [91] . Nanoparticles of various sizes
prepared by different routes and hosted in the channels of SBA - 15, exhibited a
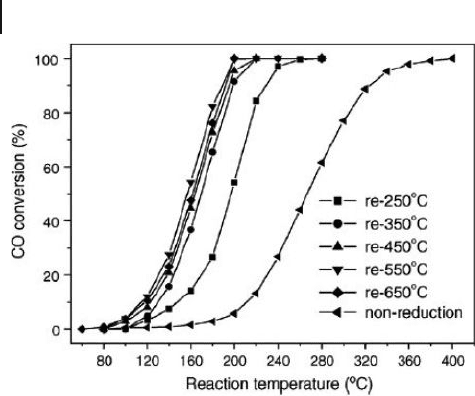
344 10 Oxidation Catalysis by Nanoscale Gold, Silver, and Copper
Figure 10.5 CO conversions versus reaction temperature over
Cu/SBA - 15 (post grafted) calcined at 500 ° C and reduced at
different temperatures. Reproduced with permission from
Ref. [92] ; © 2006, Elsevier B.V., Amsterdam.
high catalytic activity for CO oxidation, with complete conversion at 190 ° C
(Figure 10.5 ) [92] . Such high catalytic activity was mainly infl uenced by the size
and dispersion of the Cu particles.
10.4
Epoxidation Reactions
10.4.1
Gold Catalysts
Since the fi rst recognition by Hayashi [93] that Au supported on TiO
2
could
catalyze the epoxidation of propylene in the gas phase containing O
2
and H
2
, the
catalytic properties of Au/TiO
2
and related systems have attracted interest not only
from the chemical industries but also from academia. Today, propylene oxide ( PO )
is recognized as one of the world ’ s most important bulk chemicals, and is used
in the production of polyurethane and polyols. The current industrial processes
utilize two - staged chemical reactions, using either Cl
2
or organic peroxides to yield
the byproducts stoichiometrically.
From both environmental and economic points of view, the direct synthesis of
PO by using molecular oxygen has long been a major academic challenge, although
supported noble metal catalysts such as Ag/carbonates and/or titanates, Pd/TS - 1,
Pd – Pt/TS - 1 [94 – 96] , and Au/TiO
2
(Figure 10.6 ) [93, 97] , have each been reported
to be active in this process.
In 1998, using the DP technique, Haruta and coworkers [97] produced nanoscale
gold catalysts that showed a high activity towards CO oxidation, based on the
