Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

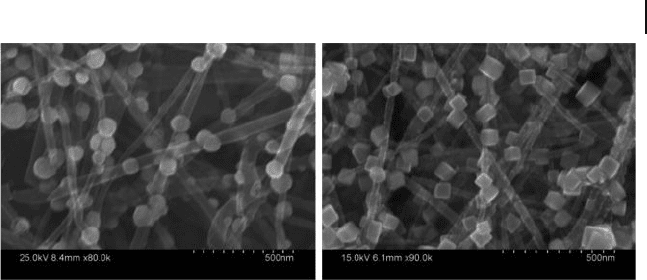
7.5 CNT–AuNP Composites 265
Figure 7.10 Scanning electron microscopy images of
synthesized CNTs that are grafted with gold nanosphere and
nanocube systems. Reprinted with permission from Ref. [56] .
response, in line with the experimentally observed absorption peaks of such
systems in the optical range.
Tello and coworkers introduced a new method, known as solvated metal atom
dispersion ( SMAD ), in combination with CVD, to prepare MWNT and AuNP
hybrid materials, and subsequently investigated their thermal stabilities [57] . In
the SMAD protocol, a colloid with small and highly reactive gold clusters was
prepared by co - evaporation of the gold metal and acetone. The colloid was subse-
quently condensed into a frozen matrix in a liquid nitrogen atmosphere, and the
matrix then allowed to warm to room temperature. The as - prepared gold clusters
were reacted with previously incorporated MWNTs in the reactor to yield MWNT –
AuNP hybrid materials which were thermally stable up to 400 ° C. Moreover, no
appreciable changes in particle size were detected as the AuNPs were covered with
amorphous carbon after annealing at 200 ° C (Figure 7.11 ).
Interestingly, whilst the average AuNP size was increased approximately from
4 to 20 nm and the nanoparticles were detached from the MWNTs, no damage
was induced on the MWNTs when the hybrid material was annealed beyond 600 ° C
(Figure 7.11 c). Surprisingly, further heating (annealing up to 800 ° C) induced a
severe transformation of the MWNTs (perhaps due to a catalytic activity of the
AuNPs) into cylindrical solid carbon nanorods (Figure 7.11 d). It would appear that
this method provides a new approach to the synthesis of both carbon nanorods
and MWNT – AuNP composites that are useful in a variety of key applications.
A new and important approach was developed recently to form naked sub - 10 - nm
AuNPs on individual 2 nm bare CNTs [58] . These assemblies were produced on
the terrace of a porous anodic alumina ( PAA ) template, on which the CNTs (single -
or double - walled) were grown by using plasma - enhanced chemical vapor deposi-
tion ( PECVD ). The AuNPs were obtained via an indirect evaporation method using
a membrane mask, consisting of a suspended, patterned silicon nitride fi lm; the
AuNPs then diffused along the PAA surface into the regions containing CNTs
(Figure 7.12 a). Three distinct regions were observed on the PAA surface: (i) a
semi - continuous gold fi lm composed of multiple grains; (ii) a transition region,
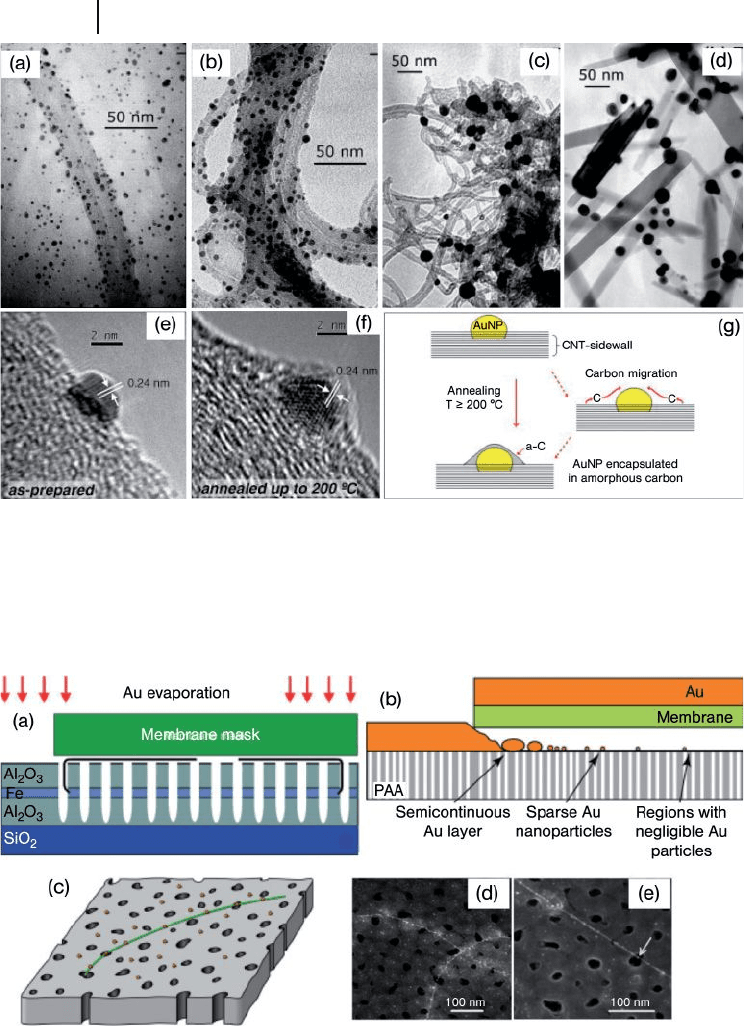
266 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
Figure 7.11 Transmission electron
microscopy images for the as - prepared
AuNP - CNT hybrids (a), and annealed up to
200 ° C (b), 600 ° C (c), and 800 ° C; (d) HR - TEM
images of AuNPs anchored on the CNT - walls
(e, f); for the as - prepared AuNP – CNT hybrids
(e), and annealed up to 200 ° C (f). A carbon
layer is encapsulating the AuNPs after the
thermal process. Lattice fringes on gold
particles are consistent with the (111) fcc
orientation (0.235 nm); (g) A schematic
representation of the process. Reprinted with
permission from Ref. [57] .
Figure 7.12 (a) Diagram of a cross - section of
the experimental set - up; (b) Schematic
representation of the three regions with
different Au particle distribution on the
porous anodic alumina (PAA) fi lm after Au
evaporation through a nitride membrane
mask; (c) Enlarged view of region sparse
AuNPs, showing one CNT emerging from a
pore and growing along the PAA surface. The
AuNPs diffuse into this region and attach to
the CNT; (d) Field - emission scanning electron
microscopy images of an area from region
sparse AuNPS, showing two crossed CNTs
decorated with AuNPs; (e) A sub - 5 - nm AuNP
(indicated by a white arrow) is on a CNT and
above the pore in the PAA fi lm. Scale
bar = 100 nm. Reprinted with permission from
Ref. [58] .

7.5 CNT–AuNP Composites 267
in which a sparse coverage of small AuNPs was observed; and (iii) a region where
negligible gold was observed. The schematic illustration of the three regions with
different gold particle distributions on the PAA fi lm after the evaporation of gold
through a nitride membrane mask is shown in Figure 7.12 b. The presence of the
various regions is attributed to the migration of gold on the surface; as the gold
atoms migrate they also aggregate, forming grains (at large surface coverage) and
small nanoparticles (at lower surface coverage) [59] . As shown schematically in
Figure 7.12 c, within region (b) the AuNPs were observed on the PAA surface and
on the CNTs. The fi eld - emission scanning electron microscopy ( FESEM ) image
of an area within region (b) shows the presence of a number of well - defi ned par-
ticles with diameters ∼ 5 nm along the CNTs, most likely due to AuNPs (Figure
7.12 d). In addition, somewhat diffuse bright regions with the dimensions of 20 –
40 nm nearer to CNTs were also observed, though these may be due to local charg-
ing effects arising during the FESEM imaging. The AuNPs attached strongly to
the CNTs, as substantiated by the observations of nanoparticles that were sus-
pended over pores or that moved along with the CNTs (Figure 7.12 e). The strong
mechanical binding between the AuNPs and the CNTs revealed a comparatively
close contact between the two objects, and also showed that this binding energy
was larger than that between the cluster and the alumina surface. This behavior
is signifi cant for understanding a strongly coupled electronic system. In contrast
to most other general methods for the direct evaporation of gold onto CNTs, defect
sites on the CNTs are not necessary in this method of creating preformed AuNPs.
This approach may provide a new strategy for functionalizing CNTs for chemical
or biological sensing and also for fundamental studies of nanoscale contacts to
CNTs. Thus, drawbacks such as the complexity in directly functionalizing CNTs,
and the inability to obtain individual SWNTs by using common bulk synthesis
methods are avoided, which confi ne the applicability of CNTs as primary elements
in sensors.
7.5.3
Interaction Between Modifi ed AuNPs and CNT s
A signifi cant feature of nanoscience and nanotechnology concerns the progress of
experimental protocols for the preparation of nanoparticles of diverse chemical
compositions, sizes, shapes, and controlled dispersity with a facile approach and
no environmental risk [60] . There are many established methods for the synthesis
of AuNPs, including conventional chemical reduction, heat - treatment, microwave
irradiation, sonochemical, photolytical, seeding growth approaches, and self -
reduction using surfactants [4, 61] . It is well known that the reaction medium,
reducing agent, and capping or protecting agent are the three key factors for the
synthesis and stabilization of metal nanoparticles in general, and for AuNPs in
particular. By selecting these factors appropriately, it would be possible to modify
the nanoparticles according to their convenient purposes. Therefore, it should
also be possible to connect the AuNPs to the surface of the CNTs through either
covalent linking or supramolecular (noncovalent) interaction. Hence, the AuNPs

268 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
prepared are modifi ed with suitable functional groups so as to link the AuNPs
to the CNT surface. These connections can be attained by using the functional
groups of the modifi ed AuNPs to covalently link with functional groups present
on the surface of the CNTs. An alternative method would be simply to adhere the
linker onto the CNT surface via supramolecular interactions such as hydrophobic
interactions, hydrogen bond linkage, π – π interactions, or electrostatic attraction.
7.5.3.1 Covalent Linkage
As CNTs are chemically inert, the triggering of their surface is a vital prerequisite
for linking the metal nanoclusters to them. Chemical functionalization is the
most common and widely used way to introduce the linkers, as well as to improve
dispersibility of CNTs, which is also signifi cant for the profi cient and uniform
deposition of nanoparticles. The functionalization of SWNTs by a chemical method
(covalent linkage) to enhance its dispersibility was introduced by Chen and
coworkers [62] .
Azamian and coworkers [63] employed carboxylate chemistry to covalently
link the AuNPs to defect sites in controllably oxidized SWNT termini and/or
sidewalls. The carboxylic acid groups created on a SWNT were converted to amides
by reaction with carbodiimide reagents [ N , N ′ - dicyclohexyl - carbodiimide ( DCC ) or
1 - ethyl - 3 - (3 - dimethyl - amino - propyl) - carbodiimide ( EDC )] and 2 - aminoethanethiol.
Generally, carbodiimide catalyzes the formation of amide bonds between carboxy-
lic acids or phosphates and amines by activating carboxyl or phosphate to form an
O - urea derivative. The introduced thiol functionality was consequently linked with
well - dispersed gold colloids (Figure 7.13 ). The attachment of AuNPs to SWNTs
sidewalls was corroborated by imaging the pristine SWNTs with atomic force
microscopy ( AFM ) before and after exposure to the coupling reagent and colloidal
AuNPs. This technique may be extended to test, with ease, the functionalization
of SWNTs with a range of groups.
A simple, direct, solvent - free approach was reported for decorating AuNPs
onto the surface of MWNTs functionalized with aliphatic dithiols such as 1,4 -
butanedithiol, 1,6 - hexanedithiol, 1,8 - octanedithiol, and 2 - aminoethanethiol [64] .
While AuNPs ( ∼ 1.7 nm) with a narrow particle size distribution were produced on
the 1,6 - hexanedithiol - functionalized MWNTs, the average size of AuNPs was
5.5 nm, obtained on MWNTs derivatized with aminothiol. This difference in
the AuNPs size was apparently due to a coalescence phenomenon of AuNPs in
aminothiol - functionalized MWNT samples; this could be the result of a nonuni-
form capping of the aminothiol over the AuNPs surface, avoiding the passivated
AuNPs. The main drawback of this approach is that attempts to attach AuNPs to
the derivatized MWNTs using water as a solvent medium were not particularly
successful, giving rise to a considerable agglomeration of gold over the nanotube
bundles, obviously due to the poor CNT dispersibility in water. Instead, the
MWNTs nicely decorated with well - dispersed AuNPs can be prepared only when
water is substituted by 2 - propanol. It is anticipated that this method may be useful
for attaching CNTs to gold tips for AFM and scanning tunneling microscopy
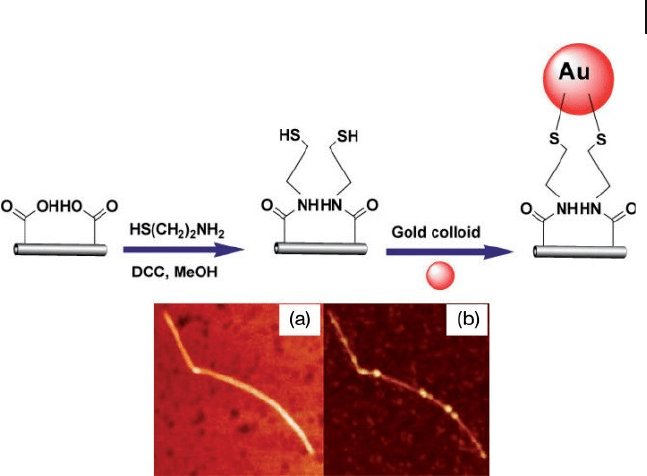
7.5 CNT–AuNP Composites 269
Figure 7.13 The chemistry used to connect the AuNPs to
oxidized SWNTs. AFM images of SWNTs before (a) and after
(b) exposure to the coupling reagents and colloidal gold
particles. DCC = N , N ′ - dicyclohexyl - carbodiimide. Adopted and
modifi ed according to Ref. [63] ; AFM images reprinted with
permission from Ref. [63] .
( STM ), and also potentially for monitoring the adsorption and concentration of
trace metal ions.
An effi cient method was developed to functionalize MWNTs with thiol groups,
after which AuNPs were anchored onto them to fabricate new composite materi-
als. Two different simple procedures were followed to produce MWNT – AuNP
composite materials, as summarized in the Figure 7.14 . The thiol - functionalized
MWNTs were stirred with already prepared AuNPs in toluene at room
temperature, and in another way AuNPs were produced in the presence of thiol -
functionalized MWNTs ( in situ formation of MWNT – AuNP composite) [65] . The
TEM images showed self - assembly of the AuNPs on the MWNTs, where the
AuNPs (Figure 7.14 a) of 25 nm were dispersed on the MWNTs. The shape and
size of the AuNPs obtained using the in situ method were different (Figure 7.14 b)
from those obtained with the former method. This might be explained by the
existence of thiol groups surrounded by AuCl
4
−
anions, which were reduced to
AuNPs in the presence of reducing agents. These AuNPs represent the cores for
further growth, as the thiol groups did not cover the whole particles.
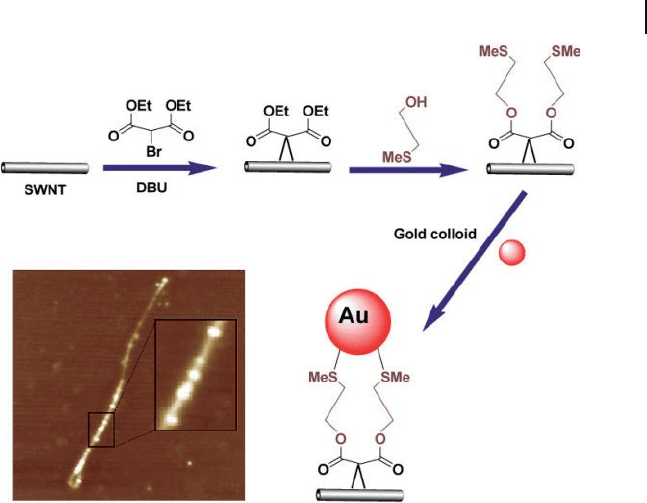
Coleman and coworkers used the Bingel reaction to functionalize the SWNTs
with a cyclopropane group [66] . For this, the cyclopropane group was tagged using
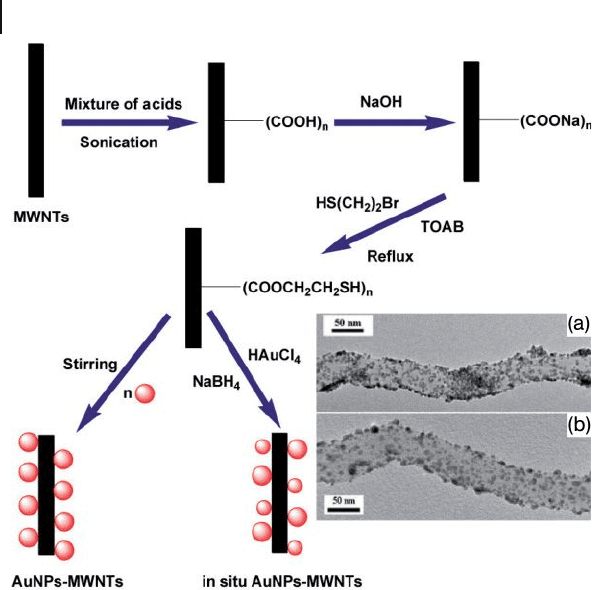
270 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
Figure 7.14 Experimental procedure for the fabrication of
AuNP – MWNT composites and TEM images of AuNP – MWNT
(a) and in situ AuNP – MWNT (b). TOAB = Tetra -
octylammonium bromide. Adopted and modifi ed according
to Ref. [65] ; TEM images reprinted with permission from
Ref. [65] .
preformed ∼ 5 nm gold colloids by exploiting the gold sulfur binding interaction,
as shown in the Figure 7.15 . AuNPs were observed both on the sidewalls and at
the ends of the SWNTs. The Bingel reaction is an example of a [2+1] cycloaddition
reaction, and is a popular method in fullerene chemistry.
Recently, a novel DNA biosensor was fabricated for the detection of DNA
hybridization based on the layer - by - layer ( LBL ) self - assembly of MWNTs and
AuNPs via covalent linkage, which exhibited an excellent specifi city and chemical
stability under the DNA - hybridization conditions [67] . The LBL assembly repre-
sents one of the simplest ways of producing fundamentally and practically interest-
ing multilayer fi lms with unique mechanical properties; it also provides a precise
control over fi lm composition and thickness, which in turn opens up many new
opportunities for achieving the ideal model surface, the properties of which are
controllable. For this, the cysteamine molecules simply act as a glue to connect
activated MWNTs and AuNPs into a 3 - D hybrid network on the Au electrode, after
which NH
2
- ssDNA ( ssDNA ; single - stranded DNA ) was immobilized onto multi-
7.5 CNT–AuNP Composites 271
Figure 7.15 Schematic illustration of the chemistry used to
connect AuNPs to SWNTs and AFM image of as - formed
AuNP – SWNT composites by using the Bingel reaction.
DBU = 1,8 - diazabicyclo[5.4.0]undec - 7 - ene. Adopted and
modifi ed according to Ref. [66] ; AFM image reprinted with
permission from Ref. [66] .
layer fi lms via the amino link at the 5 ′ end. Owing to the electron - transfer ability
of the CNTs and the catalytic activities of the AuNPs, the sensitivity of DNA bio-
sensors was improved and this DNA biosensor showed an excellent reproducibility
and stability under DNA hybridization conditions.
7.5.3.2 Supramolecular Interaction Between AuNPs and CNT s
Another strategy for preparing CNT – AuNP composites is that of the supramolecu-
lar or noncovalent functionalization of CNTs, and the subsequent attachment
of AuNPs based on noncovalent interaction such as hydrophobic – hydrophobic
interaction, weak hydrogen bond linkage, π – π stacking interaction, or electrostatic
attraction.
Hydrophobic Interactions and Hydrogen Bonding Hydrophobic interaction is the
attractive force between molecules owing to the close positioning of the nonhy-
drophilic portions of the two or more molecules. Hydrogen bonding is a type
of supramolecular interaction or a type of weak attractive (dipole – dipole) interac-
tion between an electronegative atom and a hydrogen atom bonded to another
electronegative atom such as nitrogen, oxygen, or fl uorine. It can occur between
272 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
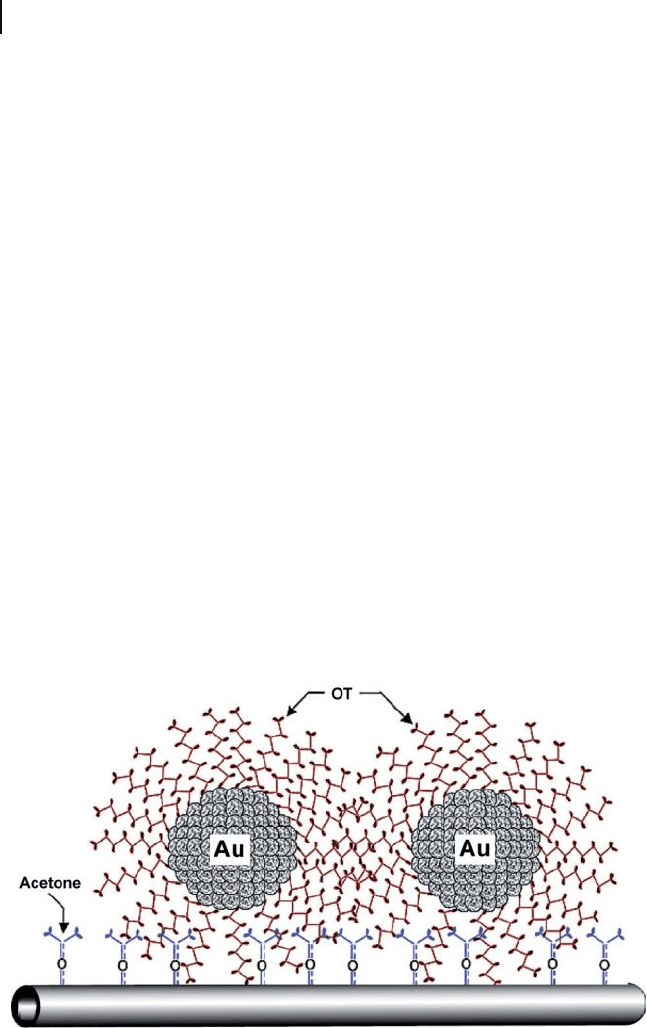
Figure 7.16 Schematic illustrating the attachment of
octanethiol (OT) - capped Au nanoclusters to acetone - activated
MWNTs. Reprinted with permission from Ref. [69] .
molecules, or within parts of a single molecule. A hydrogen bond tends to be
weaker than a covalent or ionic bond, but stronger than van der Waals forces. One
method of noncovalent functionalization of CNTs involves their association with
amphiphilic molecules through hydrophobic interaction in aqueous medium [68] .
Hydrophobic interactions between the ligands forming the monolayer that
passivate the metal surface have been used to deposit the metal nanoparticles
onto the surface of the CNTs. A novel strategy was reported to link monolayer -
protected gold nanoclusters of 1 – 3 nm diameter to the sidewalls of nonoxidized
CNTs through hydrophobic interactions between acetone - activated CNTs and
octanethiol - protected gold nanoclusters [69] . The anchorage was provided by the
interdigitation of alkyl chains of self - assembled molecular layers protecting the
gold nanoclusters and molecular moieties adsorbed on the surface of CNTs (Figure
7.16 ). These molecularly interlinked hybrid nanoblocks may be signifi cant for
exploring and manufacturing a rich variety of molecular nanostructures for poten-
tial device applications.
In addition to experimental studies, a theoretical model has been developed to
provide a detailed account of the interactions (charge transfer, van der Waals,
osmotic, elastic, nonelastic, and covalent) between tetraoctylammonium bromide -
stabilized AuNPs and alkyl - and alkylthiol - modifi ed MWNTs, so as to estimate the
coverage of AuNPs at the surface of the MWNTs under different experimental
conditions [70] . A quantitative description of the interactions between AuNPs and
MWNTs was made by comparing between the predictions of the theoretical model
and the experimental results. From such a comparison, it was concluded that as
the length of the alkyl chains at the surface of the MWNTs increased, coverage of
7.5 CNT–AuNP Composites 273
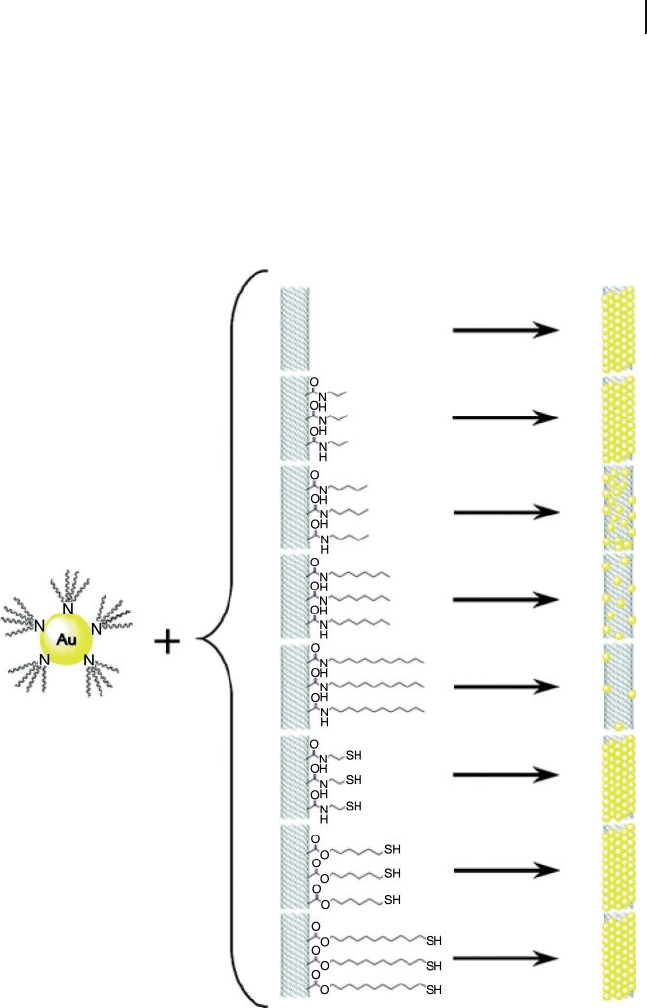
Figure 7.17 Scheme showing the templated assembly of
tetraoctylammonium bromide - stabilized AuNPs at the surface
of unmodifi ed, alkyl - modifi ed, and alkylthiol - modifi ed MWNTs.
Reprinted with permission from Ref. [70] .
the AuNPs decreased (i.e., noncovalent adsorption). In contrast, for alkylthiol -
modifi ed MWNTs, coverage of the AuNPs at their surface remained constant,
irrespective of the length of the alkyl - thiol chain (covalent adsorption) (Figure
7.17 ). The theoretical model assumption was in good agreement with the experi-
mental fi ndings, which proved the validity of the predictive model. A signifi cant
insight is that, under certain conditions, the coverage of AuNPs is very sensitive
to the nature of the MWNT surface modifi cation and the environment, pointing
274 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
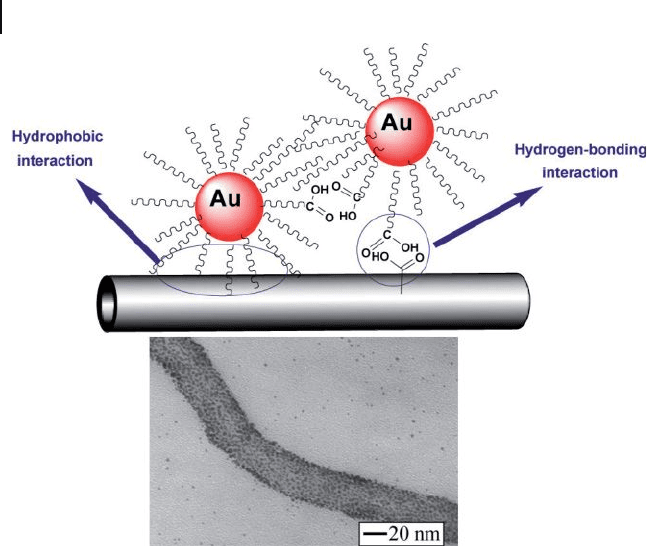
Figure 7.18 Schematic representation of the molecularly
mediated assembly of monolayer - capped AuNPs on CNTs,
and the characteristic TEM image of the AuNP – CNT
composite. Adopted and modifi ed according to Ref. [71] ; TEM
image reprinted with permission from Ref. [71] .
the way to a rational design of functional CNT - based nanoscale devices with
potentially widespread applications.
A molecularly mediated assembly of alkanethiolate - capped AuNPs onto the
surface of MWNTs through a combination of hydrophobic interactions between
the alkyl chains of the capping/linking molecules and the hydrophobic backbones
of a nanotube and the hydrogen - bonding interaction between carboxylic groups of
the capping/linking molecules and the functional groups on the surface of the
nanotube, has been described [71] (Figure 7.18 ).
Here, the simplicity and effectiveness for assembling alkanethiolate - capped
AuNPs of 2 – 5 nm core size onto CNTs with controllable coverage and spatially
isolated character were achieved by adopting such a combination of interfacial
chemistry – that is, a combination of hydrophobic and hydrogen - bonding interac-
tions between the organic shells and the CNT surfaces. The benefi t of this pathway
is that it does not require any diffi cult and tedious surface modifi cation of
CNTs. Rather, the assembly, packing density and distribution of the AuNPs onto
the surface of the CNTs depended on the relative concentrations of the AuNPs,
CNTs, and capping or linking agents. A thermal treatment to remove any organic
