Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


Self - Assembling Nanoclusters Based on Tetrahalometallate
Anions: Electronic and Mechanical Behavior
Ishenkumba A. Kahwa
365
Advanced Nanomaterials. Edited by Kurt E. Geckeler and Hiroyuki Nishide
Copyright © 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
ISBN: 978-3-527-31794-3
11
11.1
Introduction
Self - assembling systems are invaluable sources of novel materials with diverse
architectures, morphologies, physical and chemical properties, as well as potential
applications [1 – 9] . The relative simplicity of some preparative procedures, and the
precision with which thermodynamic and kinetic factors operate to produce such
materials, make self - assembly an intensely studied phenomenon [4, 7] . The major
aim of the present author ’ s research has been the preparation of novel multi - metal
materials that could: (i) effi ciently immobilize toxic metals from the environment
[10] ; (ii) trap and transport metal ions in human and animal bodies for diagnostic,
imaging, or therapeutic benefi ts [11] ; or (iii) simply act as dispersion media for
metal ions in the effi cient synthesis of multicomponent metal oxides with interest-
ing properties, such as the superconducting oxides, YBa
2
Cu
3
O
7
[12] . These studies
have covered self - assembling coordination compounds of multiple metal ions of
the 3d - , 4f - , some p - , and nearly all of the s - block series [10 – 21] . This chapter
provides a review of the synthesis of multinuclear self - assembling compounds
facilitated by crown ethers, their structures, electronic behavior, and their ther-
mally activated mechanical properties [10 – 21] .
Interest in these compounds was inspired by the potential of crown ether
chelates to bind metal ions such as M
+
, M
2+
, and M
3+
, the charge of which can then
be counterbalanced by, among others, negatively charged, metalloanions such as
[CuX
4
]
2 −
and [Cu
2
X
6
]
2 −
to produce mixed - metal compounds for the preparation of
superconducting ceramic oxides such as YBa
2
Cu
3
O
7
and their derivatives [10] . For
thallium - based superconducting ceramic oxide systems, the toxicity of thallium
and ensuing environmental health concerns were important considerations [10] .
Thus, as thallium - based superconducting ceramics demonstrated a superior
potential based on a higher transition temperature ( T
c
) (e.g., T
c
= 120 – 125 K and
100 – 105 K for Tl
2
Ba
2
Ca
2
Cu
3
O
10
and Tl
2
Ba
2
CaCu
2
O
8
, respectively) [22] compared to
90 K for YBa
2
Cu
3
O
7
) [23] , self - assembling systems that could immobilize large
amounts of toxic thallium ions achieved greater signifi cance.
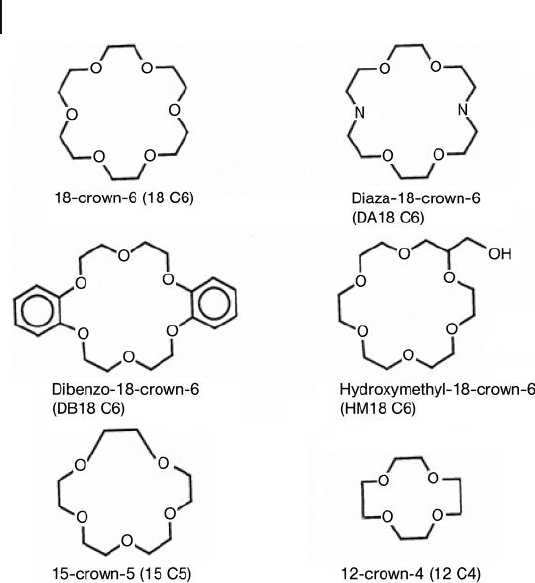
366 11 Self-Assembling Nanoclusters Based on Tetrahalometallate Anions
Scheme 11.1 Crown ethers used in these studies.
In this chapter, a review is provided of the studies conducted on the syntheses
of metal - rich Cubic F23 supramolecular complexes [(A(18 - Crown - 6))
4
(MX
4
)]
[BX
4
]
2
· n H
2
O, where A is a monovalent metal or NH
4
+
ion; M is a divalent metal
ion (normally of 3d element); B is a trivalent metal ion (Tl
3+
or Fe
3+
); and X is a
halogen (Cl or Br). The 18 - crown - 6 chelate (Scheme 11.1 ) in these and other com-
plexes exhibits a thermally activated rotation conformation reorientation motion,
which is of interest as a trigger for luminescence and magnetic on/off switching
and mechanical nanodevices. The effectiveness of Mn
2+
to serve as an effi cient
probe for a variety of coordination environments was also successfully explored,
and the results are reported.
11.2
Preparation of Key Compounds
The general preparation of the [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O series has been
reported previously [10 – 21] . The procedure involves a simple refl uxing of chelate
18C6 in alcoholic solutions containing the corresponding quantities of metal salts
(e.g., RbBr, MnBr
2
, and TlBr
3
when the complex (Rb(18C6))
4
(MnBr
4
)] [TlBr
4
]
2
· n H
2
O
is desired), and then evaporating off the excess solvent to deposit the crystalline

11.3 Structure of the [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· nH
2
O Complexes 367
Scheme 11.2 Pertinent reactions leading to supramolecular
systems: [(Tl(18 - Crown - 6))
4
(CuCl
4
)] [TlCl
4
]
2
· n H
2
O. The solvent
shells are removed, and reactions occur in air.
materials. For the complex (Tl(18C6))
4
(MX
4
)] [TlX
4
]
2
· n H
2
O, auto - oxidation of Tl
+
to Tl
3+
or auto - reduction of Tl
3+
to Tl
+
occurs in the refl uxing mixture when the
reaction vessels are open to the atmosphere (Scheme 11.2 ).
Hence, there is no need for both Tl
3+
and Tl
+
to be added into the reaction
mixture. If Fe
3+
and Tl
+
are used, no auto - oxidation of Tl
+
is observed. Overall,
compounds [(A(18C6))
4
- (MX
4
)] [BX
4
]
2
· n H
2
O with A = Tl, Na, K, Rb, NH
4
, BaX
(X = halide or OH); M = Mn, Fe, Co, Ni, Cu, Zn, and B = Tl or Fe have been
prepared. Similar results were recently obtained by another group, which demon-
strated that for M
2+
= Fe
2+
reducing conditions are required; otherwise, oxidation
to Fe
3+
occurs and the cubic compounds are not formed [24] . The crown ethers
used in the study are shown in Scheme 11.1 .
Changing the crown from 18C6 to DA18C6 or HM18C6 resulted in the forma-
tion of compounds similar to those of [(Rb(18Crown)
4
(MnX
4
)] [TlX
4
]
2
· n H
2
O, while
DB18C6, 15C5 and 12C4 failed to produce the cubic series. However, the reaction
of NaBr, 15C5, and TlBr
3
in the presence or absence of [MX
4
]
2 −
anions yielded an
elegant self - assembling compound [(Na(15C5))
4
Br] [TlBr
4
]
3
in which the Br
−
anion
played the role of concentrating [Na(Crown)]
+
cations in a manner similar to that
of tetrahedral [MX
4
]
2 −
anions [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O.
11.3
Structure of the [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O Complexes
The structure of compounds of the [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O is Cubic F23 ,
with the [A(16C6)]
+
cation perched on the triangular surfaces of the [MX
4
]
2 −
anions,
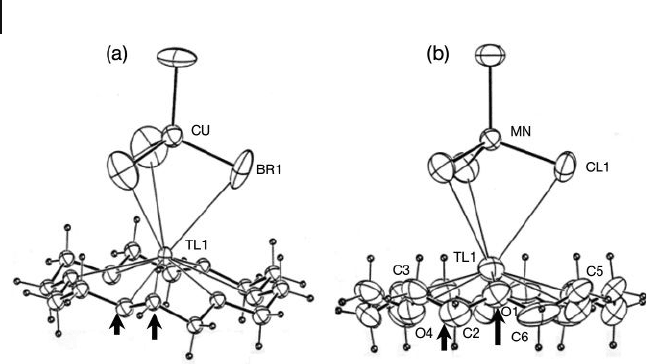
as shown in Figure 11.1 for one of the four such links. While the structures
remained Cubic F23 throughout the series [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O, there
were subtle differences in the orientation of the 18C6 in some cases, which are
temperature - dependent. For instance, for the compound [(Tl(18C6))
4
(CuBr
4
)]
[TlBr
4
]
2
· n H
2
O, the room temperature structure is similar to that of the Mn
2+
analogue, but when it is cooled to 115 K a switch in the O and C positions was
found to have occurred (Figure 11.1 ). The structures of many compounds were
studied, and the switch from the low - temperature form (that of [(Tl(18C6))
4
(CuBr
4
)]
[TlBr
4
]
2
at 115 K) to the high - temperature form (that of [(Tl(18C6))
4
(CuBr
4
)] [TlBr
4
]
2
368 11 Self-Assembling Nanoclusters Based on Tetrahalometallate Anions
Figure 11.1 Partial structures of the [(Tl(18 - Crown - 6))
4
(MX
4
)]
2+
supramolecular cations, showing the two orientations.
(a) T = 115 K, M = Cu, X = Br; (b) Room temperature, M = Mn,
X = Cl. The difference is in the 30 ° rotation for 18C6 ligand
resulting in carbon and oxygen switching positions (compare
the positions marked by the arrows). Adopted from Ref. [15] .
at room temperature) was found to depend on several factors, including the
nature of the halide, the presence or absence of crystal water, and the temperature
(Table 11.1 ) [16] .
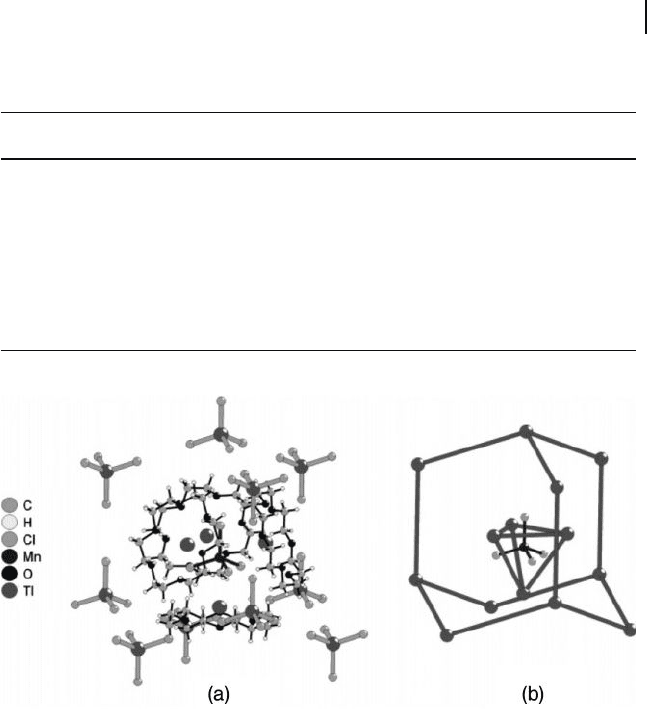
The extended cubic structure of members of the [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O
was recently [24] analyzed in detail (Figure 11.2 a); the study revealed an M
2+
nucleus trapped in a tetrahedron of four X
−
anions; the resultant [MX
4
]
2 −
is coor-
dinated on each of its four triangular faces by [A(18C6)]
+
cations with resulting
[(A(18C6))
4
(MX
4
)]
2+
cations secured inside a cavity made up of a cyclic adamantane -
like network of ten [TX
4
]
−
anions constituting six - member rings in chair conforma-
tions, as shown in Figure 11.2 b [24] .
11.4
Structure of the [(Na(15C5))
4
Br] [TlBr
4
]
3
Complex
Like the [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O, the elegant structure of the [(Na(15C5))
4
Br]
[TlBr
4
]
3
complex reveals that it is stabilized by multiple complexation networks, as
shown in Figure 11.3 [18] .
11.5
Spectroscopy of the Cubic F 23 [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O
The Cubic F23 structure [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O requires additional
support because it requires the [MX
4
]
2 −
anion, including the [CuX
4
]
2 −
, which is
11.5 Spectroscopy of the Cubic F23 [(A(18C6))
4
(MX
4
)][BX
4
]
2
· nH
2
O 369
Table 11.1 18 - Crown - 6 orientations for structures of
[( A (18 - Crown - 6))
4
( MX
4
)][ TlX
4
]
2
. The crown orientation is seen
to depend on many factors.
A M X T (K) Orientation
Tl Cu Br 295 HT
Tl Mn Cl 295 HT
Tl Cu Cl 295 HT
K Fe Cl 293 HT
Tl Cu Br 115 LT
Tl Cu Cl (with 0.25 H
2
O) 297 LT
K Mn Br 295 LT
K Zn Br 297 LT
Figure 11.2 (a) The partial extended structure of
[(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O members; (b) The network of
ten [TX
4
]
−
anions in an adamantine - type arrangement [24] .
highly susceptible to Jahn – Teller distortion [25 – 27] , to occupy a 23T site of perfect
T
d
symmetry [10] . We thus sought to determine whether the A, M, and B sites
were of the indicated oxidation states and coordination geometries by spectro-
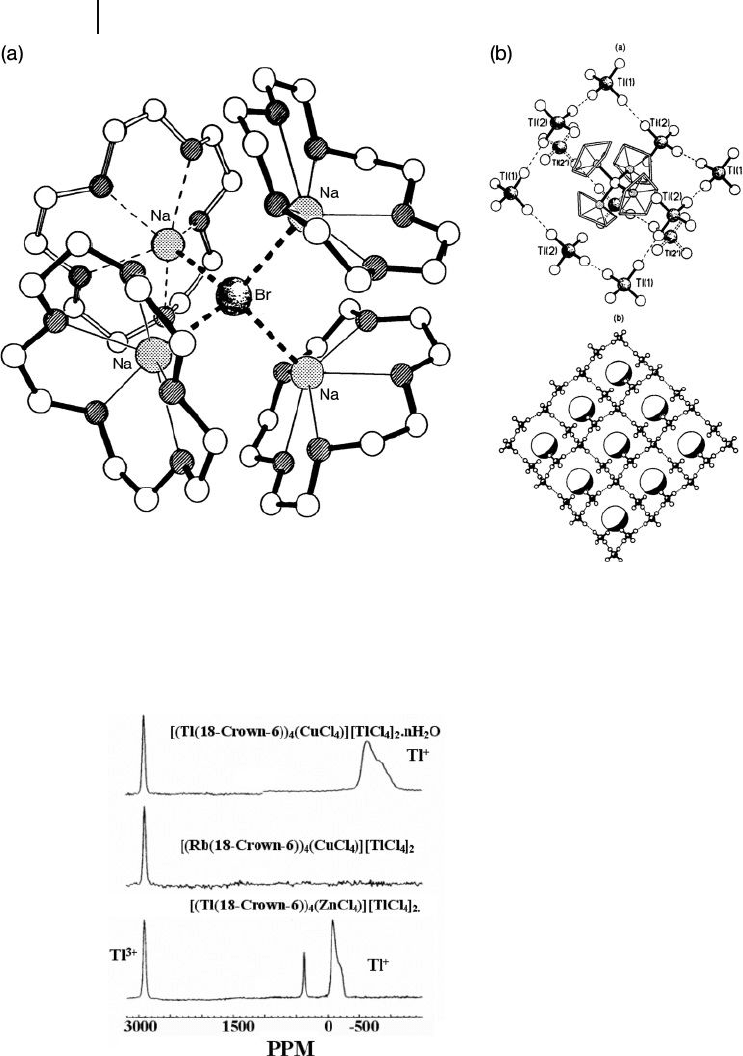
scopic means to complement the X - ray structural evidence. The presence of mixed
valence thallium centers in [(Tl(18C6))
4
(MCl
4
)] [TlCl
4
]
2
· n H
2
O was established
using solid - state nuclear magnetic resonance ( NMR ) of
205
Tl (Figure 11.4 ), which
showed Tl
3+
with chemical shifts of approximately 2900 ppm relative to Tl(NO
3
),
while that of Tl
+
occurred at − 782 ppm and − 117 ppm for the paramagnetic
[(Tl(18C6))
4
(CuCl
4
)] [TlCl
4
]
2
· n H
2
O and diamagnetic [(Tl(18C6))
4
(ZnCl
4
)]
[TlCl
4
]
2
· n H
2
O compounds, respectively. The larger chemical shift and broader
resonance of Tl
+
in [(Tl(18 - Crown - 6))
4
(CuCl
4
)] [TlCl
4
]
2
· n H
2
O, relative to that of
[(Tl(18 - Crown - 6))
4
(ZnCl
4
)] [TlCl
4
]
2
, being indicative of the cation ’ s proximity to the
paramagnetic Cu
2+
center.
370 11 Self-Assembling Nanoclusters Based on Tetrahalometallate Anions
Figure 11.3 An elegant structure of [(Na - Crown - 5))
4
Br] [TlX
4
]
3
.
(a) The cation [(Na - Crown - 5))
4
Br]
3+
; (b) Upper: the cation in a
network of [TIBr4]
−
anions. Lower: one layer of stacking
egg - tray networks of [TIBr4]
−
anions and their [(Na - Crown -
5))
4
Br]
3+
guest, shown as a partially shaded ball.
Figure 11.4 Solid - state
205
Th NMR spectra for some members
of the [(A(18C6))
4
(MCl
4
)] [BCl
4
]
2
· n H
2
O series, showing the
presence of the thallium(I) (Tl
+
) and thallium(III) (Tl
3+
)
sites [10] .
11.5 Spectroscopy of the Cubic F23 [(A(18C6))
4
(MX
4
)][BX
4
]
2
· nH
2
O 371
While the structural details of the copper complexes [(A(18C6))
4
(MX
4
)]
[BX
4
]
2
· n H
2
O, M = Cu, were similar to those of M = Co, Mn, Fe, Ni and Zn,
it was necessary to determine whether the 23T site is really T
d
or is an average
of various geometries at room temperature. The deep - blue Co
2+
cubic compound
[(Tl(18C6))
4
(CoCl
4
)] [TlCl
4
]
2
· n H
2
O is consistent with Co
2+
being in a T
d
environment.
The manganese (II) emission from the [(A(18C6))
4
(MnCl
4
)] [TlCl
4
]
2
· n H
2
O
(A = Tl or Rb) compounds peaked at about 535 nm, as is typical of the tetrahedral
[MnCl
4
]
2 −
anions [14] ; the corresponding excitation spectra were also characteristic
of T
d
[MnCl
4
]
2 −
anions (Figure 11.5 for A = Rb). The excitation spectrum
of [MnCl
4
]
2 −
emission in [(Rb(18C6))
4
(MnCl
4
)] [TlCl
4
]
2
· n H
2
O (Figure 11.5 a) is
attributed to electronic transitions from the
6
A
1
ground state to accessible higher -
energy quartets derived from the crystal fi eld splitting of
4
G,
4
D,
4
P, and
4
F levels.
Emission spectra from compounds with Cu
2+
and Fe
3+
were also typical of
4
T
1
(
4
G) →
6
A
1
emission for [MnCl
4
]
2 −
ions. However, the inner - fi lter due to charge
transfer bands of Cu
2+
and Fe
3+
led to signifi cant changes on the excitation spectra
(Figure 11.5 ).
Thus, as required by Cubic F23 symmetry, the 23T site occupied by the [MX
4
]
2 −
anions in crystalline [(A(18C6))
4
(MX
4
)] [BX
4
]
2
· n H
2
O compounds is indeed
of T
d
character. However, the Cu
2+
charge transfer bands exhibited by the
[(A(18C6))
4
(CuCl
4
)] [TlCl
4
]
2
· n H
2
O compounds at 27 000 to 30 000 cm
− 1
(
2
E ←
2
B
2
)
and 21 000 to 23 000 cm
− 1
(
2
A
2
←
2
B
2
and
2
E ←
2
B
2
) are in regions generally expected
of D
2d
[CuCl
4
]
2 −
[10] . This ion may be distorted, but this requires additional proof.
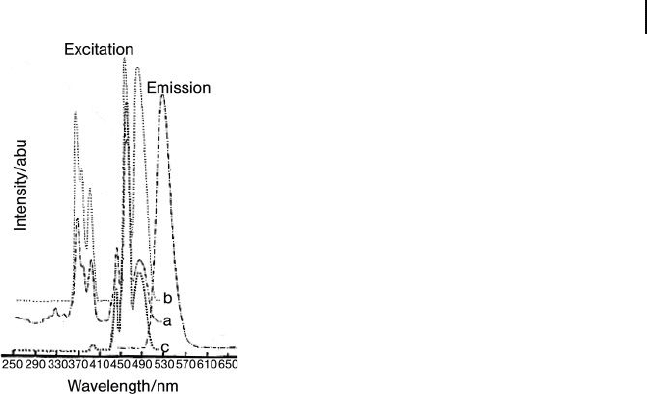
Figure 11.5 Emission and excitation spectra of
[(Rb(18C6))
4
(MnCl
4
)
1 –
x
(CuCl
4
)
x
] [(TlCl
4
)
1 –
y
(FeCl
4
)
y
]
2
· n H
2
O.
Spectrum a: x = 0, y = 0; spectrum b: x = 0.056, y = 0;
spectrum c: x = 0, y > 0) [14] .

372 11 Self-Assembling Nanoclusters Based on Tetrahalometallate Anions
11.6
Unusual Luminescence Spectroscopy of Some Cubic [(A(18C6))
4
(MnX
4
)]
[TlCl
4
]
2
· n H
2
O Compounds
Whereas, the spectral profi les of [(A(18C6))
4
(MnCl
4
)] [TlCl
4
]
2
· n H
2
O (A = Tl or Rb)
compounds (Figure 11.5 ) were typical of Mn
2+
in T
d
environments, the spectra of
several other [(A(18C6))
4
(MnX
4
)] [TlX
4
]
2
· n H
2
O compounds exhibited very interest-
ing dynamics and features that depended on the nature of the halide X
−
and the
large cation A. The spectra of the [(A(18C6))
4
(MnBr
4
)] [TlBr
4
]
2
· n H
2
O compounds
with A = K and Rb (Figure 11.6 ) show the excitation spectra being similar. However,
the emission spectrum of rubidium (A = Rb) features a green emission peaking
at 535 nm, as is normal for Mn
2+
in T
d
environments, while the potassium (A = K)
compound exhibits an unusual orange emission peaking at 575 nm, with the
normal green emission being too weak to observe.
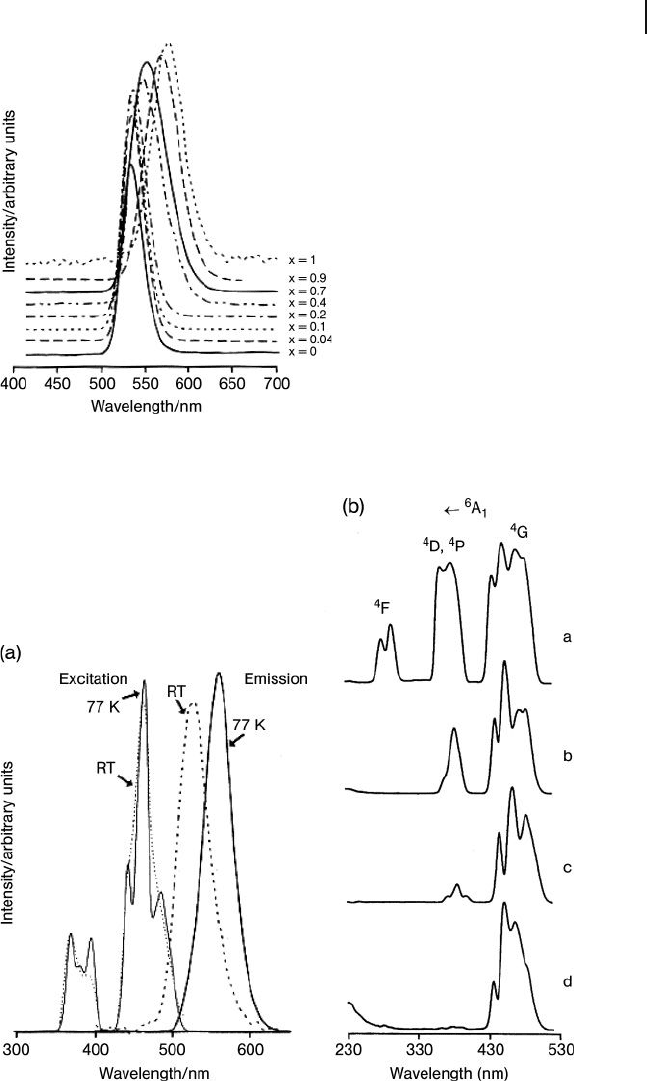
The emission spectra indeed do depend on the nature of the A cation, as shown
in Figure 11.7 , where a progressive spectral shift from the 535 nm to 575 nm emis-
sion is observed as the concentration of K
+
ions (x) increases from 0 (pure Rb
complex) to 1 (pure K complex).
Most interestingly, the ammonium compound [(NH
4
(18C6))
4
(MnCl
4
)]
[TlCl
4
]
2
· n H
2
O, which features temperature - independent spectra, shows both
emissions. The normal emission for Mn
2+
in T
d
environments is seen at room
temperature, while the strange orange emission dominates at 77 K (Figure 11.8 a).
The emission from the bromide complex [(NH
4
(18C6))
4
(MnBr
4
)] [TlBr
4
]
2
· n H
2
O
was normal, but the excitation spectrum featured an unusual broad absorption at
330 nm that was attributed to defects [21] . This led to the conclusion that the
Figure 11.6 Emission and excitation spectra of
[(A(18C6))
4
(MnBr
4
)] [TlBr
4
]
2
· n H
2
O (A = K or Rb) [16] .
11.6 Unusual Spectroscopy of [(A(18C6))
4
(MnX
4
)] [TlCl
4
]
2
· nH
2
O 373
Figure 11.7 Dependence of emission spectra of [(Rb
1 –
x
K
x
(18C6))
4
(MnBr
4
)] [TlBr
4
]
2
· n H
2
O on the molar fraction
of K [16] .
Figure 11.8 (a) The unusual emission
behavior of the [(NH
4
(18C6))
4
(MnBr
4
)]
[TlBr
4
]
2
· n H
2
O complex [16] ; (b) Compared to
[(NH
4
(18C6))
2
(MnBr
4
)] (spectrum a), the
inner - fi lter effect of defect sites on the
excitation spectral profi le of [21] : spectrum
b = (Rb(HM18C6))
4
(MnBr
4
)] [TlBr
4
]
2
· n H
2
O;
spectrum c = [(K(18C6))
4
(MnBr
4
)]
[TlBr
4
]
2
· n H
2
O; spectrum
d = [(Ba(HM18C6))
4
(MnBr
4
)] [TlBr
4
]
2
· n H
2
O.

374 11 Self-Assembling Nanoclusters Based on Tetrahalometallate Anions
strange orange emission and broad UV absorptions were associated with defect
sites. The electronic states of defect sites yielding orange emission are capable of
quenching the normal [MnX
4
]
2 −
green emission, especially at 77 K. At room tem-
perature, back energy transfer from the defect site to the normal Mn
2+
seems to
be dominant in the case of the [(NH
4
(18C6))
4
(MnBr
4
)] [TlBr
4
]
2
· n H
2
O compound.
Indeed, these defect sites were traced through a variety of compounds of [MnX
4
]
2 −
where their ability to act as inner - fi lters for [MnBr
4
]
2 −
UV absorptions is evident
(Figure 11.8 a).
11.7
Luminescence Decay Dynamics and 18 C 6 Rotations
The above temperature - dependent structural and luminescence behavior prompted
the study of the temperature evolution of luminescence decay dynamics of the
[(A(18C6))
4
(MnX
4
)] [BX
4
]
2
· n H
2
O compounds. As these fascinating decay dynam-
ics have been investigated and reported in detail elsewhere [14, 16, 21] , at this point
only the main aspects of our observations and conclusions will be presented.
The luminescence decay rates of [(A(18C6))
4
(MnX
4
)] [BX
4
]
2
· n H
2
O compounds
are infl uenced by the nature of the halogen X and metal A for spin – orbit coupling
reasons, and also the nature of defect sites, their proximity to the Mn
2+
site, and
the effi ciency with which they interact with the emitting
4
T
1
(
4
G) →
6
A
1
process of
Mn
2+
. Whilst the decay rates change with the composition of the manganese(II)
compound, the general features are a temperature - independent component up to
approximately 200 K, followed by a rapid decay which involves energy migration
over the Mn
2+
sublattice. The Arrhenius plots (Figure 11.9 ) show biphasic emis-
sion quenching processes, with activation energies of 8 – 14 kJ mol
− 1
at the start of
the emission quenching, followed by a more rapid quenching process with a
thermal barrier of 30 – 50 kJ mol
− 1
[14, 16, 21] .
Figure 11.9 Arrhenius plots (ln k
t
versus 1/ T ) for
170 < T < 300 K for [(NH
4
(18C6))
4
(MnCl
4
)] - [TlCl
4
]
2
· n H
2
O.
Normal [MnCl
4
]
2+
emission monitored at 510 nm: plots
b = 8 kJ mol
− 1
) and e = 35 kJ mol
− 1
. Unusual emission
monitored at 590 nm: plot a = 7 kJ mol
− 1
; plot c = 8 kJ mol
− 1
;
plot d = 29 kJ mol
− 1
. E (kJ mol
− 1
) ≈ 0.155 T
c
(where
T
c
= transition temperature, i.e., ≈ 210 K from the above
luminescence studies).
