Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


7.5 CNT–AuNP Composites 255
of composite material is important not only for fundamental and academic
studies of the interactions between the matrix and the metallic nanoparticles, but
also for various applications such as catalysts as well as electronic, optical, and
sensor devices.
7.5
CNT – AuNP Composites
A variety of resourceful techniques has been reported for the production of
CNT – AuNP composites (in general, the deposition of AuNPs onto the surface of
the CNT substrate), each offering different degrees of particle size control
and distribution along the CNTs. By changing the size and concentration of the
AuNPs deposited/incorporated, the electronic properties of the CNTs can in turn
be controlled. Composites of CNTs with AuNPs may be created via different path-
ways. One approach is to grow and/or incorporate the AuNPs into the hollow
cavities of the CNTs, while a second approach is to grow and/or deposit naked
AuNPs directly onto the terrace of the CNTs. In yet another process, the AuNPs
may be prepared and modifi ed with suitable functional groups that can be con-
nected to the CNT surface via covalent bonding through organic moieties; alter-
natively, the modifi ed AuNPs may simply be linked to the surface of the CNTs via
supramolecular interactions.
7.5.1
Filling of CNT s with AuNPs
In order to synthesize CNTs with predetermined characteristics, it is essential to
identify and control the mechanisms that direct CNT growth. The major challenge
here is to identify effective ways in which to fi ll the metal nanoparticles (notably
AuNPs) into the hollow CNT cavities, without affecting the latter ’ s individual
characteristic properties.
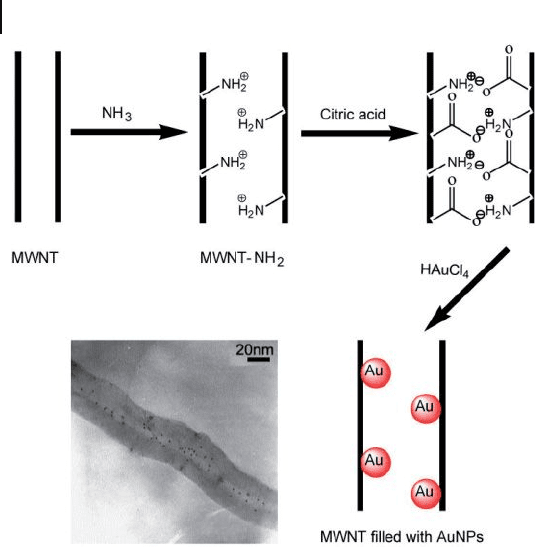
A simple procedure for producing a composite is to fi ll the MWNTs with AuNPs,
simply by mixing an aqueous citric acid solution containing NH
3
- treated MWNTs
and aqueous auric chloride solution [38] . Heat treatment in NH
3
causes most of
the nanotubes to be open, such that functional basic groups are created on their
inner walls. Mixing and ultrasonication will then help the citric acid to combine
strongly with the basic groups via electrostatic attraction, thus facilitating the in
situ reduction and subsequent attachment of AuNPs (1 – 2 nm) inside the nano-
tubes (Figure 7.2 ). These hybrid materials may in time become important for
investigating and creating a rich variety of electrical and sensor devices.
In another “ wet chemistry ” technique, a two - step procedure was used to produce
a composite material by fi lling gold metal into the cavities of the CNTs. Here, the
nanotubes were opened by oxidation with HNO
3
[39] and then stirred overnight
with a concentrated aqueous solution of AuCl
3
. The resultant CNTs, when sepa-
rated from excess concentrated solution, were calcined in a furnace under argon
256 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
at 600 ° C, whereby the AuCl
3
was decomposed to produce elemental gold [40] . Most
of the gold entrapped within the hollow CNTs was seen to be crystalline in nature,
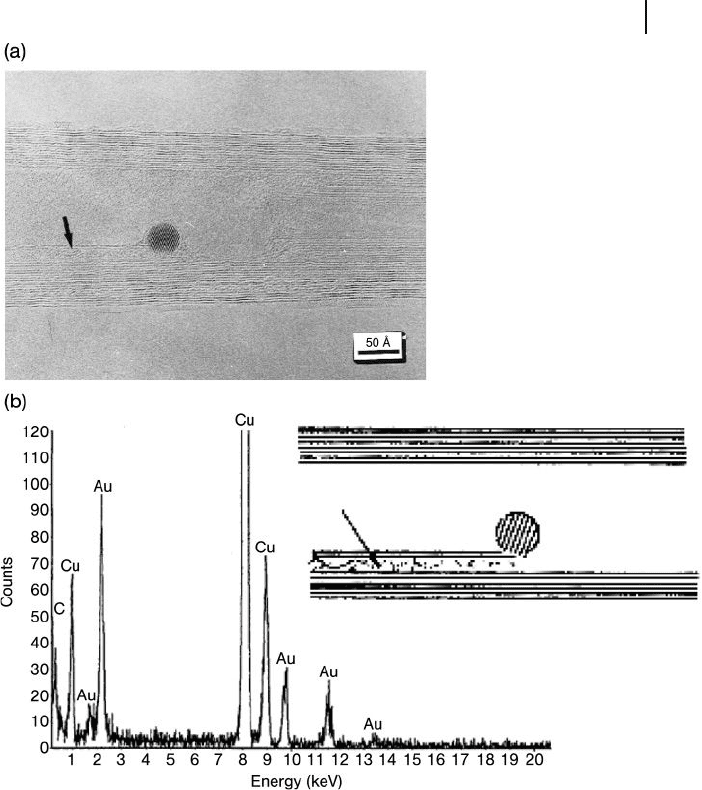
spherical in shape, and to range in size from 1 to 5 nm diameter. A HR - TEM image
of a CNT encapsulated with a gold crystal, together with the analytical energy -
dispersive spectrum ( EDS ) of the gold particle, are shown in Figure 7.3 . More
importantly, by using this method it was possible to produce a relatively high
percentage ( ∼ 70%) of opened nanotubes to be fi lled with metallic gold [33, 39] .
7.5.2
Deposition of AuNPs Directly on the CNT Surface
When depositing AuNPs onto the terrace of the CNTs, it is possible to use gold
salts as the precursors for the AuNPs; these salts are produced by a variety of
reduction processes, using either reducing agents and/or external energies such
as heat (thermal), photochemical, and light, in the presence of the CNTs. The
interactions between the AuNPs and CNTs are mostly based on van der Waals
forces which, in some cases, appear to be suffi ciently strong so as to ensure
signifi cant adhesion.
Figure 7.2 Schematic illustration for the attachment of AuNPs
to NH
3
- treated CNTs and the TEM image of AuNP - fi lled
CNTs. Adopted and modifi ed according to Ref. [38] ; TEM
image reprinted with permission from Ref. [38] .
7.5 CNT–AuNP Composites 257
Figure 7.3 (a) High - resolution transmission electron
microscopy (HR - TEM) image of a carbon nanotube fi lled with
a spherical Au crystal. The solid arrow shown in the HR - TEM
image indicates where there has been intercalation into the
gaps where carbon layers are missing; (b) ED spectrum of the
Au particle. Reprinted with permission from Ref. [40] .
An effective method was introduced by Xue and coworkers for depositing
AuNPs onto the walls of CNTs [41] . For this, AuNPs (average size 8 nm)
were grown on the surface of the CNTs by the thermal decomposition (400 ° C) of
gold salts under a hydrogen atmosphere. This synthetic strategy has also been
shown to be a generalized process that can easily be extended to the synthesis of
different types of metal nanoparticle (Pt, Ag, Pd) onto the CNT surface. It appears
258 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
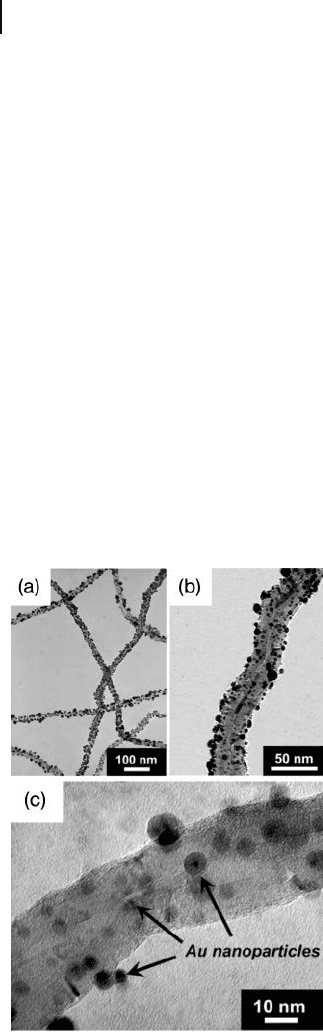
Figure 7.4 (a, b) Low - and (c) high - magnifi cation bright - fi eld
TEM images showing the decoration of MWNTs with AuNPs
of 3 – 10 nm diameter. Reprinted with permission from
Ref. [42] .
that the CNTs play a vital role here, not only as a template for tuning the metal
nanoparticles size but also acting as a support material. This fi nding was sup-
ported by the fact that larger particles were observed when metal salts were
reduced in the presence of graphite or amorphous carbon. Xue and coworkers
suggested that these composite materials might be used as effi cient catalysts for
certain environmentally advantageous reactions, as well as certain applications in
electronic devices.
Raghuveer and coworkers established a novel “ eco - friendly ” strategy of utilizing
microwave irradiation for the rapid introduction of carboxyl, carbonyl, hydroxyl,
and allyl terminal groups onto the surface of MWNTs, without using any aggres-
sive oxidants such as HNO
3
and/or ultrasonication [42] . Here, the functional
groups served as the preferred nucleation points for reducing gold ions from solu-
tion by a microwave - assisted reduction reaction. MWNTs were dispersed in water
and added to an aqueous solution containing HAuCl
4
and ethylene glycol. After
microwave irradiation, the surfaces of the MWNTs were seen to be decorated by
uniformly dispersed AuNPs (Figure 7.4 ) that ranged in size from 3 to 10 nm
(average ∼ 6 nm). The MWNTs were derivatized with nanoparticles synthesized by
an in situ gold - ion reduction during functionalization, all in a single - step process.
The notable point here was that the overall tubular structure of the MWNTs
remained intact, as was evident from TEM images. This was in great contrast to
7.5 CNT–AuNP Composites 259
the rupture and tube breakage observed during functionalization by aggressive
sonication and acid treatment [43] .
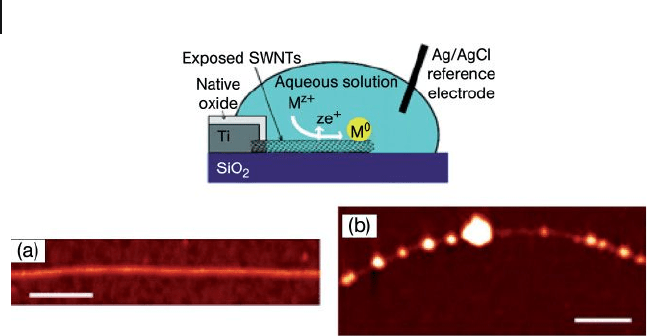
A different strategy for the formation of AuNPs on the surface of SWNTs, based
on the spontaneous reduction of metal ions in solution without the use of a reduc-
ing agent, was reported by Choi and coworkers [44] . This approach differed from
a typical electroless deposition, which requires either a reducing agent or a catalyst,
as a result of direct redox reactions between the ions and nanotubes. Electroless
deposition methods rely on a chemical (as opposed to an electrochemical) reduc-
tion process, whereby a chemical species with a redox potential suitably lower than
that of the metal species being reduced provides the driving force for the reaction
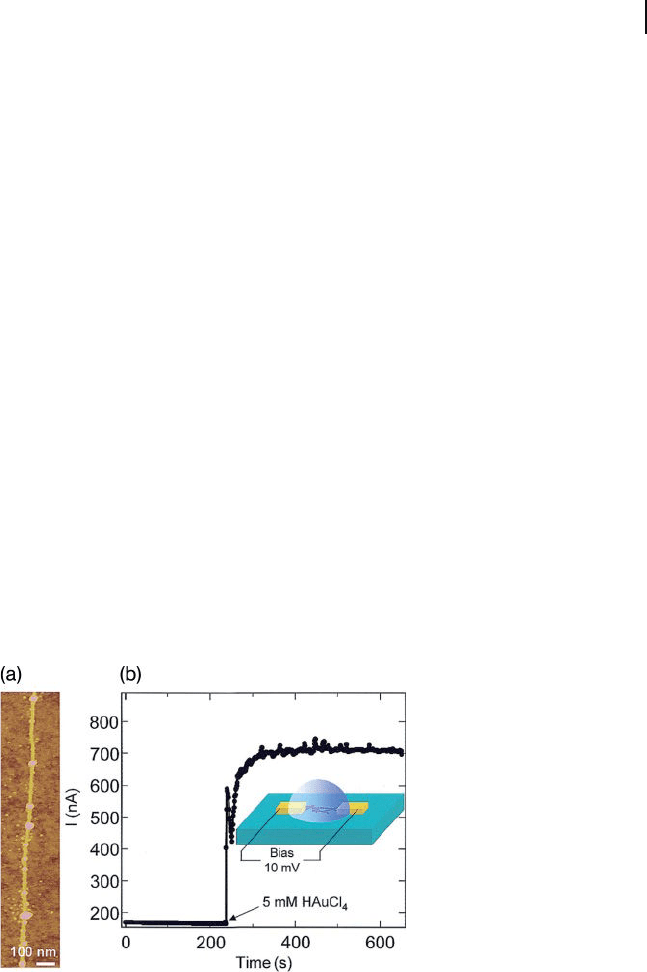
[45] . A spontaneous decoration of AuNPs (average size 7 nm) on the sidewalls of
the SWNTs was observed following their immersion in HAuCl
4
(Au
3+
) solution
for 3 min (Figure 7.5 a). The Au
3+
ion reduction and SWNT oxidation during elec-
troless metal deposition was investigated by measuring the electrical conductance
of SWNTs immersed in solutions (Figure 7.5 b). When the SWNTs act as electron
donors, hole insertion into SWNTs would be expected to cause an increase in the
electrical conductance [46] to the already p - type nanotubes due to O
2
doping under
ambient conditions [47] . Spontaneous metal deposition onto the SWNTs by an
electroless process allows for a facile, effi cient, and selective immobilization of
metal species on nanotubes, which may be useful for sensor and catalysis applica-
tions. However, this selective electroless metal deposition on SWNTs was shown
to be effective only for Au or Pt; other metal ions such as Ag
+
, Ni
2+
, and Cu
2+
, could
not be reduced in the same way, perhaps due to their lower redox potentials.
Figure 7.5 (a) Atomic force microscopy image of AuNPs
formed on an individual SWNT; (b) Monitoring the change in
current across a SWNT during exposure to a 5 mM HAuCl
4
solution. The period before the exposure corresponds to the
nanotube in a mixture of ethanol and water (1 : 1). Inset:
Schematic for the experimental set - up. Spacing between
points = 1 s. Reprinted with permission from Ref. [44] .
260 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
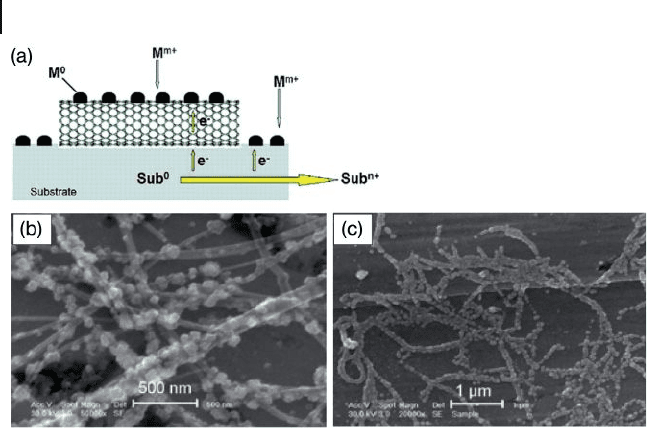
Figure 7.6 (a) Schematic illustration of the metal nanoparticle
deposition on CNTs via the substrate - enhanced electroless
deposition process; (b, c) Scanning electron microscopy
images of (b) MWNTs and (c) SWNTs supported by a copper
foil after immersion in an aqueous solution of HAuCl
4
(3.8 mM). Reprinted with permission from Ref. [48] .
Qu and Dai introduced for the fi rst time another facile, but versatile and effec-
tive, method for the electroless deposition of AuNPs on both SWNTs and MWNTs
in the absence of any additional reducing agent [48] . Upon immersion of the
copper - supported MWNTs or SWNTs into an aqueous solution of HAuCl
4
(3.8 mM), the AuNPs were deposited spontaneously onto the terrace of the nano-
tubes. The advantage of this technique over the previous method is that a large
variety of metal nanoparticles (even for metals with a lower redox potential than
that of the CNTs, such as Cu and Ag) could be reduced and decorated onto the
surface of both MWNTs and SWNTs. The general scheme of the reaction process,
together with scanning electron microscopy ( SEM ) images of AuNPs decorated
onto MWNTs and SWNTs, are shown in Figure 7.6 .
The deposition of metal nanoparticles is achieved via the redox reaction of a
galvanic cell, in which the nanotube acts as a cathode for the metal nanoparticle
deposition (M
0
, e.g., Au
0
) from the reduction of metal ions (M
m
+
, e.g., Au
3+
) in
solution, while the metal substrate (e.g., Cu) serves as an anode where metal atoms
(Sub
0
) are oxidized into corresponding ions (Sub
n
+
) followed by dissolution [49]
(Figure 7.6 ). This process, which is known as substrate - enhanced electroless depo-
sition ( SEED ), allows the electroless deposition of many metal nanoparticles onto
conducting CNTs, and indicates a great potential for the functionalization of CNTs
with various metal nanoparticles.
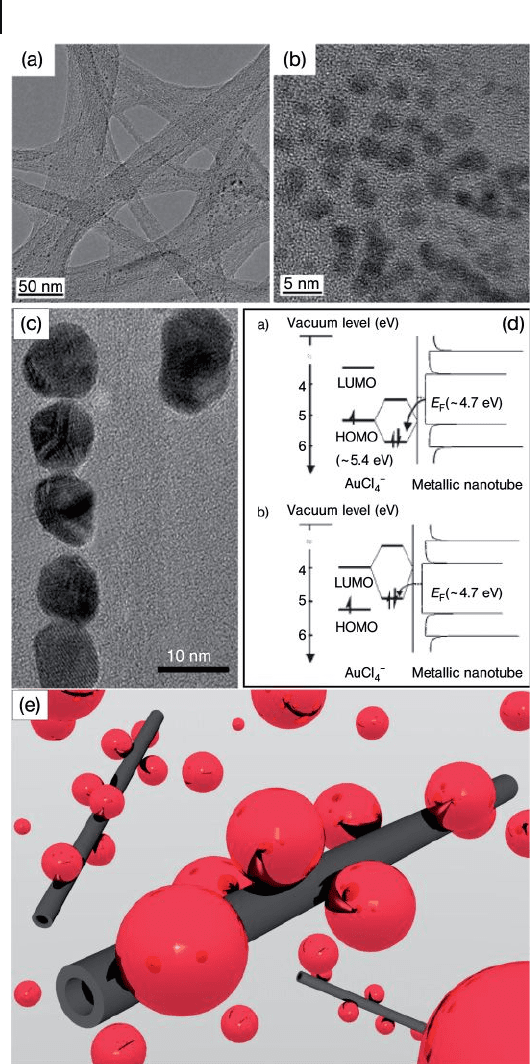
Geckeler and coworkers have established an unprecedented approach to prepare
SWNT – AuNP hybrids in homogeneous phase by using the reaction of gold salts

7.5 CNT–AuNP Composites 261
and SWNTs in the presence of a surfactant in aqueous solution, without the addi-
tion of a reducing agent [50] . The AuNPs decorated on the sidewalls of the SWNTs
were uniform in size and well dispersed (Figure 7.7 ). Statistical calculations indi-
cated the average AuNP size to be 2.94 ± 0.75 nm, with 7.5 × 1 0
− 17
g of gold being
coated on an individual nanotube that in turn contained approximately 300 AuNPs.
Interestingly, the size of the AuNPs decorated on the surface of the SWNTs could
be tailored by altering the concentration of the gold salt solution.
The chemistry of the hybrid material was described by using a frontier - orbital
picture. As the relative position of the Fermi level of nanotubes with respect to the
mixed metal ion/nanotube highest occupied molecular orbital ( HOMO ) and
lowest unoccupied molecular orbital ( LUMO ) is suitable for charge transfer, both
semiconducting and metallic CNTs may establish attractive interactions with the
metal ions, either by four - electron interactions involving two occupied orbitals, or
by zero - electron interactions involving two empty orbitals (Figure 7.7 d). It was
noted that the HOMO level of
AuCl
4
−
is partly occupied with electrons. This
method is easy to scale - up, such that a uniform size of AuNPs may be decorated
on the walls of the SWNTs, and size of the AuNPs can also be controlled. More
importantly, as the method yields water - soluble composite materials, a much
greater variety of applications can be envisaged.
A simple method has been developed recently to prepare hybrid materials from
SWNTs and AuNPs, including Pt and Rh nanoparticles. For this, nanoparticles
were deposited on the surface of the SWNTs by the mild reduction of metal salts
using poly(ethylene glycol) - 200 as the reducing agent [51] . The free surface of
the nanoparticles attached to the SWNTs was then coated with organic aliphatic
molecules such as oleylamine, which enhanced the dispersion of the resulting
hybrid material in organic solvents. This method avoids chemical functionaliza-
tion of the sidewalls and open ends of the SWNTs, and the fi nal hybrid material
may be used for the application in the catalysis of organic reactions.
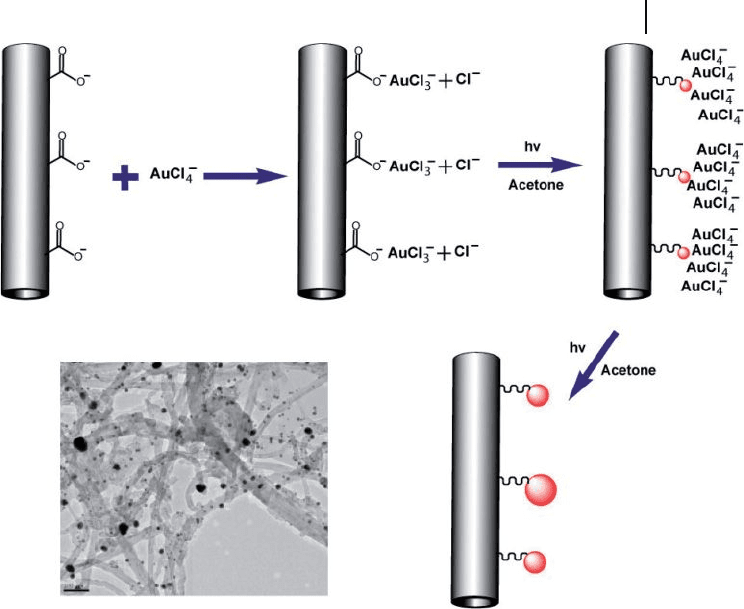
Recently, a simple UV irradiation method was developed to grow uncoated
naked AuNPs on carboxy - modifi ed MWNTs by performing UV irradiation on
mixed solution containing oxidized MWNTs, HAuCl
4
, and acetone (acting as a
photosensitive agent) at room temperature (Figure 7.8 ) [52] . The size of AuNPs
deposited on the terrace of the MWNTs was found to depend heavily on the diam-
eter of MWNTs and the solution pH. The size of the AuNPs was indirectly pro-
portional to the diameter of MWNTs and the solution pH. As a high catalytic
activity (especially for AuNPs) mainly depends on the steps, edges, and corner
sites of surface, and also on the electrical interactions between AuNPs and sup-
porting materials, the resultant composites may prove to be advantageous in cata-
lytic reactions.
Although a variety of ingenious strategies to decorate AuNPs onto CNTs is avail-
able, the electrodeposition method has its own advantages. Electrochemistry rep-
resents a potent technique for the deposition of diverse metals and/or the surface
modifi cation of CNTs, being both rapid and facile, and thus allowing the chemist
and materials scientist to control with ease the nucleation and growth of the metal
nanoparticles [45, 53, 54] . It is very feasible to control the size and distribution of
262 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
Figure 7.7 (a) Transmission electron
microscopy (TEM) image, and (b, c) HR - TEM
images of the CNT/AuNPs hybrid;
(d) Frontier - orbital picture representation
of four - electron interactions (plot a), and
zero - electron interactions (plot b) between
AuCl
4
−
and a metallic CNT. The
AuCl
4
−
frontier - orbitals are designated as HOMO and
LUMO, and the nanotube orbitals are
represented by the density of states plots.
E
F
= Fermi level. ( Reprinted with permission
from Ref. [50] ); (e) Schematic showing
decoration of the surface of CNTs with
AuNPs. (Kyungjae Lee is gratefully
acknowledged for his support in the
preparation of this image).
7.5 CNT–AuNP Composites 263
Figure 7.8 Scheme showing the growth of AuNPs on the
surface of MWNTs initiated by UV irradiation, and a TEM
image of AuNPs attached to the surface of MWNTs at pH 12.
Scale bar = 100 nm. Adopted and modifi ed according to Ref.
[52] ; TEM image reprinted with permission from Ref. [52] .
metal nanoparticles simply by varying the deposition potential, time, and sub-
strate. However, the other imaginative methods of depositing nanoparticles onto
the CNTs surface involve some tricky, tedious, and time - consuming treatments
that allow the impurities in the bath solutions to be included either into the nano-
particles or onto the terrace of the CNTs themselves, thus affecting the optical or
catalytic properties of the nanoparticles [54] . Electrochemically deposited nanopar-
ticles – particularly of noble metals such as Au, Pt, or Pd – are often of very high
purity, are formed rapidly, and have good adhesion to the CNT substrate [53, 54] .
Quinn and coworkers [53] reported a general electrodeposition method for
depositing noble metals such as Au, Pt, and Pd onto the surface of the SWNTs by
immersing the latter in a solution of the respective metal salts, namely HAuCl
4
,
K
2
PtCl
4
, and (NH
4
)
2
PdCl
4
. Whilst the size of the nanoparticles was tuned by the
concentration of the metal precursor salt and the electrochemical deposition
parameters, the coverage of the nanoparticles on the surface of the SWNTs
was controlled by the nucleation potential. The resultant composite material was
264 7 Gold Nanoparticles and Carbon Nanotubes: Precursors for Novel Composite Materials
Figure 7.9 General schematic illustration of the SWNT as an
electrode used for the electrodeposition of metal
nanoparticles, and AFM images of the predeposition (a) and
postdeposition (b) of Au. The deposition time was 20 s. Scale
bar = 300 nm. Reprinted with permission from Ref. [53] .
considered as a metallic wire, as the surface of the SWNTs was thoroughly deco-
rated by the metal nanoparticles (Figure 7.9 ). It was noted that, under the experi-
mental conditions, both the sidewalls and ends of the SWNTs were coated equally
by metal nanoparticles, even though it is believed that the CNTs sidewalls are less
reactive than the tube ends. The SWNT serves a dual function in such a way that
initially acts as the electrodeposition template, and subsequently as a wire to elec-
trically connect the deposited Au, Pt, and Pd nanoparticles.
The cubic and spherical nanoparticles of gold, with a fairly narrow size distribu-
tion, deposited on the surface of the CNTs were produced by immersing copper
foil - supported CNTs into an aqueous solution of HAuCl
4
with and without CuCl
2
at room temperature under different reaction conditions (e.g., different concentra-
tion and reaction time) [55, 56] . Both, the shape and size of the AuNPs were found
to depend heavily on the gold salt concentration and reaction time, providing
considerable room for regulating the morphological features of the resultant nano-
particles. The size of the AuNPs deposited on the surface of CNTs is approximately
60 – 100 nm, although their size can be tailored by controlling the reaction condi-
tions, especially the deposition time [56] . Gold nanospheres and nanocubes were
multisite deposited along the CNT length, with individual nanotubes even thread-
ing through the nanoparticles (Figure 7.10 ). The facile and versatile technique for
the shape - and size - controlled syntheses of AuNPs for the site - selective modifi ca-
tion of CNTs is very attractive for producing various multicomponent nanoparticle
– nanotube hybrid structures that might be useful in a wide range of potential
applications, including fuel cells, catalytic, sensing, and optoelectronic systems.
Further, in order to investigate and explain the optical response of the CNT – AuNP
hybrid from a theoretical standpoint, a 3 - D electrodynamic model was built using
the fi nite - difference time - domain [56] . These studies proposed an anisotropic
