Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

10.4 Epoxidation Reactions 345
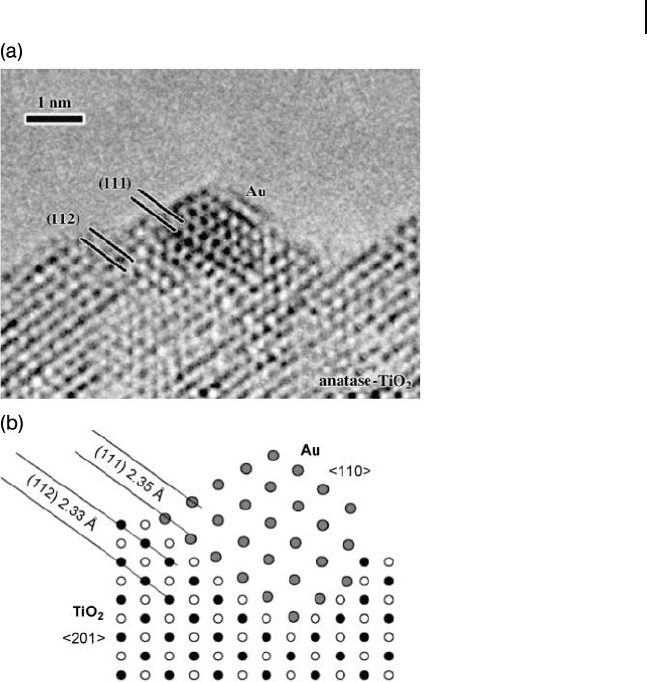
Figure 10.6 (a) Transmission electron microscopy image of
the Au/TiO
2
contact interface; (b) A schematic representation
of the interface. Reproduced with permission from Ref. [97] ;
© 1998, VSP, Leiden.
strong contact with the support at highly dispersed isolated tetrahedral Ti
4+
sites.
Moreover, this catalyst also provided a very high PO selectivity ( > 99%) in a gas
phase containing O
2
and H
2
, at ambient pressure. The same group also demon-
strated the formation of PO over an Au - based catalyst to be a typical structure -
sensitive reaction [97] , with the selective production of PO being catalyzed only
by hemispherical Au particles of a suitable size (2 nm < diameter Au nanoparti-
cle < 10 nm). In more recent investigations, these authors also showed an organi-
cally modifi ed mesoporous titanosilicate to be an effi cient support [98] , providing
a reasonably effi cient H
2
consumption, high yields, and PO selectivities in
excess of 90% [99] .
These studies initiated a growing research interest from both industry and
academia [100 – 103] , with one of the more remarkable investigations being con-
ducted by Hutchings and coworkers [102] . This group used catalytic amounts of
peroxides to initiate the oxidation of alkenes with O
2
, and making it unnecessary

346 10 Oxidation Catalysis by Nanoscale Gold, Silver, and Copper
to sacrifi ce H
2
in order to activate the O
2
. Here, Au/graphite was found to be very
active in catalyzing the expoxidation of cyclohexene, styrene, cis - stilbene, and cyclo -
octene, even in a solvent - free system. Although the selectivity could be increased
by using the correct solvent (e.g., toluene), the environment - friendly expoxidation
in solvent - free systems would be much more attractive to the chemical industry
and is certain to become another hot topic of research in the near future.
10.4.2
Silver Catalysts
Silver is considered to be an almost uniquely effective catalyst for heterogeneous
epoxidation reactions, and the mechanism of the epoxidation of ethylene with
oxygen over a silver catalyst has been the subject of extensive investigation [104 –
111] . However, despite such numerous studies and its wide use, a number
of questions remain unanswered regarding this catalytic system, including: “ How
do the supports and promoters affect the reaction? ” ; “ What is the mechanism
of the primary and secondary reactions? ” ; and “ What relationship exists between
the electron and structure factors? ” The interaction of oxygen with metal surfaces
has been suggested as one of the most important elementary steps in heterogeneous
catalysis, and several reviews of oxygen adsorption, active oxygen species, promoter
effects and reaction mechanisms on silver catalysts have been produced [112] . The
oxygen species on silver were found to play a key role in ethylene epoxidation, and
extensive studies have been performed to establish details of the interaction of
oxygen with silver surfaces, namely whether the chemisorbed oxygen is atomic or
molecular [113] . Details of surface molecular, surface atomic, subsurface atomic,
and bulk atomic oxygen species have each been reported in the literature.
Signifi cant efforts to improve selectivity have included the use of different silver
precursors, of different preparation techniques, and of different promoters. Such
information is gathered following the continuous addition of a chlorine - containing
hydrocarbon species to the gaseous reactants as a moderator, which also acts to
depress the overall reaction rates. Campbell reported that small amounts of pro-
moters (e.g., Cl) would increase the ethylene oxide selectivity [114, 115] . For an
industrial oxidation of ethylene, alkali metal ions are an important additive when
using a silver catalyst, and alkali or alkaline earth promoters (e.g., cesium) can
provide further substantial improvements [116 – 118] . Campbell [116] reported the
role of a cesium promoter in silver catalysts for the selective oxidation of ethylene.
The oxidation of ethylene in solution, catalyzed by polymer - protected silver col-
loids, and the promotion effect by alkali metal ions on colloidal silver catalysts,
have also been studied [119] . Colloidal dispersions of silver nanoclusters, when
protected by poly(sodium acrylate), caused increases in the rate of oxidation, the
reaction temperature and also the catalytic activity following the addition of Cs(I)
and Re(VII) ions [120] .
The infl uence of silver nanoparticle size on catalytic activity is due not only to an
enhanced surface area but also to particular electronic properties, which differ from
those of bulk silver. The effect of silver particle size on the reaction rate is a well -

10.5 Selective Oxidation of Hydrocarbons 347
known property of supported silver catalysts [121, 122] . For example, when monitor-
ing the distribution of Ag supported on alumina, silver particles of 30 – 70 Å on
alumina or silica showed higher activities. Previously, small silver clusters had been
shown to be the most effective, probably due to the electronic and other properties
of silver (atomic environment, electron work function, electric conductance, etc.),
and differed considerably from bulk silver in this respect. Although the majority of
studies have used bulk silver samples, uncertainty remains as to the nature of the
active sites for ethylene epoxidation on a commercial catalyst. Enlargement of the
silver particles has been found to decrease the amount of subsurface oxygen, and
result in the appearance of nucleophilic oxygen. These fi ndings have been used to
provide a possible explanation for the size effect in ethylene epoxidation over Ag/
Al
2
O
3
catalysts (Figure 10.7 ) [123 – 126] . The kinetic study and shape - controlled cata-
lytic epoxidation of olefi ns by these nanoparticles on several supports such as α -
Al
2
O
3
, CaCO
3
and spherical particles of TiO
2
(all obtained using the St ö ber method)
were investigated for a non - allylic olefi n (e.g., styrene) and for an allylic olefi n (e.g.,
propene), using molecular oxygen and N
2
O as oxidants.
Silver supported on titania was found to be active for propene epoxidation using
hydrogen/oxygen mixtures at 50 ° C [127, 128] . The direct aerobic oxidation of
various alkenes catalyzed by H
5
PV
2
Mo
10
O
40
polyoxometalate - stabilized silver nano-
particles supported on Al
2
O
3
showed a higher selectivity towards epoxide in the
liquid phase [129] . Styrene represents a useful alkene model for studying the reac-
tion mechanism of terminal alkene epoxidation; the size and morphology of the
nanoparticles was reported to affect the catalytic behavior of silver catalysts sup-
ported on α - Al
2
O
3
and MgO, in the selective oxidation of styrene in the gas phase
[130 – 134] . The epoxidation of styrene to its oxide by molecular oxygen was studied
using a Cs - loaded silver nanowire catalyst, and resulted in the desired product with
greater selectivity.
10.5
Selective Oxidation of Hydrocarbons
The selective oxidation of alkanes with molecular oxygen represents a major chal-
lenge [135, 136] , as the production of the more valuable oxidized products relative
to the low cost of the raw materials is of economic interest. The chemical inertness
of hydrocarbons makes the activation of C – H bonds especially diffi cult, and usually
requires dramatic reaction conditions such as high temperature and pressure. The
oxidation of cyclohexane is of special interest industrially, because the process
produces KA - oil as an intermediate; this is a mixture of cyclohexanone and
cyclohexanol that is important in the petroleum chemical industry. KA - oil is used
for the production of adipic acid and ε - caprolactam, which are key materials in the
respective manufacture of 6,6 - nylon and 6 - nylon [137] .
Modern industrial methods typically require both high pressure and tempera-
ture when using a soluble cobalt catalyst. A high selectivity for cyclohexanone
and cyclohexanol can only be achieved at a low conversion, as these products are
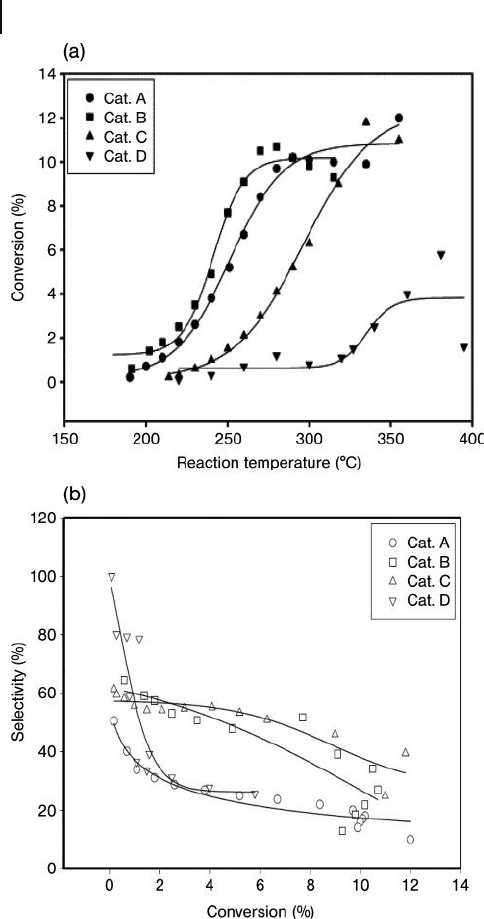
348 10 Oxidation Catalysis by Nanoscale Gold, Silver, and Copper
Figure 10.7 (a) Conversion of ethylene; (b)
Selectivity and conversion of Ag on γ - Al
2
O
3
with catalysts prepared by different methods:
A, precipitation; B, modifi ed precipitation; C,
water – alcohol; D, microemulsion silver
loading (7.5 – 8.6%). Reproduced with
permission from Ref. [126] ; © 2003, Elsevier
B.V., Amsterdam.
10.5 Selective Oxidation of Hydrocarbons 349
substantially more reactive than the cyclohexane reactant. Thus, under mild condi-
tions it is diffi cult to achieve, simultaneously, a high conversion and selectivity.
Whilst it is desirable to identify a good catalyst in order to activate the reaction
between oxygen and cyclohexane, the technology by which cyclohexane is oxidized
by O
2
to produce KA - oil has not yet been improved [138] . In an effort to reduce
the use of environmentally harmful elements, heterogeneous catalysts prepared
by immobilizing Mn
III
, Co
III
, Cr
III
and Fe
III
ions on metal oxides have been
developed for the oxidation of cyclohexane [139 – 141] , although these systems were
subsequently found to suffer from leaching under the reaction conditions used.
Mesoporous materials were reported as effi cient support materials because in
the pores, cyclohexane is oxidized more readily than cyclohexanol, such that the
selectivity is enhanced.
10.5.1
Gold Catalysts
Zhao and coworkers were the fi rst to apply gold nanoparticles for this application,
and reported that a supported gold catalyst could activate cyclohexane at 150 ° C,
with selectivities in the region of 90% [142, 143] . Under similar reaction condi-
tions, Kake Zhu and coworkers found that gold nanoparticles immobilized by a
variety of methods in the channels of SBA - 15 showed a good performance in cata-
lyzing the aerobic oxidation of cyclohexane in a solvent - free system (Figure 10.8 )
[144] , with the highest conversion being reported as 32%. In order to enhance
selectivity, the aerobic oxidation of cyclohexane was performed below 100 ° C and
catalyzed by supported Au, Pt, and Pd catalysts [145] . Although, the selectivity for
cylohexanone and cyclohexanol was found to decline rapidly with the enhanced
conversion and longer reaction times, the gold catalysts provided an identical
performance to the Pt and Pd catalysts.
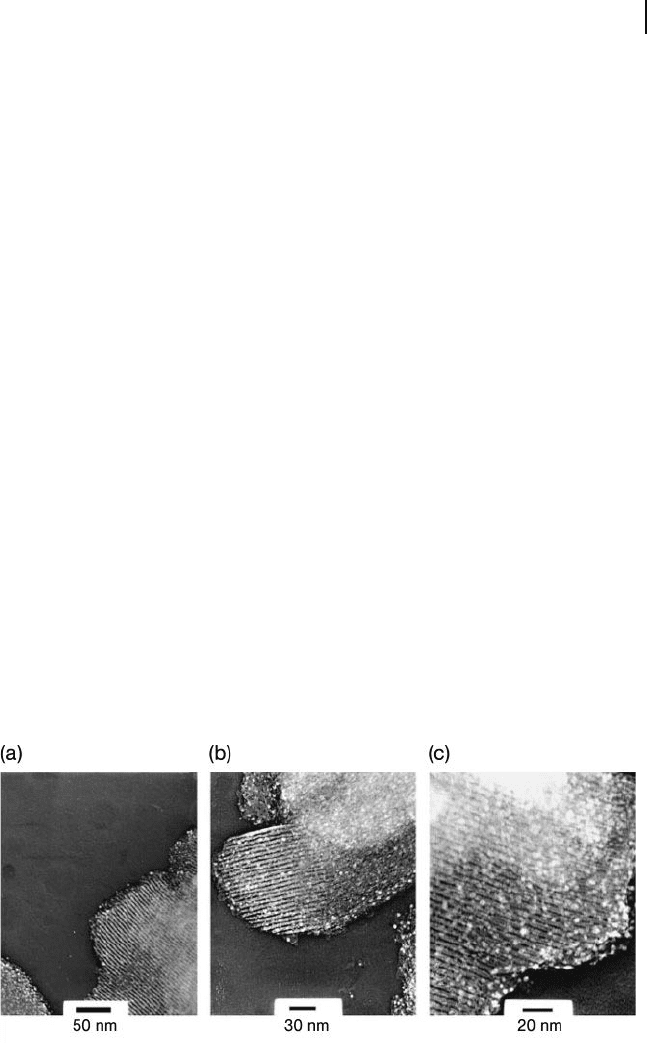
Figure 10.8 Representative transmission electron microscopy
images. (a) Au – APS/SBA - 15; (b, c) Au – SH/SBA - 15 at low
(b) and high (c) magnifi cation. Reproduced with permission
from Ref. [144] ; © 2005, Springer, Netherlands.

350 10 Oxidation Catalysis by Nanoscale Gold, Silver, and Copper
10.5.2
Silver Catalysts
Silver catalysts have also found use in hydrocarbon oxidation reactions. Since
the granting of the fi rst patent in 1931 for the manufacture of ethylene oxide with
an Ag catalyst [146] , the industrial production of ethylene oxide by direct oxidation
in gas phase has become widely used, with ethylene being converted into ethylene
glycol or a variety of other derivatives. In fact, the selective oxidation of ethylene
over a supported material represents one of the few uses of silver as an industrially
important catalyst, whilst also providing fundamental interests in the surface
sciences [112, 147] .
Silver has, however, rarely been considered as a catalyst for the selective oxida-
tion of saturated hydrocarbons. Yet, nanoscale silver supported on MCM - 41 for
the liquid - phase oxidation of cyclohexane was found to be an effective catalyst
in the absence of a solvent [148] , with a higher turnover number and improved
selectivity compared to other support systems of Ag/TS - 1 and Ag/Al
2
O
3
.
10.5.3
Copper Catalysts
Copper nanoparticles supported on natural zeolites of different structure and
origin have been utilized for the complete oxidation of hydrocarbons over a tem-
perature range of 170 to 250 ° C. Although a complete conversion was reported,
this was shown to depend on the Si/Al ratio of the zeolite matrix and the different
nanospecies of copper present [149] .
10.6
Oxidation of Alcohols and Aldehydes
The oxidations of alcohols and polyols are important processes in industrial chem-
istry, and it is not surprising that a signifi cant effort is currently being expended
within the scientifi c community to improve present - day technologies, and in par-
ticular to create processes that are more “ green. ” The objective of these research
investigations has been the development of a catalytic system that would compete
against a stoichiometric approach involving concentrated and toxic oxidizing
agents. Supported platinum and palladium catalysts are well known as effective
catalysts for the oxidation of polyols under acidic or basic conditions. Yet, sup-
ported gold nanoparticles were also found to be very effective for the oxidation of
alcohols, including diols, in the presence of a base [150 – 157] . These catalysts could
also be used in the oxidation of sugars, glucose, and sorbitol [158, 159] . When
using dioxygen as the oxidant, Carrettin et al . reported a 100% selectivity for the
oxidation of glycerol to glycerate, this being catalyzed by an Au catalyst supported
on graphite under relatively mild conditions, and with yields approaching 60%
[160 – 162] . In these studies the presence of a base was also found to be essential
for both activity and selectivity.

10.6 Oxidation of Alcohols and Aldehydes 351
10.6.1
Gold Catalysts
It has been commonly observed that the oxidation of alcohols catalyzed by
supported gold catalysts show better performances under basic conditions in terms
of high selectivities and reasonable activities, although the mechanism behind this
chemistry remains unclear. Another interesting observation is that the types of
support used for the gold catalysts, which have important roles in the oxidation of
CO and epoxidation, appear to have little effect in the oxidation of alcohols. In fact
Rossi and coworkers [163] , when monitoring the oxidation of glucose to gluconic
acid, found that unsupported colloidal AU particles were equally active as Au nano-
particles supported on active carbon, under the same conditions. A subsequent
study extended the application of colloidal gold catalysts to the oxidation of 1,2 - diols
[164, 165] . The disadvantage of an unsupported colloidal gold catalyst is its poor
long - term performance compared to a supported gold nanocatalyst, due to prob-
lems of aggregation. Recently, gold nanoclusters stabilized by polymers [166]
showed good activity in the aerobic oxidation of benzyl alcohol in aqueous media.
However, Corma and coworkers [167, 168] proposed that the support of Au/CeO
2
catalysts could help to stabilize a reactive peroxy intermediate from O
2
, and thus
could enhance the activities of gold catalysts in the selective oxidation of alcohols
to aldehydes/ketones, and of aldehydes to acids. One further interesting point here
was that the gold catalysts functioned well in solvent - free systems without any
additional base, a point which differed considerably from earlier fi ndings.
One of the most signifi cant advances in the fi eld of alcohol oxidation, provided
by the group of Hutchings [169] , indicated that an Au/Pt alloy supported on TiO
2
was a highly active catalyst for the oxidation of benzyl alcohol, cinnamyl alcohol,
and vanillyl alcohol. In particular, for the oxidation of benzyl alcohol, the perform-
ance of the Au - Pd/TiO
2
catalyst was remarkably superior to that of Au/TiO
2
and
Pd /TiO
2
in terms of both conversion and selectivity (Figure 10.9 ).
10.6.2
Silver Catalysts
The silver - catalyzed partial oxidation of methanol to formaldehyde, which is an
industrially important chemical transformation of alcohols to carbonyls in the gas
phase, and is signifi cant in the synthesis of drugs, vitamins, fragrances, and many
complex syntheses, was fi rst employed on an industrial scale by BASF AG in 1905
[170, 171] . Since that time, several silver - based catalysts, including bulk and sup-
ported systems, have been developed for the oxidation of alcohols. The main
problems encountered problems with bulk silver are low catalytic activities at low
temperatures, or the products of cracking and/or overoxidation at higher reaction
temperatures. Various attempts have been made to improve the catalytic perform-
ance of silver - based catalysts, either by adding additives to the bulk silver catalysts
or by dispersing the silver particles on supports [172 – 175] . Supported silver would
be expected to enhance the dispersion and stability of silver, and thus also enhance
its catalytic activity at relatively low temperatures. The design of a catalyst combin-
352 10 Oxidation Catalysis by Nanoscale Gold, Silver, and Copper
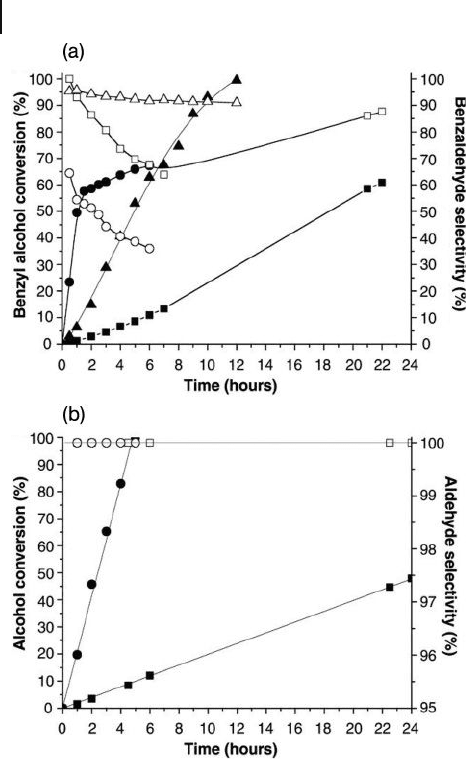
Figure 10.9 (a) Benzyl alcohol conversion and
selectivity in benzaldehyde with the reaction
time at 373 K and 0.1 MPa p O
2
. Squares
indicate Au/TiO
2
; circles indicate Pd/TiO
2
;
triangles indicate Au – Pd/TiO
2
. Solid symbols
indicate conversion, open symbols indicate
selectivity; (b) Au – Pd/TiO
2
- catalyzed reactions
at 363 K, 0.1 MPa p O
2
, for cinnamyl alcohol
(squares) and vanillyl alcohol (circles). Solid
symbols indicate conversion, open symbols
indicate selectivity to the corresponding
aldehydes. Reproduced with permission from
Ref. [169] ; © 2005, RSC Publishing,
Cambridge.
ing the advantages of both bulk silver catalysts (conventional electrolytic silver)
and supported silver catalysts, with better performance, remains a major research
challenge in the area of alcohol catalytic oxidation [176] .
Supported platinum and palladium nanoparticles are generally acknowledged
as effective catalysts for the oxidation of polyols [177] . In situ electrolytic nanosil-
ver/zeolite fi lm/copper grid catalysts have shown higher catalytic oxidation proper-
ties towards mono, di, and other types of alcohols, and with higher selectivities

10.7 Direct Synthesis of Hydrogen Peroxide 353
[176, 178] . The silver nanoparticles, when generated in situ , highly dispersed on
the zeolite fi lm and then precoated on a copper grid, demonstrated excellent activi-
ties towards polyhydric alcohol at low temperature. Such performance effectively
avoids the problems of both overoxidation and C – C bond cracking at high tem-
peratures, or mild oxidation at low temperatures (as in the case of the practical
electrolytic silver catalyst). Au – Ag alloy clusters (size range 1.6 – 2.2 nm) with
various Ag contents (5 – 30%) and prepared using a coreduction method in the
presence of poly( N - vinyl - 2 - pyrrolidone), were investigated in the aerobic oxidation
of p - hydroxybenzyl alcohol as a model reaction to understand the effect of Ag on
the catalytic activity of Au clusters [179] . The rate constants per unit surface area
for the Au – Ag:PVP clusters with a small Ag content ( < 10%) were larger than those
of monometallic Au:PVP clusters of comparable size. Spherical nanoparticles
anchored on the external walls of a multiwalled carbon nanotube (Ag/MWNT)
composite electrode exhibited a high catalytic activity for the electro - oxidation of
methanol [180] .
10.7
Direct Synthesis of Hydrogen Peroxide
As noted above, there has been much recent interest in the design of new hetero-
geneous catalysts for selective oxidation under ambient conditions, and these typi-
cally use hydrogen peroxide as the oxidant [96] . At present, hydrogen peroxide is
produced by the sequential hydrogenation and oxidation of alkyl anthraquinone,
with the annual global production approximating 1.9 × 1 0
6
tons. However, a
number of problems are associated with the anthraquinone route, including the
cost of the quinone solvent system and a periodic need to replace the anthraqui-
none because of hydrogenation. In view of this, considerable interest has been
expressed in the direct manufacture of hydrogen peroxide from the catalyzed reac-
tion of hydrogen and oxygen. At present, a degree of success has been achieved
using Pd as a catalyst, especially when halides are used as promoters. Typically,
dilute solutions of hydrogen peroxide are produced, with earlier studies indicating
that the Pd catalyst could be combined with an oxidation catalyst, TS - 1, such that
the hydrogen peroxide produced could be used in situ . To date, however, no com-
mercial process exists for the direct manufacture of hydrogen peroxide. Hutchings
and coworkers were the fi rst to show that Au/Al
2
O
3
catalysts were effective for the
direct reaction, and thus far the best catalysts identifi ed have been Pt – Au alloy
supported catalysts (Table 10.2 ); these showed a better performance than pure Pd
or Au catalysts [181, 182] . The suggestion was made that the enhanced activity
observed by the addition of Pd to Au was due to an enhanced activation of hydro-
gen. However, if too much Pd was added, the decomposition activity of the hydro-
gen peroxide was also enhanced, such that the rate declined.
Subsequently, Ishihara et al . [183] have shown that Au/SiO
2
and Au – Pd/SiO
2
catalysts are also effective for this reaction at only 10 ° C. In recent studies, Hutch-
ings and coworkers have shown that the selectivity for H
2
utilization could be

354 10 Oxidation Catalysis by Nanoscale Gold, Silver, and Copper
Table 10.2 Formation of H
2
O
2
from the reaction of H
2
/ O
2
over A u and P d catalysts [182] .
Catalyst
Solvent
a
Temperature
( ° C)
Pressure
(MPa)
O
2
/H
2
(molar ratio)
H
2
O
2
mmol g (catalyst)
− 1
h
− 1
Au/Al
2
O
3
C H
3
OH 2 3.7 1.2 1530
Au : Pd (1 : 1)/
Al
2
O
3
CH
3
OH 2 3.7 1.2 4460
Pd/Al
2
O
3
C H
3
OH 2 3.7 1.2 370
Au/ZnO scCO
2
35 9.2 1.0 9
Au : Pd (1 : 3)/
ZnO
scCO
2
35 9.2 1.1 7
Au : Pd (1 : 1)/
ZnO
scCO
2
35 9.2 0.8 12
Au : Pd (3 : 1)/
ZnO
scCO
2
35 9.2 0.9 8
Pd/ZnO scCO
2
35 9.2 1.3 0
a scCO
2
= supercritical CO
2
.
signifi cantly enhanced when Fe
2
O
3
and TiO
2
were used as supports [184, 185] .
Indeed, with short reaction times the selectivity may exceed 95% for the reaction
of dilute H
2
/O
2
mixtures (1 : 1; 5 vol%) diluted with CO
2
(95 vol%). Very high rates
of reaction were observed with noncalcined Au – Pd/TiO
2
catalysts, but these proved
to be unstable as they lost both Au and Pd during the reaction and could not be
successfully reused. However, if the catalysts were calcined at 400 ° C prior to use,
very stable reusable materials were obtained. Detailed structural investigations of
these active stable catalysts, using X - ray photoelectron spectroscopy ( XPS ) and
transmission electron microscopy ( TEM ), showed that the catalysts have a core –
shell structure with a gold - rich core and a palladium - rich shell. It was concluded
that the Au was acting as an electronic promotor for the Pd - rich surface of the
Au – Pd nanocrystals.
10.8
Conclusions
Although the catalytic processes described in this chapter have provided a good
overview of the oxidation catalysis properties of Group 11 metals, this is a far from
comprehensive survey of the fi eld. However, this group of metals has provided
