Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


7.5 CNT–AuNP Composites 275
capping/linking shells of the AuNPs induced an agglomeration of the AuNPs that
attached effectively to the CNTs surface. These composite materials were very
stable, with even sonication in hydrophobic solvents being unsuccessful in dis-
sociating the composite material. In time, this approach may fi nd important
implications for the design of nanostructured and nanocomposite catalyst and
sensory materials. Since it is possible to control the size, shape, loading, and dis-
persion of the AuNPs on the surface of the conductive CNTs by using a combina-
tion of hydrophobic and hydrogen - bonding interactions, this system might also
become important for addressing some of the fundamental issues in fuel cell
catalysis applications.
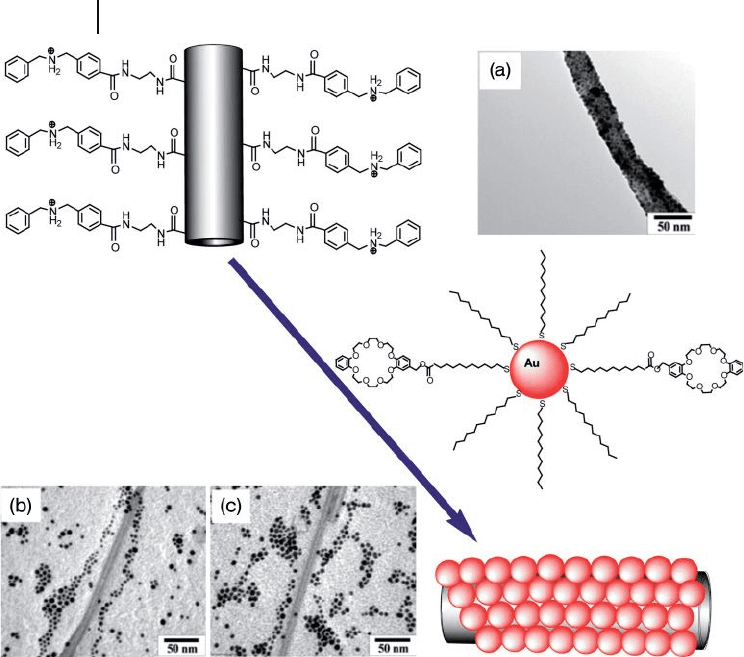
Sainsbury and Fitzmaurice [72] reported a smart, noncovalent, self - assembly
coverage of dibenzylammonium - cation - modifi ed MWNTs with crown - modifi ed
(dibenzo[24]crown) AuNPs, where ammonium cations thread the crown ethers
present on the AuNPs surface (Figure 7.19 ). From the different experimental
conditions utilized, it was found that the MWNT - templated self - assembly of a solid
nanowire was observed only when uncomplexed cations were present on the
surface of the MWNTs and uncomplexed crown was present on the surface of the
AuNPs (Figure 7.19 a – c). This signifi cant observation led to the perception that
the possibility of driving force for the templated nanowire assembly via charge
transfer from the crown - modifi ed AuNPs to the cation - modifi ed MWNT could
be excluded. Therefore, it was concluded that the templated self - assembly of
nanowires was driven by the formation of the surface - confi ned to pseudorotaxanes
that resulted from the electron - poor cation threading the electron - rich crown. In
order to initiate the templated self - assembly of a gold nanowire in solution, a
dispersion of crown - modifi ed AuNPs in chloroform was added to a freshly soni-
cated suspension of cation - modifi ed MWNTs in chloroform. The stable dispersion
of dodecane - thiol - modifi ed AuNPs was prepared using the well - known method
introduced by Brust and coworkers [73] . The nearly size - monodisperse fraction
was subsequently modifi ed by the exchange of the adsorbed thiol for thiol -
incorporating crown molecules (dodecanethiol incorporating a crown moiety
in the terminal position) [74] . The MWNTs were modifi ed by amide - coupling
reactions either between the carboxy - modifi ed MWNTs and ethylenediamine,
or between the remaining amine of the coupled ethylenediamine and the
cation precursor [ N - (4 - carboxydibenzylamine) carbamate]. This approach may
offer the vision of diverse nanoscale components for which many applications can
be anticipated.
A facile approach has been developed to prepare a hybrid material of
dodecanethiol - protected AuNPs decorated on the surfaces of SWNTs and MWNTs
simply by reacting dispersions of both components in dichloromethane [75] . The
as - prepared AuNP – SWNT and AuNP – MWNT systems exhibited a strong elec-
tronic coupling, which may infl uence important physico - chemical properties.
Although the nature of the electronically coupled state was not clearly understood,
it was anticipated that it might involve a charge - transfer character, in which the
AuNPs functioned as an electron acceptor, receiving additional electron density
from the SWNTs or MWNTs. The well - known absorption features of SWNTs led
276 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
Figure 7.19 A cation - modifi ed MWNT and the
noncovalent self - assembly of AuNPs with a
crown ether - modifi ed monolayer, and TEM
images of (a) crown - modifi ed AuNPs
adsorbed at the surface of a cation - modifi ed
MWNT. The same AuNPs are not adsorbed at
the surface of (b) a cation - precursor - modifi ed
MWNTs or (c) an unmodifi ed MWNTs.
Adopted and modifi ed according to Ref. [72] ;
TEM images reprinted with permission from
Ref. [72] .
to considering the dispersion of AuNP – SWNT instead of the less - explored and
less - understood MWNTs characteristics.
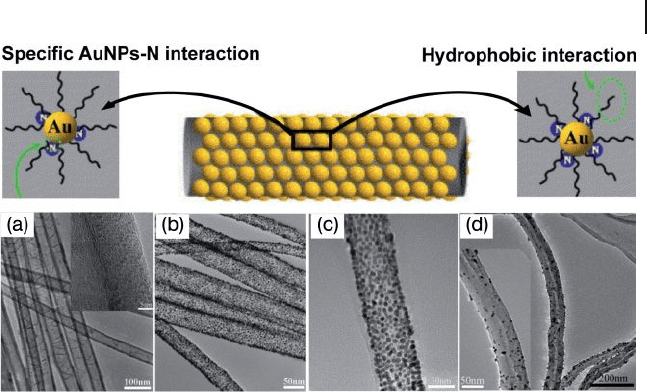
In contrast to normal CNTs, Li and coworkers [76] produced bamboo - like carbon
nanotube s ( BCNT s) that were decorated with a high - density and uniform assembly
of AuNPs on the surface, by using a simple and effective method. BCNTs pro-
duced by the pyrolysis of iron(II) phthalocyanine were sonicated in toluene for
homogeneous dispersion, and then mixed, by gentle shaking, with the toluene
solution of AuNPs protected by organic shell. The resultant composite materials,
AuNP – BCNTs, were separated by centrifugation and washed repeatedly with
toluene. Apart from the hydrophobic interaction between the alkyl chains of the
capping/linking molecules and the hydrophobic backbones of the nanotubes, the
specifi c interaction between AuNPs and the nitrogen atoms present on the surface
7.5 CNT–AuNP Composites 277
Figure 7.20 Schematic illustration of the direct assembly of
AuNPs on BCNTs. (a) TEM image of BCNTs; (b, c) BCNT –
AuNP nanohybrids; (d) AuNPs supported on an undoped
CNTs. The inset in (a) shows a higher - magnifi cation image.
Reprinted with permission from Ref. [76] .
of the BCNTs is responsible for the high - density and uniform assembly of AuNPs
on BCNTs (Figure 7.20 ). It is important to note that, compared to the N - doped
BCNTs, the pristine undoped CNTs were not effi cient for the direct, highly dense
and uniform immobilization of AuNPs, due mainly to an absence of specifi c
AuNP – N interactions or other activation processes. As evidently seen from the
TEM images, the assembled AuNPs (average size 3.9 nm) were quite uniform, well
dispersed, and decorated or packed onto the walls and ends of BCNTs with high
density. The unique properties of the BCNTs, combined with this simple and
straightforward assembly approach, may facilitate the use of CNTs in a variety of
pivotal fi elds, including biosensors and fuel cell catalysis.
π – π Stacking Interactions This is a type of noncovalent interaction, which is
mainly caused by the intermolecular overlapping of p - orbitals in π - conjugated
systems (e.g., organic compounds or polymers containing aromatic moieties). The
results of different studies [68, 77] have confi rmed that organics or polymers with
phenyl groups could interact with CNTs through π – π stacking, which is a stronger
interaction than the van der Waals forces. Compared to various aromatic com-
pounds, pyrene derivatives are more susceptible to stack on the surface of the
CNTs via π – π stacking interactions [78 – 80] . In this way, the intrinsic electronic
properties of the CNTs are preserved, and the surface functional groups on the
nanotubes can easily be varied by changing the pyrene derivatives.
By using bifunctionalized pyrene derivatives such as 17 - (1 - pyrenyl) - 13 -
oxo - hepta - decanethiol ( PHT ) as an interlinker, AuNPs were decorated on the
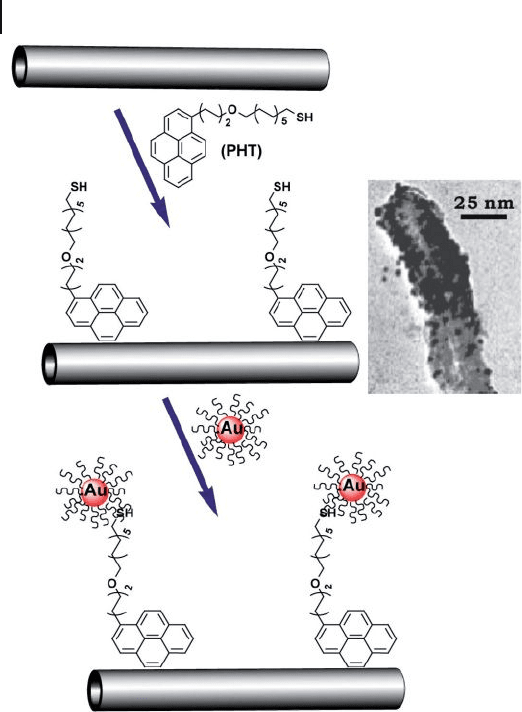
278 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
Figure 7.21 Illustration of the connection of AuNPs onto
MWNTs through pyrene derivatives as an interlinker, and
TEM image of MWNTs decorated with AuNPs.
PHT = 17 - (1 - pyrenyl) - 13 - oxo - heptadecanethiol. Adopted and
modifi ed according to Ref. [78] ; TEM image reprinted with
permission from Ref. [78] .
surface of the didecylamine - modifi ed MWNTs, where PHT bound to the surface
of MWNTs via a π – π stacking interaction between its pyrenyl unit at one end and
the sidewall of MWNTs, while the other terminal thiol group was interacted to the
surface of AuNPs (Figure 7.21 ). In this way, AuNPs were self - assembled onto the
surface of nanotubes via an organic linker. It should be noted that, while the fl uo-
rescence of the resultant composite material was entirely quenched, the Raman
response of the CNTs was enhanced considerably. These important consequences
imply that there are charge - transfer interactions between the CNTs and AuNPs
through the organic interlinker.
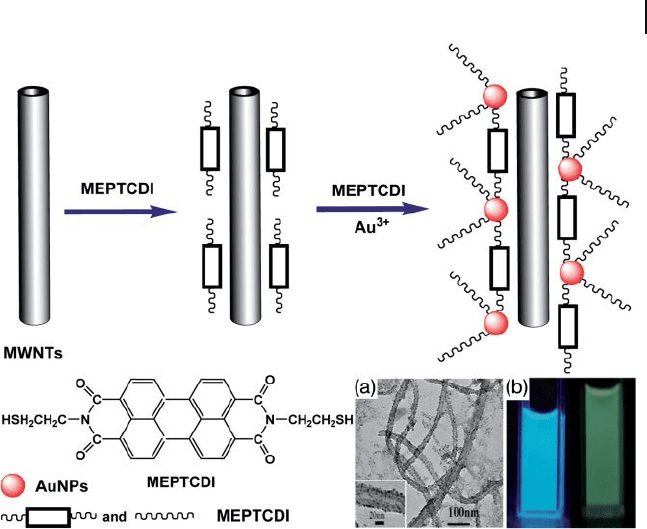
7.5 CNT–AuNP Composites 279
Figure 7.22 Representation for the fabrication
processes of the AuNP – MWNT hybrids and
molecular structure of MEPTCDI. (a) TEM
image of AuNP – MWNT hybrids; (b) Emission
from the AuNP – MWNT hybrid with MEPTCDI
in water (left) and MEPTCDI in DMF (right)
under UV irradiation at 365 nm.
MEPTCDI = N , N ′ - bi(2 - mercaptoethyl) -
perylene - 3,4,9,10 - tetracarboxylic diimide.
Reprinted with permission from Ref. [81] .
Very recently, the in situ - solution method was proposed for the synthesis of
water - dispersable AuNP – MWNT hybrids with high density and well - distributed
AuNPs by using an optoelectronic - active compound of N , N ′ - bi(2 - mercaptoethyl) -
perylene - 3,4,9,10 - tetra - carboxylic diimide ( MEPTCDI ) as an interlinker and stabi-
lizer for the formation of the AuNP – MWNT hybrids [81] . Here, the MEPTCDI
with both phenyl and mercapto groups, played a dual role as an interlinker (Figure
7.22 ) between the MWNTs and AuNPs (noncovalently wrapping of the MWNTs
through π – π stacking) and as a stabilizer for controlling the nucleation and growth
of AuNPs on the surface of the MWNTs. This endowed the AuNPs anchored
on the surface of the MWNTs with a good stability and dispersability, as well as
fl exibility of size - control (Figure 7.22 a).
AuNP – MWNT hybrids were prepared using an in situ procedure in such a way
that the MWNTs were dispersed in aqueous solution containing sodium dodecyl
benzen sulfonate ( SDBS ) by sonication and a dimethylformamide ( DMF ) solution
of MEPTCDI was added to the MWNTs suspension, followed by the addition of
an aqueous solution of HAuCl
4
, and stirred for 12 h at room temperature. The
nanohybrids formed were further purifi ed by centrifugation and redispersed in
water for further characterization. The as - prepared AuNP – MWNT hybrid with

280 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
MEPTCDI showed an interesting photoluminescence property under UV irradia-
tion at 365 nm (Figure 7.22 b). Based on several blank experiments and reported
information [82] , it was concluded that the strong luminescence of the hybrids did
not result from the atomic Au clusters or from defects of the CNTs or from the
organically functionalized CNT hybrids, but rather originated from the AuNP –
MWNT hybrids with MEPTCDI, which may be due to energy transfer occurring
between the MWNTs and AuNPs or between AuNPs and the MEPTCDI. This
property of AuNP – MWNT hybrids in water may expand the potential applications
as building blocks to a wider range such as light - emitting sources, biomedical
labels, or even tracking materials for drug delivery, and so on.
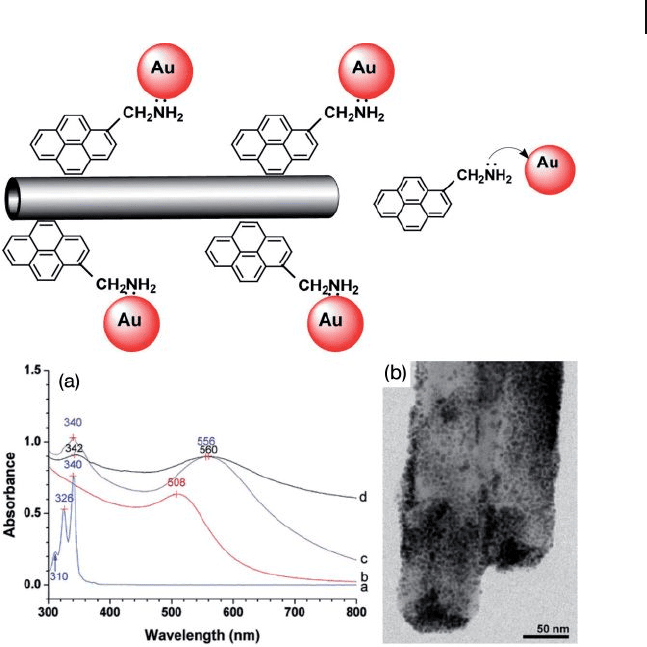
AuNPs of 2 – 4 nm diameter were densely decorated on the walls of MWNTs
using pyrenealkylamine derivatives such as 1 - pyrenemethylamine as the inter-
linker in aqueous solution [83] . While the pyrene chromophore is noncovalently
attached to the terrace of nanotubes through a π – π stacking interaction, the
alkylamine substituent of 1 - pyrene - methylamine connects to the AuNPs (Figure
7.23 ). UV - visible absorption and luminescence spectroscopy were employed to
monitor the formation of functionalized AuNPs and AuNP – CNT composites. Fol-
lowing surface modifi cation of the AuNPs with 1 - pyrenemethylamine, the absorp-
tion value of the pyrene chromophore was greatly decreased and the surface
plasmon resonance ( SPR ) of AuNPs showed a red shift from 508 to 556 nm (Figure
7.23 a), owing to interparticle plasmon coupling. Further, the photoluminescence
property of 1 - pyrenemethylamine and its emission intensity were drastically
quenched after formation of the AuNP – CNT composites, most likely due to the
energy and/or electron transfer from the excited pyrene fl uorophore to the AuNPs.
This facile strategy for the formation of a high - density assembly of AuNPs onto
the surface of CNTs (Figure 7.23 b) has been applied to other linking organic
molecules with similar structures, such as N - (1 - naphthyl)ethylenediamine and
phenethylamine, thus demonstrating the general applicability of this approach for
preparing AuNP – CNT composites. The resultant composites may become impor-
tant for applications in low - temperature CO oxidation and other catalytic reactions.
Further, the present strategy of depositing spherical AuNPs may be extended to
produce CNT – Au nanorod heterojunctions for electronic connection and molecu-
lar sensing applications.
Electrostatic Interactions Electrostatic interactions are noncovalent dipole –
dipole or induced dipole – dipole interactions that can be either stabilizing or desta-
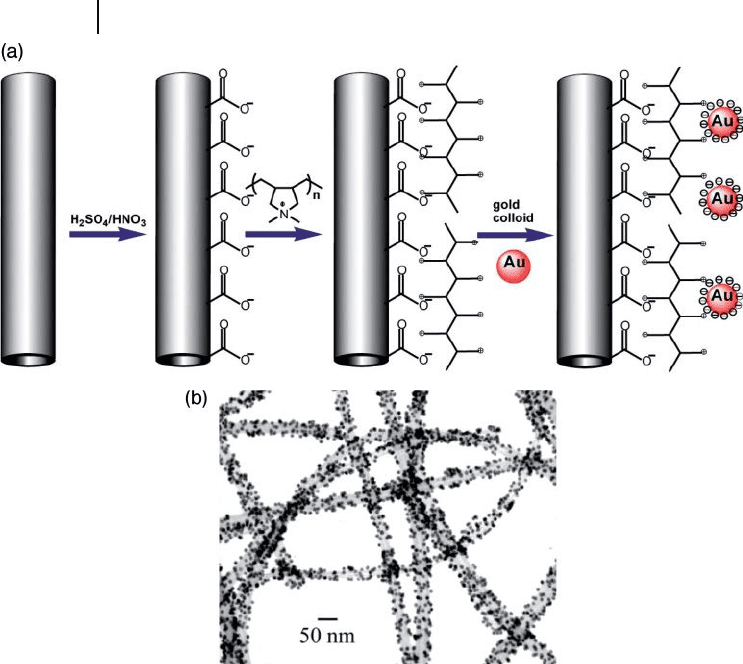
bilizing. A simple and profi cient method has been introduced for the selective
anchoring of AuNPs on the surface of nitrogen - doped MWNTs by electrostatic
adsorption [84] . Nitrogen - doped MWNTs produced using a pyrolysis method were
initially chemically modifi ed by acid treatment (mixture of H
2
SO
4
and HNO
3
)
and the resultant oxidized MWNTs subsequently treated with a cationic polyelec-
trolyte, namely poly - (diallyldimethylammoniumchloride) ( PDDA ). The polyelec-
trolyte was adsorbed onto the surface of the nanotubes by an electrostatic interaction
between the carboxyl groups on the chemically oxidized nanotube surface and
the polyelectrolyte chains. Negatively charged AuNPs of 10 nm were attached
7.5 CNT–AuNP Composites 281
Figure 7.23 AuNP assembly on CNTs
through the 1 - pyrenemethylamine interlinker.
(a) UV - visible absorption spectra of: (curve a)
free 1 - pyrenemethylamine in ethanol; (curve
b) aqueous AuNP solution; (curve c) a
mixture of 1 - pyrenemethylamine and AuNPs;
(curve d) solution containing AuNP − CNT
composites; (b) TEM image of the resulting
AuNP − CNT composites. Reprinted with
permission from Ref. [83] .
to the surface of the nanotubes via electrostatic interactions between the polyelec-
trolyte and AuNPs (Figure 7.24 ). In this way, novel composites with homogene-
ously distributed AuNPs on the surface of nitrogen - doped MWNTs were obtained.
Therefore, by selecting different types of polyelectrolytes the surfaces of the
CNTs can be modifi ed to be either negatively or positively charged, such that
accordingly different types of other nanoparticle (e.g., semiconductor nanocrystals,
magnetic nanoparticles) can be selectively anchored to the nanotube surfaces.
This strategy of decorating nanotubes can be used to identify the location of func-
tional groups on the CNT surfaces, while the resultant composite materials may
be used in various domains such as catalytic, electronic, optical, and magnetic
applications.
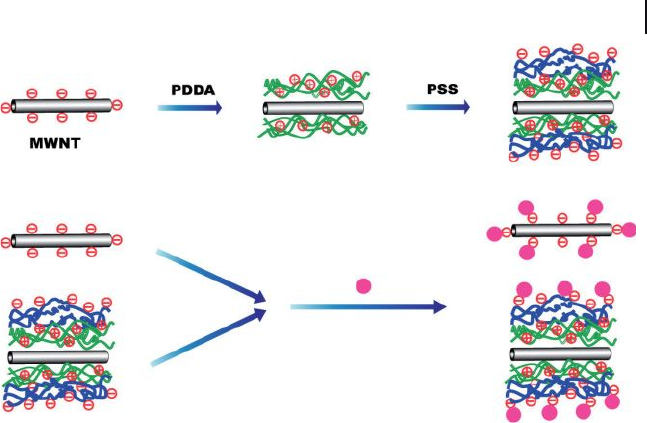
Another example of the electrostatic approach is the formation of AuNP – MWNT
composites by the LBL self - assembly technique using polyelectrolytes [85] . To this
282 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
Figure 7.24 (a) Schematic view of the process for anchoring
AuNPs onto CNTs; (b) TEM image of AuNP – CNT hybrid
structures. Reprinted with permission from Ref. [84] .
end, chemically functionalized MWNTs were wrapped with a layer of a positively
charged polyelectrolyte (PDDA), followed by a layer of a negatively charged poly-
electrolyte such as poly(sodium 4 - styrene sulfonate) ( PSS ). Consequently, posi-
tively charged AuNPs prepared by the phase - transfer method [86] were immobilized
on negatively charged MWNTs prepared by LBL self - assembly due to electrostatic
interactions (Figure 7.25 ). Positively charged AuNPs also interacted with chemi-
cally functionalized MWNTs alone (without polyelectrolyte coating) through elec-
trostatic interaction with the carboxylic acid groups present on MWNTs sidewalls.
Hence, it is expected that positively charged AuNPs could be used to detect nega-
tively charged defect sites on CNTs. However, the binding interaction between
MWNTs and AuNPs is much more powerful and effective for the LBL approach
than the direct approach.
The noncovalent attachment of silica - coated AuNP monolayers and multilayers
onto CNT templates was achieved by using the polymer - wrapping technique,
7.5 CNT–AuNP Composites 283
combined with the LBL assembly process [87] . First, a stable dispersion of indi-
vidual CNTs was obtained by dispersing the MWNTs in water by sonication in the
presence of PSS, which acts as the wrapping polymer. PSS has negatively charged
sulfonate groups, which serve as primers for the homogeneous adsorption of the
cationic polyelectrolyte PDDA. In the LBL approach, the interactions accountable
for the assembly are mostly electrostatic, and permit an exploitation of the surface
properties of silica to obtain close - packed monolayers and multilayers. As a result,
the MWNTs were entirely packed with dense monolayers of silica - coated AuNPs.
Such composite nanowires were optically labeled and might have key applications
as components of nanoelectronic circuits and waveguides. The repetition of the
LBL process allows a good control of the thickness of the nanocomposites by
increasing the number of layers deposited. Another strategy for functionalizing
SWNT or MWNT surfaces noncovalently with polymer multilayers and AuNPs
was also reported by Carrillo and coworkers [88] .
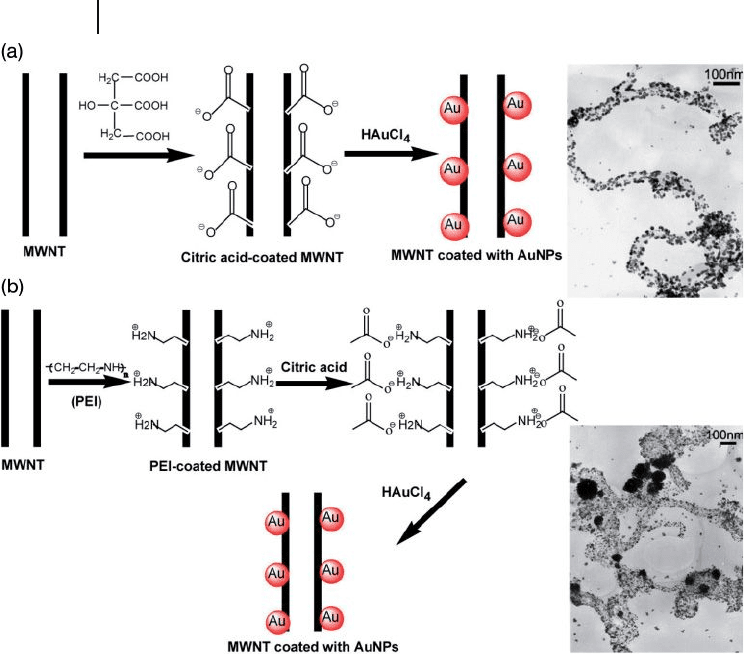
Jiang and Gao reported a versatile method for the selective attachment of AuNPs
onto the surface of the MWNTs [38] . By using cationic poly(ethyleneimine) ( PEI )
or anionic citric acid ( CA ) as the dispersant, the surface properties of the MWNTs
were modifi ed to yield a basic or acidic surface. Then, by electrostatic interactions,
AuNPs of approximately 10 nm were successfully anchored onto the sidewalls of
the MWNTs (Figure 7.26 ). In the case of the CA - coated MWNTs, the CA forms
an adsorption layer around the outer walls of the CNTs and then reduces in situ
the HAuCl
4
to produce the AuNPs. The PEI treatment of the nanotubes and expo-
sure to the HAuCl
4
solution generated nitrogen - containing functional groups on
Figure 7.25 Schematic illustration of the LBL self - assembly
technique. PDDA = poly(diallyldimethylammoniumchloride);
PSS = poly(sodium 4 – styrene sulfonate). Adopted and
modifi ed according to Ref. [85] .
284 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
Figure 7.26 The process for attaching AuNPs to: (a) Citric
acid - coated CNTs; (b) Poly(ethyleneimine) (PEI) - coated CNTs.
The TEM images show the respective products. Adopted and
modifi ed according to Ref. [38] ; TEM images reprinted with
permission from Ref. [38] .
the outer walls of the MWNTs. This strategy may be extended to attach other
nanoparticles (e.g., semiconductor, electrical and magnetic nanoparticles) onto the
terrace of the CNTs by choosing different types of suitable dispersant.
Another straightforward in situ reduction procedure for the fabrication of CNT -
supported AuNP composite nanomaterials was demonstrated by Hu and cowork-
ers [89] . Here, linear PEI had a dual role as a functionalizing agent for the MWNTs
as well as a reducing agent for the formation of AuNPs (Figure 7.27 ). The driving
force involved in the functionalization of PEI on the surface of CNTs is a combina-
tion of the electrostatic interaction between the oppositely charged CNTs and the
PEI and the physisorption process, which is analogous to the polymer - wrapping
process [87] . This synthetic method engages a mild heat - treatment process, which
persuades the in situ reduction of HAuCl
4
on the MWNTs sidewalls and does
not require the additional steps of oxidizing the MWNTs with mixed acids and
