Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

8.4 Improved Methods for Attachment and Growth of AuNPs on ITO 305
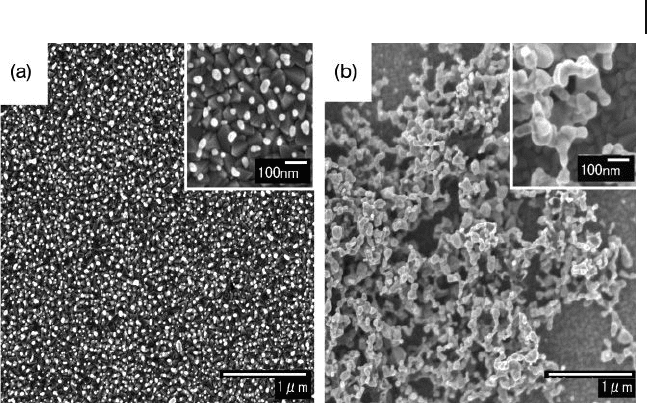
Figure 8.5 FE - SEM images of AuNP - attached ITO surfaces
prepared using the cast seed - mediated growth method. The
repeated cycles of casting were (a) three times and (b) 10
times. The insets show the higher - magnifi cation images.
Reproduced with permission from Ref. [28] ; © 2006, Elsevier.
surfaces having a higher density and a narrower size distribution. The FE - SEM
image is shown in Figure 8.5 a. On the other hand, a 10 - cycle cast seeding process
formed the connected or networked nanostructures of AuNPs (see Figure 8.5 b),
while the optical properties were also different from those of the dispersed AuNP -
attached ITO [28] . The cast seeding approach provided a facile and practical strat-
egy for attaching AuNPs on the ITO surfaces while controlling the amount of Au
loading and without using certain organic binder molecules.
Furthermore, by adjusting the concentration of citrate ions in the seed solution
from 1 m M to 50 m M by adding trisodium citrate after the preparation of the Au
nanoseed solution, dramatic changes could be observed in the SEM images and
in the actual colors of the ITO substrates, which in turn indicated changes in the
nanostructures of the AuNPs formed on the ITO surfaces [29] . The attachment
of smaller AuNPs with a higher density was observed when 25 m M citrate ions
were added in the seed solution (see Figure 8.6 ). In contrast, larger AuNPs were
seen to attach when the concentration of citrate ions was increased to 50 m M .
On the basis of this difference and the FE - SEM images observed just after seeding,
it was inferred that the citrate ions affected not only the growth process but also
the seeding process [29] .
Whilst the repulsive power expected from the increased negative charges of
citrate ions was not signifi cant, a rather dense attachment was promoted as the
particular effect of citrate ions. Such control of the AuNP attachment on ITO
would be practically effective because the dense attachment could be achieved by
simply changing the composition of the seed solution.
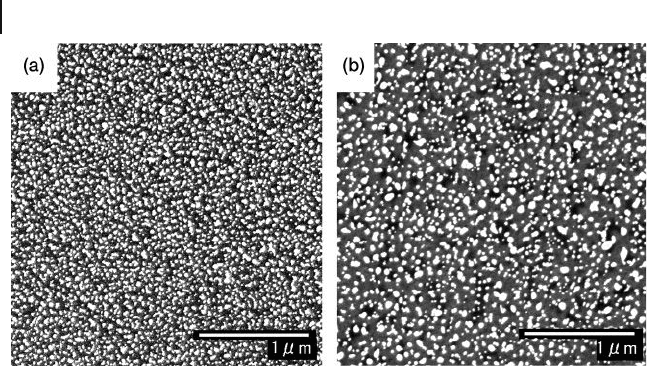
306 8 Recent Advances in Metal Nanoparticle-Attached Electrodes
Figure 8.6 FE - SEM images of AuNP - attached ITO surface.
The sample was prepared by adding (a) 25 m M and
(b) 50 m M citrate ions into the seed solution before
seeding, followed by the growth treatment. Reproduced
with permission from Ref. [29] ; © 2006, Elsevier.
8.5
Attachment and Growth of AuNPs on Other Substrates
The seed - mediated growth method for surface modifi cation with AuNPs could be
applicable to the surface treatments of various types of substrate, mainly because
that the modifi cation of AuNPs was found to be possible on all of the examined
materials, including glassy carbon ( GC ), mica, stainless, epoxy resin, phenol resin,
simple glass, and ZnO fi lm. FE - SEM observations of these materials revealed that
the AuNPs attached and grew on the surfaces, although the grown size of the
AuNPs and the formation of rod - like particles varied depending on the substrates.
Thus, attachment of the Au nanoseed particles by simple immersion into the Au
colloid solutions is considered to proceed commonly via physisorption, and not by
specifi c chemical bonding.
As an example, Figure 8.7 shows the FE - SEM images of AuNP - attached GC
surfaces observed after seed - mediated growth treatment [30] . Here, although the
seeding time was fi xed at 2 h for all samples, the growth period was changed from
5 min to 24 h. This resulted in the dense attachment of AuNPs on the GC surfaces,
and shorter growth times when compared to the cases where the ITO surfaces
were modifi ed. In addition, fl at and smaller AuNPs were clearly formed on the
GC surfaces (see Figure 8.7 a – c). Interestingly, a longer (24 h) treatment in the
growth solution promoted the growth of some microcrystals in local areas, as
shown in Figure 8.7 d. This would be a refl ection of ripening in the growth solu-
tion, to promote crystal growth and to form the bold bodies. The AuNP - attached
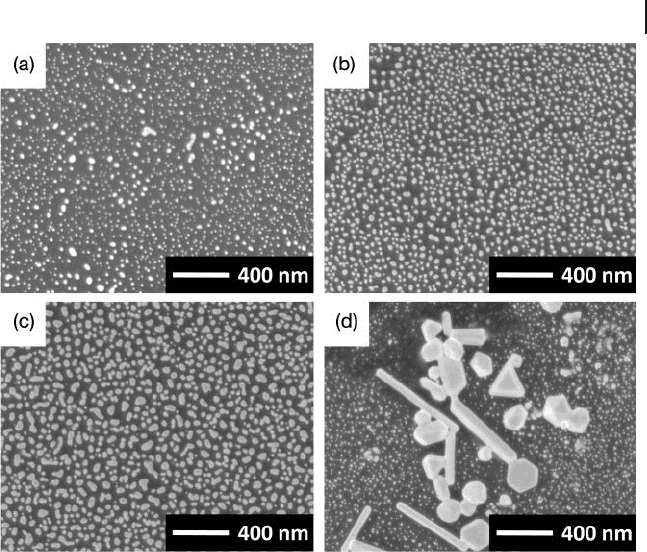
8.5 Attachment and Growth of AuNPs on Other Substrates 307
Figure 8.7 FE - SEM images of AuNP - attached GC surfaces.
Samples were prepared using the seed - mediated growth
method, with seeding for 2 h. The growth period was
(a) 5 min, (b) 2 h, (c) 8 h, and (d) 24 h. The observation of
(d) was focused on an area where bold crystals were
dominantly formed. Reproduced with permission from
Ref. [30] ; © 2009, Japan Society for Analytical Chemistry.
GC electrodes prepared using the seed - mediated growth method were used for
the electrochemical detection of nitrite [31] .
The method of attachment used for AuNPs on solid surfaces was applied to the
slightly different fabrication of a functional electrode. The technique was used to
attach AuNPs onto mesoporous TiO
2
fi lms prepared via a liquid - phase deposition
process on GC substrates [32] . Whilst the TiO
2
fi lm strongly inhibited the electron
transfer process of the [Fe(CN)
6
]
3 −
/[Fe(CN)
6
]
4 −
redox couple, electrochemical meas-
urements indicated that the overpotential for the reduction of maleic acid was
signifi cantly decreased when the electrode surface was covered with TiO
2
fi lm, due
to the electrocatalytic activity. After attaching the AuNPs by the seed - mediated
growth method (Figure 8.8 ), however, the sluggish heterogeneous electron trans-
fer kinetics at the TiO
2
fi lm was effectively improved while the catalytic activity of
the TiO
2
fi lm was retained [32] . This example showed the effect of AuNPs on the
nature of less - conducting electrode materials.
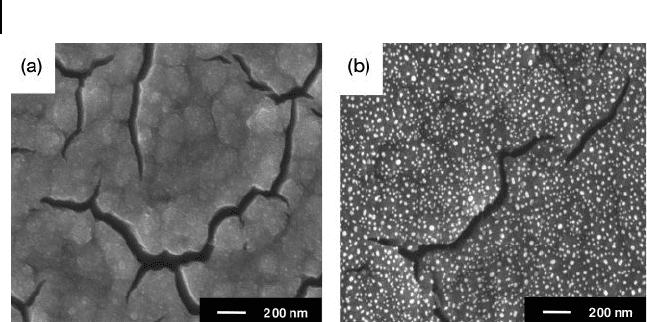
308 8 Recent Advances in Metal Nanoparticle-Attached Electrodes
Figure 8.8 FE - SEM images of (a) TiO
2
fi lm and (b) AuNPs
attached and grown on the surfaces of TiO
2
fi lm. When the
TiO
2
fi lm had been prepared on the GC substrate by
liquid - phase deposition, the AuNPs were attached and grown
using the seed - mediated growth method. Reproduced with
permission from Ref. [32] ; © 2005, Electrochemical Society.
8.6
Attachment and Growth of Au Nanoplates on ITO
Although previously, all approaches and trials for preparation have focused on
the seeding process, it is likely that a modifi cation of the growth process should,
potentially, also be capable of altering the nanostructures of AuNPs on the ITO
electrode surfaces.
As a new strategy for attaching Au nanoplates onto the ITO surfaces, a 2 - D
crystal growth of Au was permitted through a liquid - phase reduction from Au
nanoseed particles attached to the ITO surface, using poly(vinylpyrrolidone) ( PVP )
rather than CTAB as a capping reagent in the growth solution [33] . By controlling
the PVP concentration it was possible to form Au nanoplates (see Figure 8.9 ) with
a surface coverage of up to 30%, although variously shaped Au nanocrystals were
formed concurrently on the ITO surface. The Au nanoplates were single crystalline
in nature, with (111) basal planes and an edge - length of up to approximately
∼ 2 μ m, growing parallel to the ITO surface [33] . The concentration of PVP in the
growth solution was a key factor in the Au nanoplate formation, as spherical or
irregularly shaped AuNPs were formed at either higher or lower concentrations
of PVP [33] . The absorption spectra of the Au nanoplate - attached ITO implied
anisotropic and specifi c optical characteristics of the modifi ed ITO glasses.
The Au nanoplate - attached ITO electrode showed some interesting characteris-
tics; for example, an electrochemical response to cytochrome c was observed
without using promoter molecules. However, in order to better examine the
functions of the Au nanoplates, an increase in their coverage is important. Hence,
improved attachment and coverage methods for Au nanoplates are currently
under investigation.
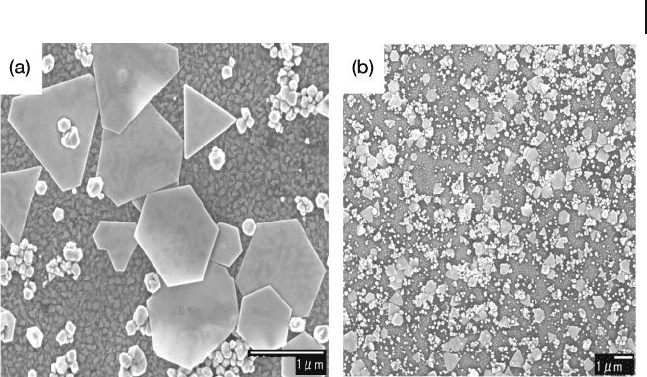
8.7 Attachment and Growth of Silver Nanoparticles (AgNPs) on ITO 309
Figure 8.9 FE - SEM images of Au nanoplates attached
and grown on ITO surfaces. (a) High - magnifi cation and
(b) low - magnifi cation images. Reproduced with permission
from Ref. [33] ; © 2006, American Chemical Society.
8.7
Attachment and Growth of Silver Nanoparticles ( AgNPs ) on ITO
By applying the same seed - mediated growth method, silver nanosphere and
nanorod particles were successfully attached to the ITO surfaces [34] . As with
AuNPs, the attachment of AgNPs could be performed without using a bridging
reagent (such as MPTMS), by a simple two - step immersion into fi rst the seed
solution, and second into the growth solution containing AgNO
3
, CTAB, and
varying amounts of ascorbic acid [34] .
The formed nanostructures were found to be very sensitive to the ascorbic acid
content of the growth solution. For example, with an ascorbic acid concentration
of 0.64 m M the AgNPs grew on the ITO surface but retained a moderate dispersion
(Figure 8.10 a). However, when the concentration was raised to 0.86 m M , Ag
nanorod and nanowire formation on the ITO surfaces was much less dispersed
(Figure 8.10 b).
The attachment of AgNPs onto the ITO surfaces was suffi ciently strong for
further use, for example as a working electrode, and consequently AgNP/ITO
electrodes were used in a number of electrochemical measurements. As a result,
it was confi rmed that the outer spheres of the Ag nanoparticles involved in
the redox reaction showed the typical oxidation and reduction waves of Ag metal
[34] . In addition, the redox behavior of [Fe(CN)
6
]
3 −
/[Fe(CN)
6
]
4 −
was improved
on the AgNP/ITO electrode, refl ecting the low electron transfer resistance of Ag
(see Figure 8.11 a). This, in turn, indicated that the AgNPs promoted electron
transfer reactions by their presence on the conducting ITO surface. The AgNP/
ITO electrode was also investigated for reduction of the methyl viologen dication
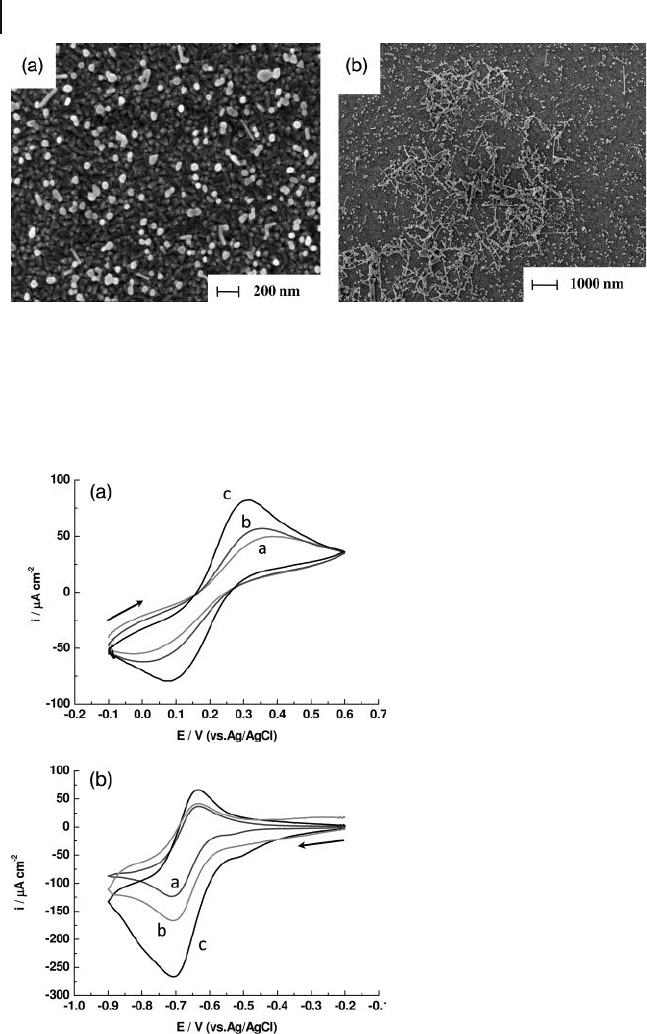
310 8 Recent Advances in Metal Nanoparticle-Attached Electrodes
Figure 8.10 FE - SEM images of AgNP - attached ITO surfaces.
The ITO substrate was fi rst immersed in the seed solution for
2 h and then into the growth solution containing (a) 0.64 m M
or (b) 0.86 m M ascorbic acid for 24 h. Reproduced with
permission from Ref. [34] ; © 2005, American Chemical
Society.
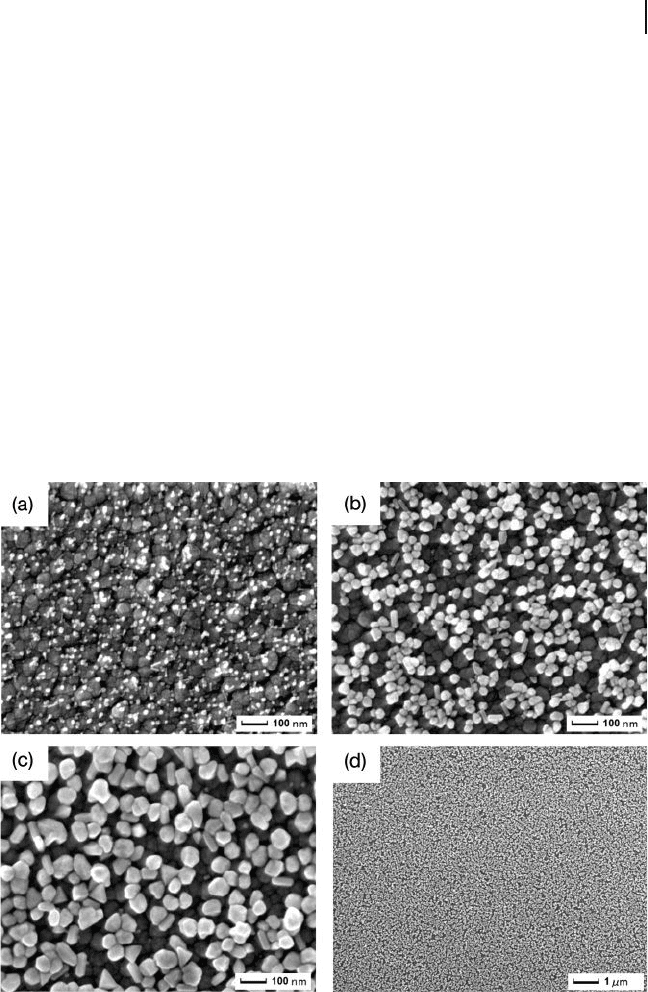
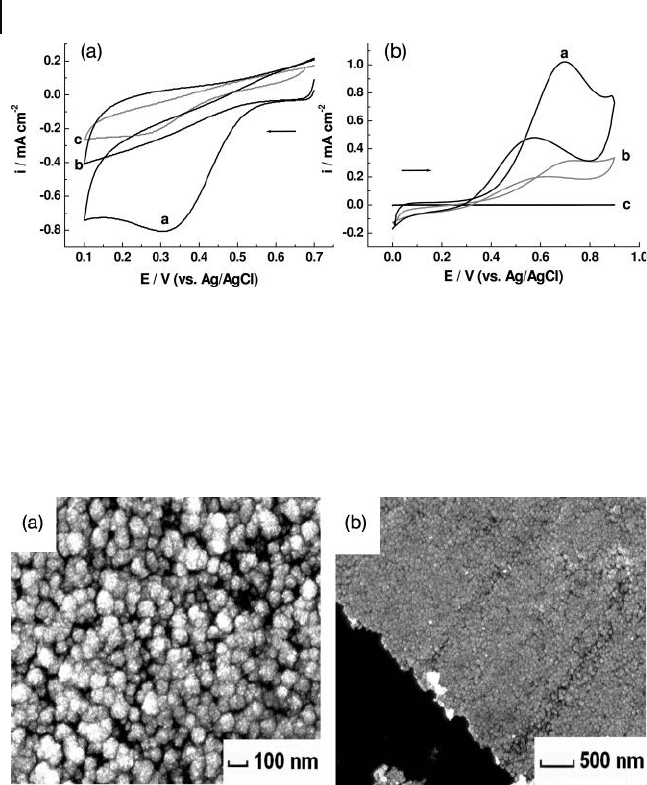
Figure 8.11 (a) Cyclic voltammograms of
0.5 m M [Fe(CN)
6
]
3 −
and 0.5 m M [Fe(CN)
6
]
4 −
in
0.1 M phosphate - buffered solution (pH 7.0)
recorded using: (curve a) a bare ITO
electrode; (curve b) an Ag seed - attached ITO
electrode; and (curve c) an AgNP/ITO
electrode. Scan rate = 50 mV s
− 1
; (c) Cyclic
voltammograms of 0.5 m M methyl viologen
dication in 0.1 M Na
2
SO
4
recorded using:
(curve a) a bare ITO electrode; (curve b) a
conventional Ag electrode; and (curve c) an
AgNP/ITO electrode. Scan rate = 100 mV s
− 1
.
Reproduced with permission from Ref. [35] ;
© 2006, American Chemical Society.
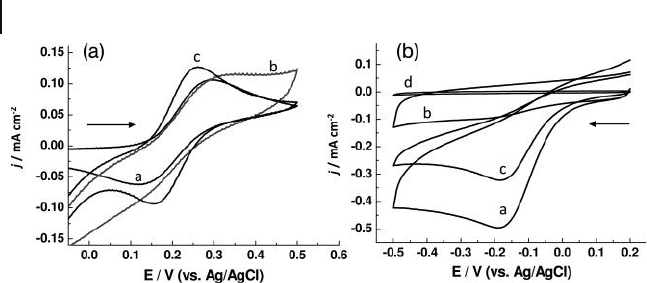
8.8 Attachment and Growth of Palladium Nanoparticles on ITO 311
(Figure 8.11 b), in order to determine the native adsorption features of the
fabricated AgNP/ITO electrodes [34] .
8.8
Attachment and Growth of Palladium Nanoparticles PdNPs on ITO
Palladium nanoparticle s ( PdNP s) were also successfully attached and grown on
ITO surfaces using the seed - mediated growth method [35] . The FE - SEM images
recorded after treating the Pd nanoseed particle - attached ITO substrates in a
growth solution for 4 and 12 h, respectively, are shown in Figures 8.12 a and b.
Crystal growth of PdNPs occurred as the immersion time in the growth solution
was increased, with Pd nanocrystals of 60 – 80 nm being identifi ed after 24 h
(Figures 8.12 c and d). The major characteristic of the formed nanostructure of Pd
was that the nanocrystals tended to adhere to each other. Moreover, such aggrega-
tion occurred not only on the surface but was also 3 - D in nature; that is, some
nanocrystals were seen to grow above the basal nanocrystals. The nanostructure
Figure 8.12 FE - SEM images of PdNP - attached ITO surfaces.
The ITO substrates were immersed into the seed solution for
2 h and then into the growth solution for: (a) 4 h; (b) 12 h; and
(c) 24 h; (d) A low - magnifi cation image of the surface of panel
(c). Reproduced with permission from Ref. [35] ; © 2006,
American Chemical Society.
312 8 Recent Advances in Metal Nanoparticle-Attached Electrodes
of Pd grown on ITO was quite different from that of Au and Ag, mainly because
the AuNPs and AgNPs tended to form in dispersed states (see above). Hence, the
inference was that the identity of the metal changed the aggregation characteristics
during the growth process, despite using CTAB as the same capping reagent in
all growth procedures.
Due to the dense nanoparticle attachment (see Figure 8.12 c), the PdNP/ITO
electrodes had a signifi cantly lowered charge transfer resistance compared to that
of a bare ITO, and the redox reaction of [Fe(CN)
6
]
3 −
/[Fe(CN)
6
]
4 −
was observed to be
reversible in 0.1 M phosphate - buffered solution (Figure 8.13 a) [35] . The electro-
catalytic property of PdNPs attached on ITO was confi rmed for the reduction of
oxygen (Figure 8.13 b). In addition, some typical responses were observed in 0.5 M
H
2
SO
4
with the PdNP/ITO electrodes, refl ecting both the characteristics of the
NPs and the thin layer on a nanoscale [35] . The proposed preparation method for
PdNP - attached ITO surfaces should be promising for catalytic applications, as well
as electrochemical uses.
8.9
Attachment of Platinum Nanoparticles PtNPs on ITO and GC
Although attempts were made to prepare platinum nanoparticle s ( PtNP s) on ITO
surfaces using the seed - mediated growth method, some preliminary studies
showed this approach to be diffi cult [36] . The problems were caused by the appear-
ance of brown precipitates in the growth solution, despite using the same principle
(i.e., the mixing of K
2
PtCl
4
, CTAB and ascorbic acid), before the seed - attached ITO
was immersed.
Figure 8.13 (a) Cyclic voltammograms of
1.0 m M [Fe(CN)
6
]
4 −
in 0.1 M phosphate -
buffered solution (pH 7.0) with: (curve a) a
bare ITO electrode; (curve b) a bulk Pd
electrode; and (curve c) a PdNP/ITO
electrode prepared via 24 h growth. Scan
rate = 50 mV s
− 1
; (b) Cyclic voltammograms
recorded with: (curves a, b) the PdNP/ITO
electrode prepared via 24 h growth in (curve
a) air - saturated and (curve b) N
2
- saturated
0.1 M KCl solutions, and with (curve c) a Pd
bulk electrode and (curve d) a bare ITO
electrode in air - saturated 0.1 M KCl solution.
Scan rate = 50 mV s
− 1
. Reproduced with
permission from Ref. [35] ; © 2006, American
Chemical Society.
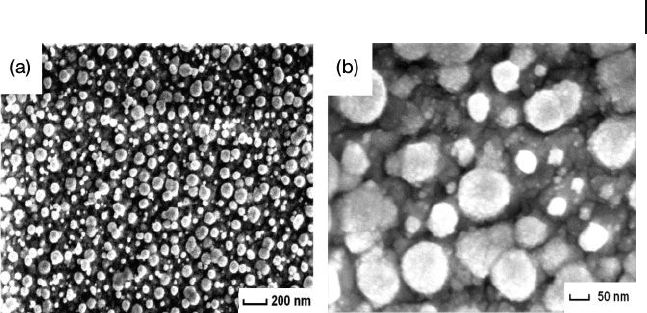
8.9 Attachment of Platinum Nanoparticles on ITO and GC 313
Figure 8.14 FE - SEM images of PtNP - attached ITO surfaces.
The ITO substrate was immersed in the growth solution
containing 0.25 m M K
2
PtCl
4
and 5 m M ascorbic acid for 24 h.
(a) Low - magnifi cation and (b) high - magnifi cation images of
the same surface. Reproduced with permission from Ref. [36] ;
© 2006, American Chemical Society.
However, with further investigation the attachment of PtNPs on ITO was
achieved by employing a rather simple method, namely a one - step in situ chemical
reduction of PtCl
4
2−
by ascorbic acid, but without using CTAB [36] . The FE - SEM
images of the PtNP - attached ITO surfaces prepared via this in situ reduction
method are shown in Figure 8.14 . Here, the attached PtNPs were spherical and
showed an agglomerated nanostructure which was composed of small nanoclus-
ters. Based on the morphological changes which were dependent on the growth
time, PtNPs were shown to grow via a progressive nucleation mechanism [36] .
Characteristically, when PtNP/ITO was used as a working electrode, the
charge transfer resistances were found to be signifi cantly lowered due to the PtNP
growth. Hence, for the typical redox system of [Fe(CN)
6
]
3 −
/[Fe(CN)
6
]
4 −
, the PtNP/
ITO electrodes exhibited electrochemical responses which were similar to that
of a bulk Pt electrode [36] . It was also apparent that the PtNP/ITO electrodes
had signifi cant electrocatalytic properties for oxygen reduction and methanol oxi-
dation (see Figures 8.15 a and b, respectively) [36] . Those PtNPs which demon-
strated an agglomerated nanostructure should show promise as a new type of
electrode material.
The in situ reduction method used to prepare PtNPs was also applied to the
modifi cation of GC surfaces. This resulted in a thin continuous Pt fi lm which was
composed of small nanoclusters that had a further agglomerated nanostructure
of small grains, and could be attached onto the GC surface [37] . FE - SEM images
of the Pt nanocluster fi lm ( PtNCF ) are shown in Figure 8.16 . The electrochemical
results obtained indicated that the current values for Pt oxidation, Pt oxide reduc-
tion and hydrogen - related redox reactions, when recorded with the PtNCF
electrode, were almost twice those with the Pt nanocluster dispersedly - attached
GC (PtNC/GC) electrode, but this refl ected the higher Pt loading (Figure 8.17 a)
[37] . The electrocatalytic ability of the PtNCF for methanol oxidation was also
314 8 Recent Advances in Metal Nanoparticle-Attached Electrodes
Figure 8.15 (a) Cyclic voltammograms
recorded using (curves a, b) a PtNP/ITO
electrode prepared via 24 h growth in: (curve
a) air - saturated and (curve b) N
2
- saturated
0.5 M H
2
SO
4
solutions, and (curve c) a Pt bulk
electrode for the air - saturated 0.5 M H
2
SO
4
solution. Scan rate = 50 mV s
− 1
; (b) Cyclic
voltammograms obtained for 0.1 M methanol
oxidation in the N
2
- saturated 0.5 M H
2
SO
4
solution recorded with: (curve a) the prepared
PtNP/ITO electrode; (curve b) a Pt bulk
electrode; and (curve c) a bare ITO electrode
Scan rate = 50 mV s
− 1
. Reproduced with
permission from Ref. [36] ; © 2006, American
Chemical Society.
Figure 8.16 FE - SEM images of PtNCF attached on GC. The
concentration of ascorbic acid for preparation was 5.1 m M .
(a) High - magnifi cation and (b) low - magnifi cation images. The
left lower corner of (b) was scratched to show the fi lm
thickness. Reproduced with permission from Ref. [37] ;
© 2007, Elsevier.
apparently higher than that of the PtNC/GC or PtNP/ITO electrodes (Figure
8.17 b). In addition, the electrocatalytic performance of PtNCF, when expressed in
term of Pt content, was clearly superior to that of Pt black formed on GC [37] .
Taken together these results indicated that, in spite of the continuous nanos-
tructures, the nanograins of PtNCF functioned effectively for catalytic electrolysis.
At present, PtNCF may be regarded as an interesting thin - fi lm material, which
can be easily prepared via a one - step chemical reduction.
