Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

6.5 Metal Oxide Nanotubes 225
6.5.4
Nanotubes of Vanadium and Niobium Oxides
Vanadium oxides have received considerable attention because of their structural
fl exibility and useful catalytic, electrochemical, and other properties. The structure
of V
2
O
5
permits the intercalation of various cationic species in the interlamellar
space [121] . The cations include alkylammonium ions, which are readily interca-
lated between the layers under hydrothermal conditions [122] . This system is thus
analogous to graphite and layered dichalcogenides. Nesper and coworkers [123,
124] synthesized nanotubules of alkylammonium intercalated VO
x
by hydrother-
mal means. The vanadium alkoxide precursor was hydrolyzed in the presence of
hexadecylamine and the hydrolysis product (lamellar structured composite of the
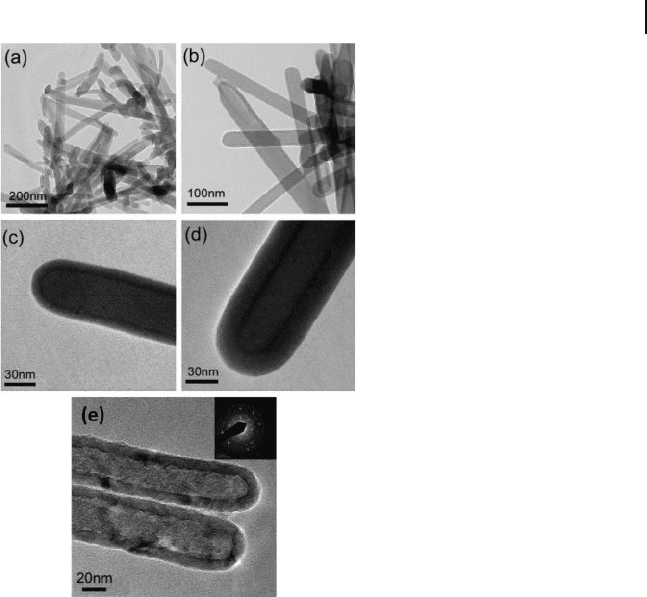
Figure 6.15 TEM images of a) GaQ
3
nanowires prepared by
thermal evaporation. b,c) GaQ
3
– Al
2
O
3
core – shell nanowires
fabricated by 100 cycles of ALD, and (d) same as (c), after 200
cycles of ALD. e) TEM image of the alumina nanotubes after
heat treatment at 900 ° C for 1 h. The inset shows the electron
diffraction pattern of alumina. Reproduced with permission
from [120] . Copyright 2007, ACS.

226 6 Synthesis of Inorganic Nanotubes
surfactant and the vanadium oxide) yielded VO
x
nanotubes along with the inter-
calated amine under hydrothermal conditions. Vanadium in these materials is
in the mixed - valent state, and is redox active. The template cannot be removed
by calcination as the structural stability is lost above 523 K. It is possible to par-
tially extract the surfactant under mild acidic conditions. Nesper et al. have shown
that the alkylamine intercalated in the intertubular space could be exchanged
with other alkylamines of varying chain lengths as well as α , ω - diamines [124] .
Such mixed valent VO
x
nanotubes are also obtained under hydrothermal condi-
tions using V
2
O
5
and 3 - phenylpropylamine [125] . Most of the VO
x
nanotubes
obtained by the hydrothermal method are open ended. Very few closed tubes had
fl at or pointed conical tips. Cross - sectional TEM images of the nanotubular phases
show that instead of concentric cylinders (i.e., layers that fold and close within
themselves), the tubes are made up of single or double layer scrolls that provide
a serpentine - like morphology [124, 126] . Non - symmetric fringe patterns in the
tube walls exemplify that most of the nanotubes are not rotationally symmetric
and carry depressions and holes in the walls. Diamine - intercalated VO
x
nanotubes
are multilayer scrolls with narrow cores and thick walls, composed of vanadium
oxide layers. Diamine - containing VO
x
nanotubes show a smaller number of holes
in the wall structure and the tubes are well ordered with uniform distances
throughout the tube length [124] . The scroll - like structures of VO
x
are not real
nanotubes of the type formed by carbon or metal dichalcogenides. Vanadium
oxide nanotubes that contain primary monoamines with long alkyl chains have
been prepared by employing non - alkoxide vanadium precursors such as VOCl
3
and V
2
O
5
. The amine complexes of the vanadium precursors are then hydrolyzed.
Hydrothermal treatment of the precursors gives good yields of VO
x
nanotubes
that incorporate the amines [127] . In general the inner diameter varies between
15 and 50 nm in the VO
x
nanotubes, independent of the precursor. The outer
diameters are similar, generally between 50 and 150 nm, with a range of tube
lengths. Starting from the alkoxides, the tube length varies from 1 to 15 μ m. In
the samples obtained starting with VOCl
3
and V
2
O
5
, the average length of the
tubes is shorter (1 – 3 μ m). The distribution of the number of layers is similar in
all the nanotubes, generally between 6 and 15 layers. The layers are preformed
in the lamellar phase and roll up during the autoclave treatment, because the
tetragonal structure of the layers gives rise to a more or less square - shaped growth.
However, since many scrolls consist of multiple independent layers and the
maximum length approaches 15 μ m, growth along the tube axis may occur as
well. Mn – V oxides have been prepared by mixing V
2
O
5
with dodecylamine in the
presence of ethanol and water. The amine templates are easily substituted or
ion - exchanged with ions like Mn
2+
in an aqueous alcohol solution to yield Mn – V – O
nanotubes [128] . Most of the nanotubes had open ends, while some of them had
closed ends, with the side of the tubes wrapped around the end to close it. The
Mn
2+
ions replace the organic cations in the structures and hence are intercalated
in between the layers.
Arrays of V
2
O
5
· n H
2
O nanotubes with diameters of 200 nm and 5 μ m length have
been fabricated by template - based physical wetting of V
2
O
5
sols [129] . Urchine - like
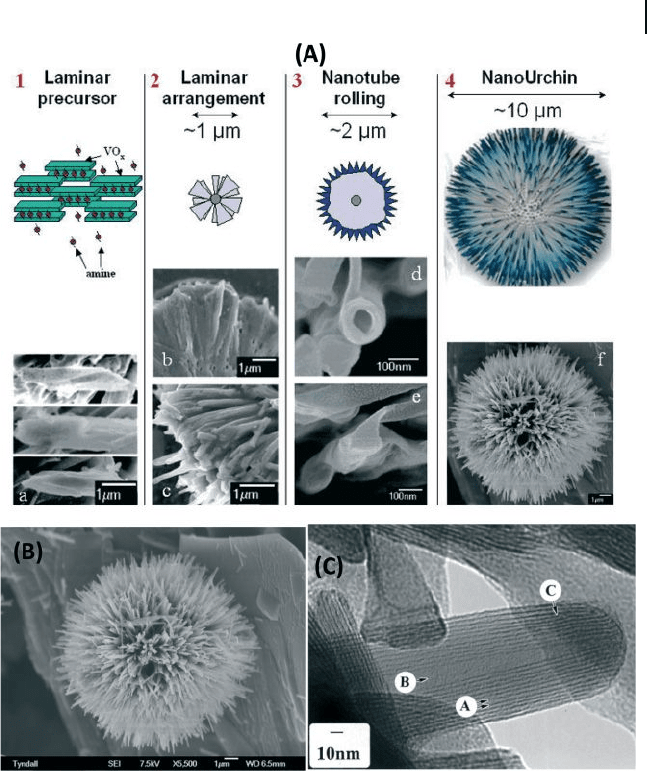
6.5 Metal Oxide Nanotubes 227
nanostructures (Fig. 6.16 ) that consist of high - density spherical nanotube radial
arrays of vanadium oxide nanocomposites, were successfully synthesized by a
simple chemical route using an ethanolic solution of vanadium tri - isopropoxide
and alkylamine hexadecylamine for 7 days at 180 ° C [130] . M – Nb
2
O
5
nanotube
arrays have been prepared starting from H – Nb
2
O
5
nanorods, taking advantage of
the phase transformation accompanied by the formation of voids [131] .
Figure 6.16 Schematic summary of the stages
of growth of VO
x
nano - urchins. FESEM image
of an individual nano - urchin. This fully grown
nano - urchin is 12 μ m in diameter and is
covered in VO
x
nanotubes with a volumetric
density of ∼ 40 sr
− 1
. HRTEM image of an early
stage nanotube. Lattice planes are resolved
(A) and have a measured lattice spacing
a = 2.85 nm. The hollow center (B) extends to
the tip of the nanotube. A lattice plane
termination dislocation is also observed (C).
Reproduced with permission from [130] .
Copyright 2006, ACS.

228 6 Synthesis of Inorganic Nanotubes
6.5.5
Nanotubes of other Transition Metal Oxides
A hydrothermal reaction of KMnO
4
in HCl gives rise to α - MnO
2
nanotubes [132] .
These MnO
2
nanotubes are single - crystalline and possess a tetragonal structure,
with an average outer diameter of around 100 nm. The wall thickness is 30 nm
and the length goes up to several micrometers. The nanotubes show nearly perfect
tetragonal cross sections, consistent with the crystal structure.
By the reaction of FeCl
3
with water in the presence of polyisobutylene bis - suc-
cinimide (L113B) or the surfactant span80 as well as butanol under solvothermal
conditions, Fe
2
O
3
nanotubes are produced [133] . Fe
2
O
3
nanotubes (rhombohedral
structure) prepared from the surfactant (span80 as the template) were tube - like
with diameters of 18 – 29 nm, wall thickness of 3 – 7 nm, and lengths of 110 – 360 nm.
They have a multi - walled structure, with an interlayer spacing of 2.76 Å . α - Fe
2
O
3
nanotubes are also obtained by the deposition of a metal salt solution and NaOH/
NH
3
in the pores of templates with the initial formation of an insoluble metal
hydroxide precursor, and its subsequent transformation by dehydration and crys-
tallization to metal oxide nanotubular structures [134] . The α - Fe
2
O
3
nanotubes so
obtained are polycrystalline and have diameters and lengths of ∼ 260 ± 60 nm and
6 ± 3 μ m, respectively. These nanotubes possess a rhombohedral structure and
consist of small mis - oriented single - crystalline nanocrystalline domains. Ordered
Fe
2
O
3
nanotube arrays can also be prepared by ALD in an AM [135] . In this
method, the thermal decomposition of a homoleptic dinuclear iron( iii ) tert - butox-
ide complex (Fe
2
(O t Bu)
6
) is carried out in the presence of water inside a self -
ordered porous anodic AM to yield arrays of nanocrystalline Fe
2
O
3
tubes with
aspect ratios up to 100. Polycarbonate membranes are used to obtain nanoparticle –
nanotube arrays of Fe
2
O
3
by employing electrodeposition followed by calcination
[136] . The arrays have lengths in the 5 – 6 μ m range and a diameter of ∼ 200 nm,
which corresponds closely to the pore dimension and pore diameter, respectively,
of the membrane. The open ends of the arrays demonstrate the hollow structure
of the product. The nanotubes have a hexagonal structure.
Ferromagnetic γ - Fe
2
O
3
nanotubes have been synthesized by a template process
with the aid of a high magnetic fi eld [137] . These are polycrystalline, with a cubic
spinel structure and lengths of about 30 μ m, a wall thickness of 20 nm, and a
diameter in the 300 – 400 nm range. α - Fe
2
O
3
is fi rst formed at 500 ° C by the thermal
decomposition of Fe(NO
3
)
3
inside the template and α - Fe
2
O
3
is then transformed
into γ - Fe
2
O
3
in the presence of the high magnetic fi eld. Porous alumina templates
are also used to obtain Fe
3
O
4
nanotubes [138] . The hydrothermal reaction of
Fe(NO
3
)
2
in ethanol at pH 12 gives rise to α - FeOOH nanotubes [139] . The so
obtained α - FeOOH nanotubes were ∼ 10 nm in outer diameter and ∼ 6 nm in inner
diameter. Electron microscopic images of the nanotubes clearly show the resolved
interplanar spacing of about 4.18 Å , which corresponds to the spacing between the
(110) planes of orthorhombic α - FeOOH.
Needle - like Co
3
O
4
nanotubes have been prepared by employing a one - step self -
supported topotactic transformation of nanoneedles of β - Co(OH)
2
(Fig. 6.17 ) [140] .
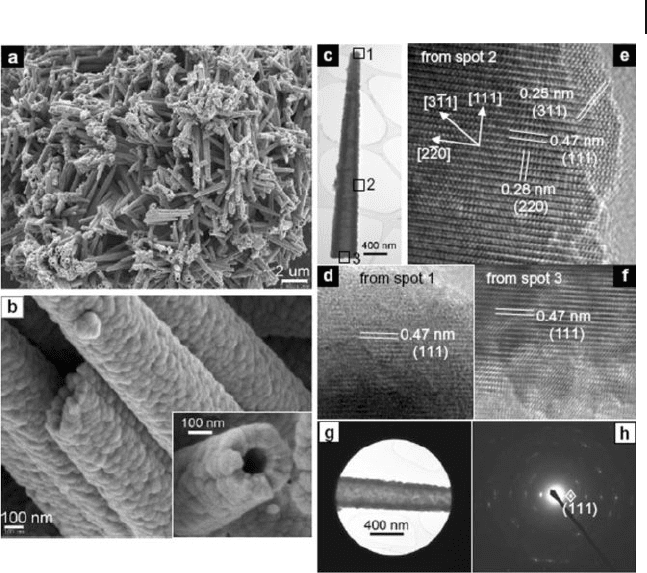
6.5 Metal Oxide Nanotubes 229
The nanotubes can be as long as 10 μ m with a variable diameter in the range of
150 – 400 nm. The nanotubes are cylindrical and constructed from Co
3
O
4
building
blocks of less than 100 nm. The high - resolution (HR) TEM images in Figures
6.17 d – f correspond to spots 1 to 3, respectively, of an individual nanotube (Fig.
6.17 c). All show (111) lattice fringes perpendicular to the tube axis with an inter-
plane spacing of 0.47 nm, while the two other sets of lattice fringes seen in Figure
6.17 e are (220) and (311) planes, which correspond to interplanar spacings of 0.28
and 0.25 nm, respectively. On the basis of structural analysis, it is found that the
tube axis is along the [111] direction with possible small mis - orientations for indi-
vidual Co
3
O
4
nanocrystals. The quasi - single - crystallinity of the cubic Co
3
O
4
nano-
tubes is also confi rmed by the SAED pattern (Fig. 6.17 h) and the corresponding
TEM image in Figure 6.17 g. Co
3
O
4
nanotubes are also prepared in the pores of
AM by CVD, starting with cobaltacetylacetonate [141] . These nanotubes are highly
ordered with a uniform diameter in the range of 100 – 300 nm and lengths of up
to tens of micrometers. The nanotubes are composed of cubic polycrystalline
Figure 6.17 a,b) FE - SEM images of needle - like
Co
3
O
4
nanotubes. The inset in (b) shows a
cross - sectional view of a nanotube.
c) Low - magnifi cation TEM image of an
individual needlelike Co
3
O
4
nanotube.
d – f) HREM images taken from spots 1 – 3,
respectively, of the nanotube shown in (c).
g) TEM image showing a selected area of a
Co
3
O
4
nanotube for electron diffraction and
h) the corresponding SAED pattern.
Reproduced with permission from [140] .
Copyright 2008, Wiley - VCH.

230 6 Synthesis of Inorganic Nanotubes
Co
3
O
4
. Porous AMs have been used to obtain CoFe
2
O
4
nanotube arrays by employ-
ing a sol – gel procedure [142] . These nanotubes are several micrometers in length
with a mean outer diameter of 50 nm, which corresponds to the diameter of the
alumina pore. The wall thickness is 15 nm.
NiO nanotubes can be fabricated through a MOCVD route using an AM as the
template and Ni(tta)
2
tmeda (Htta = 2 - thenoyl - trifl uoroacetone, tmeda = tetrameth-
ylendiamine) as the Ni source [143] . The nanotubes are polycrystalline and have
the bunsenite structure of NiO. The length is around 1 μ m, with an outer diameter
of ∼ 200 nm with a wall thickness of 20 nm. The nickel – ammine complex
( Ni NH
3
2
()
+
x
) has also been used as a precursor to produce NiO nanotubes in an
AM [144] . These NiO nanotubes are about 60 μ m in length, with an outer diameter
of about 200 nm and a wall thickness of 60 nm. They are polycrystalline with a
face - centered cubic structure.
Synthesis of WO
3
nanotubes has been carried out by various methods, in par-
ticular starting from tungstic acid hydrate nanotubes without using any templates
[145] . WO
3
nanotubes are obtained on slow calcination at 450 ° C. The tungstic acid
hydrate nanotubes obtained from the solvothermal reaction have outer diameters
of 300 – 1000 nm and lengths of 2 – 20 μ m. The nanotubes have a nearly rectangular
pore of around 250 nm and the surface of the nanotube is not smooth. The poly-
crystalline nanotubes when calcined at 450 ° C for 3 h, retain the open ended
tubular structure and the overall dimensions of their precursor nanotubes. The
nanotubes have a triclinic structure. The calcined nanotubes are free - standing and
show no signs of aggregation. The annealed nanotube walls consist of individual
nanoparticles arranged one - dimensionally with numerous self - supported pores,
which are formed by the incomplete aggregation of nanoparticles. The sidewalls
of the nanotubes are, therefore, porous. Although the diameter of the nanoparti-
cles is approximately 40 – 80 nm, the nanotubes are 2 – 20 μ m long. HR - TEM images
of WO
3
nanotubes show that the lattice fringes of the nanocrystals have a spacing
of 0.309 nm, which corresponds to the interplanar distance of the (112) plane of
triclinic tungsten trioxide.
6.5.6
Nanotubes of other Binary Oxides
MgO nanotubes can be synthesized by introducing Sn as a catalyst in the CVD
process. The nanotubes are formed by the vapor – liquid – solid (VLS) mechanism
[146] . MgO nanotubes can also be formed by the thermal evaporation of a mixture
of Zn and Mg powders [147] . In
2
O
3
nanotubes are prepared by annealing InOOH
nanotubes [148] , having obtained the latter under solvothermal conditions starting
with InCl
3
in the presence of a surfactant and formamide in anhydrous ethanol.
In
2
O
3
tubular nanotubes are also generated by CVD starting with nanoporous
structures of InP [149] . The In
2
O
3
nanotubes are single crystalline with a cubic
structure and have outer diameters of around 200 – 300 nm and lengths of around
2 – 6 μ m and retain the size and square shape of the pores. Porous In
2
O
3
nanotubes
can also be prepared by layer - by - layer assembly/deposition on carbon nanotube
templates followed by calcination. The layer - by - layer assembly is used to form a
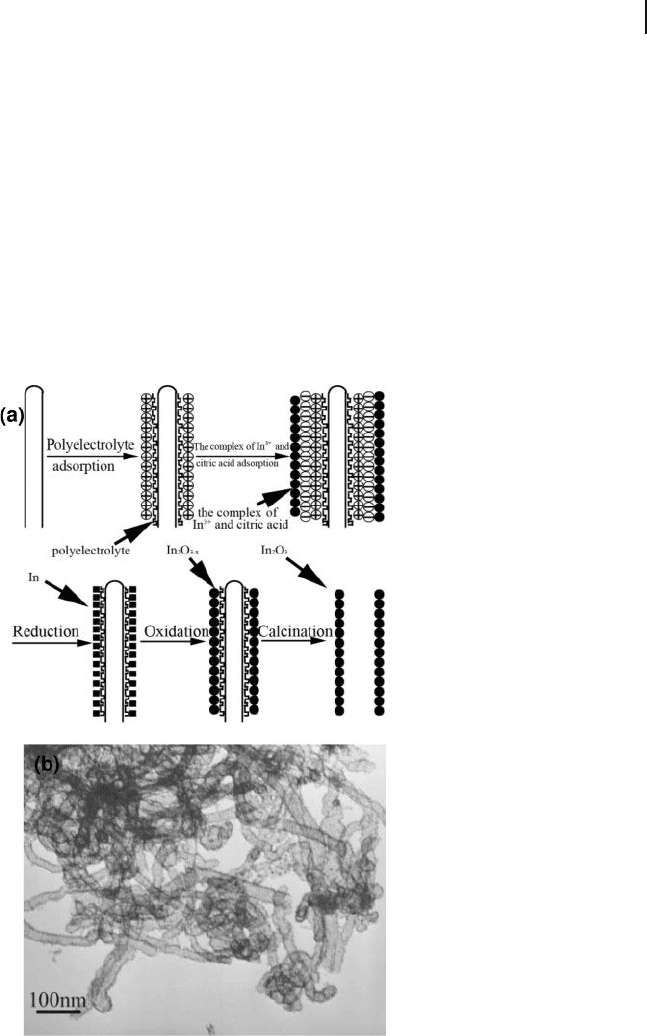
6.5 Metal Oxide Nanotubes 231
polyelectrolyte on the surface of pristine nanotubes, which enhances the adsorp-
tion of the metal - complex species on the surface of the carbon nanotube as a result
of electrostatic attraction between the charged species [150] . In a typical procedure,
the layer - by - layer assembly is formed with a polyelectrolyte such as sodium
poly(styrene sulfonate) (PSS) and poly(diallyldimethylammonium chloride)
(PDDA) on the surface of pristine carbon nanotubes. A solution mixture of InCl
3
and citric acid is added into the solution of the polyelectrolyte - modifi ed carbon
nanotubes. A solution of NaBH
4
is added into the above - mentioned solutionto
reduce In
3+
into indium, which is readily oxidized into In
2
O
3 −
x
because of the
oxygen dissolved in the solution from the surrounding ambient air. The porous
In
2
O
3
nanotubes are obtained by calcinations (Fig. 6.18 ). Thus, uniform, porous
and polycrystalline In
2
O
3
nanotubes with diameters of 30 – 60 nm can be formed
Figure 6.18 a) Schematic diagram for the growth process of
In
2
O
3
nanotubes. b) TEM image of regular In
2
O
3
nanotubes
prepared by the calcination of In
2
O
3
/polyelectrolyte/carbon
nanotube nanocomposites at 550 ° C in O
2
for 3 h. Reproduced
with permission from [150] . Copyright 2007, Wiley - VCH.

232 6 Synthesis of Inorganic Nanotubes
using layer - by - layer assembly on carbon nanotube templates, followed by calcina-
tion. In
2
O
3
nanotubes have a cubic structure and comprize nanoparticles of about
5 nm. The wall thickness is about 9 nm. This technique can also be used for the
preparation of nanotubes of other oxides such NiO, SnO
2
, Fe
2
O
3
, and CuO [150] .
Y
2
O
3
nanotubes are prepared by a non - aqueous electrochemical method involv-
ing oxide transfer to Y
III
precursors [151] . These horn - shaped nanotubes (nano-
horns) with a narrow size distribution coexist with small nodular deposits. The
horn - shaped structures are hollow. The diameter of these structures tapers from
approximately 900 nm at the base to less than 100 nm at the tip. The nanohorns
have lengths that range from 3.9 to 16.5 μ m. The thickness of the walls of the
cylinder is approximately 250 nm, and the inner diameter is approximately 400 nm.
These open cap structures have diameters similar to the nanohorn base diameters
and are commonly observed at the initial stages of growth. A hydrothermal pro-
cedure has also been employed starting with Y(OH)
3
nanotubes, followed by cal-
cination of the hydroxide [152] .
The one - pot synthesis of SnO
2
nanotubes has been accomplished at room tem-
perature starting from Sn nanorods [153] . The method involves the Kirkendall
effect. The SnO
2
nanotubes are polycrystalline, tetragonal with diameters that
range from 50 to 60 nm, and lengths from 300 to 500 nm. A 10 – 20 nm increase
in the diameter of SnO
2
nanotubes compared with that of the starting Sn nano-
rods is seen as a result of the outward fl ow of Sn during the oxidation. A solution
phase synthesis of SnO
2
nanotubes using surfactant - assisted micelles as templates
has been reported [154] . In this method, a mixture of SnCl
4
with PVP and dime-
thyl sulfoxide (DMSO) is refl uxed in the presence of Na
2
S. The nanotubes consist
of nanocrystalline particles that have a face - centered cubic structure, a diameter
of ∼ 200 nm, and lengths up to a few micrometers. Electrosynthesis of SnO
2
nano-
tubes employing the track - etched polymer polycarbonate membranes has been
carried out [155] . A gold electrode modifi ed with a porous polycarbonate mem-
brane is immersed in an aqueous tin chloride solution. Electrochemistry is
employed to control the local pH within the pores and drive the precipitation
reaction. Removal of the gold and dissolution of the polymer yields 1D polycrys-
talline tin oxide particles. The crystallinity of the material is enhanced by annealing
at 650 ° C. The particles are hollow, with a wall thickness of approximately 10 nm.
Nanotubes result from the continuous side - wall precipitation along a reaction
front, which are polycrystalline SnO
2
(rutile structure), with a diameter in the
100 nm range and a length between 0.4 and 1.4 μ m
2
. Nanotubular indium - tin
oxide (ITO) has been prepared by the sol – gel process using cellulose paper as a
template [156] .
ZrO
2
nanotube arrays with diameters of about 130 nm and lengths up to 190 μ m
are obtained by anodizing zirconium foil in a mixture of formamide and glycerol
that contains NH
4
F and 3 wt % water [157] . The as - prepared ZrO
2
nanotube arrays
are amorphous, with the coexistence of monoclinic and tetragonal phases when
annealed from 400 to 600 ° C. Monoclinic zirconia nanotubes were obtained at
800 ° C with retention of shape. By employing a sol – gel method and AM, yttria -
stabilized ZrO
2
nanotubes have been prepared [158] . The length and the diameter

6.5 Metal Oxide Nanotubes 233
of these nanotubes are 50 μ m and 200 nm, respectively, in agreement with the
dimensions of the template pores. The wall thickness of the nanotubes depends
on the impregnation time. The nanotubes after sintering at 800 ° C are polycrystal-
line with a cubic structure.
Formation of CeO
2
nanotubes has been reported by a few workers. CeO
2
nano-
tubes are produced by a two - step procedure involving precipitation at 100 ° C and
ageing at 0 ° C for 45 days [159] . CeO
2 −
x
nanotubes are crystalline, having a cubic
fl uorite structure. They tend to align the (111) planes parallel to the axis direction.
The diameter of the nanotubes ranges from 5 to 30 nm, while the length goes up
to several micrometers. The thickness of the wall of the nanotubes is about 5.5 nm.
CeO
2
nanotubes are also obtained by annealing layered Ce(OH)
3
nanotubes by a
simple oxidation – coordination – assisted dissolution process of the Ce(OH)
3
nano-
tubes/nanorods. One - dimensional structures of Ce(OH)
3
are synthesized by the
hydrothermal treatment of Ce
2
(SO
4
) · 9H
2
O with a 10 M NaOH solution at 130 ° C.
Starting from Ce(OH)
3
nanorods (as well as from narrow cavity nanotubes), it is
possible to obtain CeO
2
nanotubes with large cavities by a simple oxidation and
dissolution process [160] . These nanotubes with open ends have a cubic structure
and display an outer diameter of about 15 – 25 nm and a length of about 100 nm.
Nanoparticles of about 8 nm are attached to the walls and the thickness of the wall
is about 5 – 7 nm. The inner diameter of the nanotubes is about 10 – 15 nm. In this
method, freshly prepared 1D Ce(OH)
3
is exposed to air at room temperature for
24 h. The partially oxidized Ce(OH)
3
nanotubes with narrow cavities are dispersed
in distilled water and subjected to ultrasonication for 2 h after adding 15% H
2
O
2
.
Partial oxidation of the Ce(OH)
3
is essential to form the ceria tubular structures.
Electrosynthesis of CeO
2
nanotubes using AMs has been reported by employing
a non - aqueous electrolyte [161] . ThO
2
nanotubes are reported by the sol – gel
method by using a porous alumina template [162] .
6.5.7
Nanotubes of Titanates and other Complex Oxides
The hydrothermal method has been employed to synthesize nanotubes of monoc-
rystalline BaTiO
3
[163] , and tubular PbTiO
3
[164] . Pulsed laser ablation also yields
PbTiO
3
nanotubes within the pores of an AM [165] , while sol – gel electrophoretic
deposition of an acetic acid - based highly stabilized lead zirconate titanate (PZT)
sol in AMs gives rise to PZT nanotubes [166] . The PZT sol with a near - morpho-
tropic phase boundary composition and no polymeric addition was prepared using
lead acetate trihydrate, zirconium, and titanium tetra - butoxides. By anodization of
a Ti – Zr alloy, ZrTiO
3
nanotubes are obtained [167] .
Mallouk et al. [168] . and Peng et al. [169] . have reported the synthesis of niobate -
based nanotubes at low temperatures by the exfoliation of acid - exchanged K
4
Nb
6
O
17
with tetra( n - butyl)ammonium hydroxide (TBA
+
OH
−
) in aqueous solution. In the
presence of excess acid, around 80% of the potassium ions in K
4
Nb
6
O
17
are
replaced by protons. The remaining potassium ions appear to reside in the slowly
exchanging interlayer galleries that alternate along the stacking axis [168] . The

234 6 Synthesis of Inorganic Nanotubes
bilamellar colloid initially formed by reacting this material with TBA
+
OH
−
is
unstable relative to the formation of unilamellar sheets and tubules. Exfoliation
initially produces a bilayer colloid and then transforms (depending on conditions)
irreversibly into tubules, unilamellar sheets, or a mixture of the two. The concen-
tration of the colloid and the pH are important factors in controlling the
coiling equilibrium. Both H
+
and alkali ions help to precipitate the aggregated
tubules. The individual tubules are formed by the rolling of sheets of exfoliated
K
4 −
x
H
x
Nb
6
O
17
( x ≈ 3.2). The tubules are 0.1 – 1 μ m in length, with outer diameters
that range from 15 to 30 nm. The colloids and the precipitated tubules are both
highly stable.
Potassium hexaniobate nanotubes have also been fabricated from polycrystal-
line K
4
Nb
6
O
17
at room temperature using the intercalating and exfoliating methods
[169] . These are multilayer crystalline nanotubes with interlayer spacings from
0.83 to 3.6 nm, depending on the intercalating molecules such as tetra( n - butyl)
ammonium hydroxide (TBA
+
OH
−
) and alkylamines (C
n
H
2
n
+1
NH
2
). The number
of layers in the wall is in the range of 3 to 8. The outer diameter varies between
20 and 90 nm for the nanotubes obtained with different alkylamines, and the
length of the nanotubes ranges from a few hundred nanometers to several
micrometers. When a single - layer ( – Nb
6
O
17
– )
n
sheet rolls up into a nanotube,
C
n
H
2
n
+1
NH
2
( n ≠ 1) molecules are already adsorbed on both sides of the sheet
and then reside in the interlayer spaces of the nanotube. A model of the spiral
structural growth of these nanotubes has been proposed and the tube axis found
to be parallel to the [100] direction of the K
4
Nb
6
O
17
crystal. Spiral nanotubes of
potassium niobate are obtained by introduction of a polyfl uorinated cationic
azobenzene derivative, trans - [2 - (2, 2, 3, 3, 4, 4, 4 - heptafl uorobutylamino)ethyl]
{2 - [4 - (4 - hexylphenylazo)phenoxy]ethyl} dimethylammonium (abbreviated as C
3
F
7
-
Azo+), into the layered niobate interlayer by a two - step guest – guest exchange
method, with methyl viologen (MV
2+
) - K
4
Nb
6
O
17
as the precursor [170] . When
MV
2+
- intercalated niobate was used as the precursor, the polyfl uorinated C
3
F
7
-
Azo+ results in the quantitative formation of spiral nanotubes from exfoliated
nanosheets of the niobate, by rolling along the sandwiched microstructure. Nano-
tubes of FePO
4
have been prepared under solvothermal conditions in the pres-
ence of a sodium dodecyl sulfate (SDS) surfactant [171] . Iron phosphate nanotubes
have mesoporous walls and diameters of 50 – 400 nm and lengths of several
micrometers. The walls of the nanotubes range from 20 to 40 nm in thickness.
The removal of the surfactant by acetate exchange and heat treatment results
in amorphous mesoporous nanotubes of FePO
4
. Mesoporous NiPO
4
nanotubes
have been prepared in the presence of a cationic surfactant and different bases
by the sol – gel method [172] . The solution – liquid – solid (SLS) method has been
exploited to obtain tin - fi lled In(OH)
3
nanotubes using liquid droplets of an In – Sn
mixture [173] .
Nanotubes and how they are formed of other complex oxides reported are:
In
2
Ge
2
O
7
by thermal evaporation [174] , InVO
4
nanotubes using templates [175] ,
WO
3
– H
2
O nanotubes with the aid of intercalated polyaniline [176] , chrysotile
nanotubes by the hydrothermal method [177] , aluminogermanate nanotubes by a
simple solution procedure [178] , α - FeOOH nanotubes by employing reverse
