Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


6.5 Metal Oxide Nanotubes 215
newly formed Ga is in the form of small - sized liquid clusters. The Ga clusters
are transported by the Ar gas to the low temperature region, where they deposit
as liquid droplets on the inner walls of the graphite crucible. The Ga droplets
are the favored sites for the adsorption of ZnS vapor generated in the system.
On supersaturation, ZnS segregates and gives rise to ZnS nanowires. With an
increase in temperature, the decomposition of the SiO results in the formation
of Si vapor, which is oxidized by the residual O
2
to SiO
2
. This is transported by
the Ar gas and is deposited on the surface of the ZnS nanowires, which results
in ZnS/SiO
2
hetero - nanowires. Because of the high temperature, the inner ZnS
nanowires evaporate, and hollow SiO
2
nanotubes remain. This method can also
be used for nanotubes of other inorganic nanomaterials such as ZnS and GaN.
CdSe nanocrystals are used as seeds in a one - step thermal evaporation process
to prepare long, high density SiO
2
nanotubes [79] . SiO
2
nanotubes generally have
smooth surfaces, uniform thickness, and round cross sections of both the interiors
and exteriors which are amorphous in nature. The nanotubes are open - ended
and have diameters of about 80 – 110 nm and a length of several hundreds of
micrometers. High purity SiO
2
nanotubes are obtained by using track - etched
membrane templates that involve O
2
plasma treatment. Oxygen plasma pre -
treatment ensures pore - fi lling of the precursor solution and covalent bonding
between template and precursor, while pyrolysis of the template – nanostructure
composite completely removes organics and produces inorganic nanostructures
[80] . These nanotubes are also amorphous and have diameters of 100 nm and
lengths of 2 – 6 micrometers.
Hybrid SiO
2
nanotubes with walls that contain chiral aromatic rings are obtained
by using self - assemblies of appropriate amphiphiles [81] . Mesoporous SiO
2
nano-
tubes with helical channels have been prepared by the self - assembly of surfactants
in the presence of chiral molecules [82] . These are formed by the self - assembly of
the achiral surfactant sodium dodecyl sulfate (SDS) in the presence of ( R ) - (+) - and
( S ) - ( − ) - 2 - amino - 3 - phenylpropan - 1 - ol (APP) (Fig. 6.10 ). TEM combined with com-
puter simulations confi rm the presence of ordered chiral channels winding around
the central axis of the tubes with an inner diameter of 100 nm.
Double - walled silica nanotubes have been obtained by biomimetic synthesis
under mild conditions. Pouget et al. [83] . use the peptide lanreotide in which the
silica phase and the lanreotide nanotube grow synergistically in a concerted
manner by mutually neutralizing their charges (positive on the lanreotide and
negative on on the silica) (Fig. 6.11 ). This requires kinetic coupling of the two
chemical processes. The presence of an X - ray diffraction peak at 0.35 Å
− 1
, charac-
teristic of the β - sheet organization in the lanreotide wall surface, gives a mean
diameter of 24.6 nm. The walls of the tubes are separated by 2 nm, which corre-
sponds to the thickness of the lanreotide tube. The walls of the silica tubes are
thin (1.4 nm) with lengths up to 3 μ m, in agreement with the TEM data shown in
Figure 6.11 . Calcination at 600 ° C converts hybrid organic – inorganic nanostruc-
tures into pure silica double - walled nanotubes. A dynamic template mechanism
can explain these results. Silica deposition occurs on both sides of the lanreotide
molecule, and stops immediately after neutralization of the surface charge. This
216 6 Synthesis of Inorganic Nanotubes
is a well - controlled process and yields a hierarchical structure from the nanometer -
scale to the macroscopic level. The double - walled silica tubes form bundles 1 – 3 μ m
in length, and bundles of closely packed aligned nanotubes as much as a centi-
metre long. Shape - coded SiO
2
nanotubes are obtained by using porous alumina
along with the sol – gel method [84] . The template synthesis of shape - coded nano-
tubes begins with the fabrication of a porous alumina fi lm that contains well -
defi ned cylindrical pores with two or more different diameter segments created
by multistep anodization of the aluminum substrate. The nanotubes are fabricated
with a surface sol – gel method that controls the wall thickness at a single - nanom-
eter level. Amorphous silica nanotubes seeded by copper sulfi de nanoparticles
have been synthesized by using a supercritical organic solvent [85] . Addition of
copper sulfi de nanocrystals, monophenylsilane, and small amounts of water and
oxygen to supercritical toluene at 500 ° C at 10.3 MPa yields silica nanotubes.
6.5.2
T i O
2
Nanotubes
Amongst the nanotubes of various metal oxides, those of TiO
2
have been investi-
gated most widely. TiO
2
nanotubes can be prepared hydrothermally, by anodiza-
tion of titanium by template - assisted growth as well as seeded growth. Porous
alumina templates are specially useful for fabricating dense, uniform, aligned
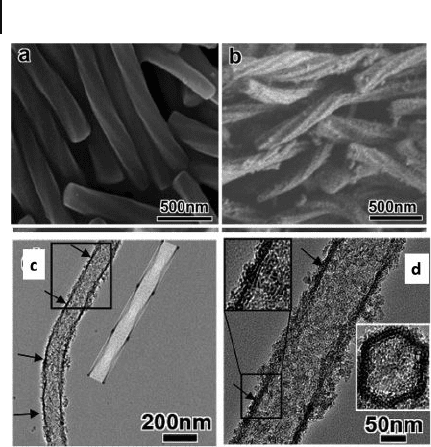
Figure 6.10 a,b) SEM images of calcined
silica nanotubes with chiral mesoporous wall
structure synthesized with different ( R ) - (+) -
APP/SDS molar ratios of 0 and 0.2,
respectively. These materials were synthesized
at 30 ° C for 6 h and then allowed to age for 1
day at 90 ° C. c) Low and d) high magnifi cation
TEM images of calcined samples. Reproduced
with permission from [82] . Copyright 2007,
ACS.
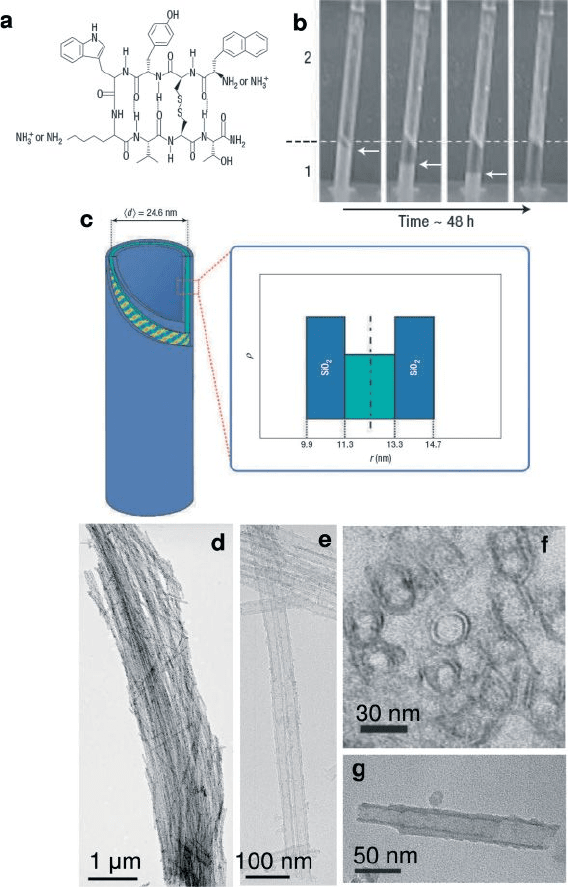
Figure 6.11 Silica mineralization of lanreotide:
a) Structure of the lanreotide octapeptide
showing the two charged amine sites. b)
Time - lapsed pictures of the capillaries during
the mineralization process. Initially, a volume
of lanreotide gel at 5% (w/w) poured into the
bottom of the capillary (region 1) and is
covered with the same volume of a 30%
(w/w) TEOS/water mixture (region 2) (tube
1). On ageing, the lanreotide turbid gel
recedes (white arrows), to yield a transparent
region, while long white fi bres appear in
region 2, starting from the interface (tubes 2
and 3). After 48 h, the lanreotide gel has
completely disappeared and only two phases
separated by a white ring are observed: the
lower clear one containing a dilute lanreotide
solution and the upper one fi lled with
mineralized fi bres (tube 4). c) Schematic
representation of a multi - scale organization of
a silica – lanreotide nanotube with an inner and
an outer 1.4 nm thick silica shell and a central
2.0 nm thick lanreotide tube as deduced from
radial density ( ρ ) profi les. d) TEM image of
double - walled silica nanotube replica obtained
after the calcination of silica – lanreotide
nanotubes. e) TEM image of a 7 μ m long
bundle of dried mineralized nanotubes.
f) Cross - sectional TEM image of dried fi bres,
revealing several concentric circles attributable
to the double - wall structure. g) TEM image of
a fragment of dried nanotube, showing that
the internal and external cylinders are
independent and free to slide. Reproduced
with permission from [83] . Copyright 2007,
Nature Publishing Group.

218 6 Synthesis of Inorganic Nanotubes
arrays of TiO
2
nanotubes on substrates such as glass, silicon, and polymers. Free -
standing porous alumina templates have been employed for atomic layer deposi-
tion (ALD) of ordered TiO
2
nanotube arrays on various substrates (Fig. 6.12 ) [86] .
The diameter and length of the nanotubes, as well as the distance between two
neighboring nanotubes, can be controlled by varying the dimensions of the tem-
plate and the anodization conditions. Typically, hexagonally packed pores with a
diameter of ∼ 65 nm and interpore distance of ∼ 110 nm are used. The synthesis of
highly ordered arrays of TiO
2
nanotubes by potentiostatic anodization of Ti has
been reviewed by Grimes [87] . This appears to be an excellent method wherein
anodic oxidation is carried out in a dimethyl sulphoxide (DMSO) medium that
contains hydrofl uoric acid, potassium fl uoride, or ammonium fl uoride as the
electrolyte [88] . Self - aligned, hexagonally close packed TiO
2
nanotube arrays,
1000 μ m in length with high aspect ratios ( ∼ 10 000) are obtained by the anodization
of titanium. These are polycrystalline in the anatase structure after annealing in
oxygen at 280 ° C for 1 h. Such nanotubes can be transformed into self - standing
membranes [89] .
The formation of TiO
2
membranes by the anodization of Ti foil in fl uorine -
containing ethylene glycol has been described [90] . This method yields self - organ-
ized, free - standing TiO
2
nanotube arrays with ultra - high aspect ratio of the
diameter/length ( ∼ 1500) by simply using solvent - evaporation - induced delamina-
tion of the TiO
2
barrier layer formed between the TiO
2
membrane and Ti foil
during anodization. The resulting membrane consists of highly ordered, vertically
aligned, one - side open TiO
2
nanotube arrays with pore diameter, wall thickness,
and length of around 90 nm, 15 nm, and 135 μ m, respectively. The as - grown TiO
2
nanotubes are amorphous and transform into the anatase structure after anneal-
ing at high temperature in air. Aligned TiO
2
nanotubes with novel morphologies,
such as bamboo - type reinforced nanotubes and 2D nanolace sheets, obtained by
an anodization process carried out under alternating - voltage conditions in fl uo-
ride - containing electrolytes, have been reported [91] . The experiment was carried
out under constant voltage conditions, and after 2 h of anodization at 120 V in an
electrolyte that consists of 0.2 mol L
− 1
HF in ethylene glycol, yielded a regular layer
of aligned, individual TiO
2
nanotubes with thickness of about 10 μ m and diameter
of 150 nm. If the voltage is lowered to 40 V, the growth of the nanotubes slows
down and may even stop. A bamboo - type structure can be grown under certain
conditions (i.e., when the voltage is alternated between 120 and 40 V). The spacing
between the bamboo rings can be altered by means of changing the time for which
the sample is held at 120 V, and spacing is reduced from 200 to 70 nm by reducing
the holding time. If anodization takes place for a long time at a low voltage, nano-
tubular features with a reduced diameter start to grow. This can be exploited to
grow a double - layer structure. In this case, branching of the main tube with a
diameter of 150 nm into several (typically 2 – 3) smaller tubes of about 50 nm in
diameter occurs. The structures can be transformed to the anatase structure
without losing structural integrity by annealing in air at 450 ° C. Anodization under
constant - voltage conditions leads to an ordered layer that consists of smooth tubes
with a defi ned cylindrical or hexagonal cross section [92, 93] . Fluoride - free aqueous
6.5 Metal Oxide Nanotubes 219
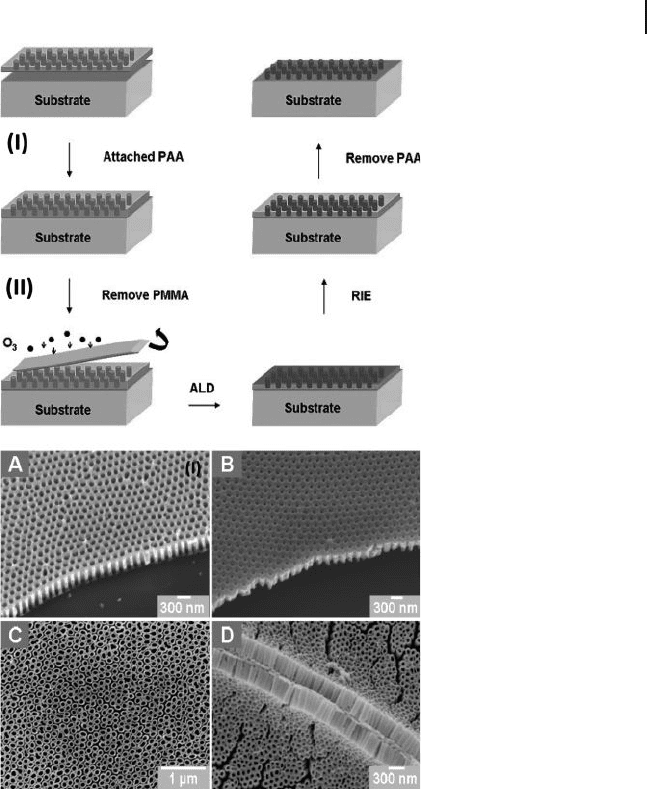
Figure 6.12 I) Schematic of the process to
fabricate highly ordered TiO
2
nanotube arrays
on substrates using ALD on a free - standing
porous alumina template. Free - standing AM
supported with a poly(methyl methacrylate)
(PMMA) layer was fi rst attached onto the
substrate. The PMMA was then removed for
ALD on the AM template. Substrates were
alternatively exposed to TiCl
4
and water vapor
for ALD at a pressure of 1 × 1 0
− 3
Torr. The
TiO
2
overlayer was etched by RIE, and fi nally
highly ordered ALD TiO
2
nanotube arrays were
released from the AM template. II) Dense,
uniform, highly ordered, and well - aligned ALD
TiO
2
nanotube arrays on Si and a fl exible
polyimide fi lm. SEM images of a highly
ordered free - standing AM template ( ∼ 300 nm)
attached on Si before (A) and after (B)
150 - cycle TiO
2
ALD. C) Highly ordered TiO
2
nanotube arrays released from the template
on the Si substrate. D) Cross - section of
well - aligned TiO
2
nanotube arrays on a
bending polyimide fi lm. Reproduced with
permission from [86] . Copyright 2008, ACS.

220 6 Synthesis of Inorganic Nanotubes
HCl electrolyte is also used to obtain vertically oriented TiO
2
nanotube arrays [94] .
These nanotube arrays, obtained by using a 3
M HCl aqueous electrolyte with an
anodization potential of 20 V, have an inner pore diameter of 15 nm, wall thickness
of 10 nm, and length up to 600 nm. The nanotubes are polycrystalline and have an
anatase structure, with a rutile barrier layer separating the tubes from the underly-
ing metal foil.
Sulfur - doped TiO
2
nanotubular arrays are obtained by potentiostatic anodization
of Ti foils followed by the annealing of TiO
2
tubular arrays in a fl ow of H
2
S at
380 ° C [95] . Ordered, vertically oriented B - doped TiO
2
nanotube arrays are prepared
by forming a nanotube - like TiO
2
fi lm in the anodization process on a Ti sheet
followed by CVD treatment with trimethylborate vapor [96] . A double - template -
assisted sol – gel method has been used to prepare TiO
2
nanotube arrays with nano-
pores on their walls [97] . In this method, poly(ethylene glycol) dissolved in a TiO
2
sol is used as a soft template to form nanopores on the walls of TiO
2
nanotube
arrays, which were templated from ZnO nanorod hard templates by the dip -
coating technique. The microstructure of nanoporous TiO
2
nanotube arrays can
change from end - opened to end - closed by increasing the number of dip - coating
cycles.
Gas - phase ALD of metal oxides in combination with a micro - contact printing
( μ - CP) technique has been used to attain precise atomic - level control over the
dimensions (wall thickness) of nanotubes as well as the one - step fabrication of the
free - standing oxide nanotubes [98] . In this procedure, octadecyltrichlorosilane
(OTS) molecules were transferred onto both sides of the surfaces of the template
by the μ - CP technique and self - assembled monolayers (SAMs) formed on the
surfaces, thus exposing chemically inert methyl surfaces. The ALD process allows
atomic - level control over the thickness of the wall of the nanotubes, and the OTS
self - assembled mono - layers function as resistant layers to materials deposition,
thus allowing free - standing cylindrical nanotubes to be collected after dissolution
of the template without an additional polishing step. This method has been used
to obtain high aspect ratio ( ∼ 300) nanotubes of TiO
2
, ZrO
2
, and Al
2
O
3
. Crystalline
and homogeneous CeO
2
naoparticles have been used as seeds to grow TiO
2
nano-
tubes from the hydrolysis of aqueous titanium sulfate solution [99] . Layer - by - layer
deposition of a water - soluble titania precursor (titanium( iv ) bis(ammonium
lactato) dihydroxide, along with the oppositely charged poly(ethylenimine) gives
rise to multilayer fi lms. The tubular structure is obtained by depositing inside the
cylindrical pores of a polycarbonate membrane followed by calcination [100] .
Nanotubular TiO
2
can be obtained by using uncharged or negatively charged l -
lysine - based organic gelators as templates [101] . By heating nanotubes of titanic
acid (obtained by heating P25 - TiO
2
(Degussa) with an NaOH solution at 110 ° C)
in ammonia, N - doped TiO
2
nanotubes with an anatase structure are obtained [102] .
The use of carbon nanotubes to obtain oxide nanotubes is well documented [103,
104] . This method has been used to obtain pure rutile nanotubes [105] . The
method involves coating of the carbon nanotubes with amorphous titania through
a sol – gel process followed by heating in air to convert titania into anatase. Further
heating in nitrogen transforms the anatase predominantly to rutile and fi nally
6.5 Metal Oxide Nanotubes 221
heating in air again at a higher temperature removes the carbon nanotubes and
transforms the remaining anatase to rutile. Brookite - type TiO
2
nanotubes are
obtained by heating titanate nanotubes obtained by the reaction of TiO
2
powder
with NaOH solution, followed by hydrothermal treatment [106] . Transparent thin
fi lms of titanate nanotube arrays with super - hydrophilic characteristics are grown
on sapphire substrates by the hydrothermal reaction of sputter - deposited Ti fi lms
in an aqueous NaOH solution [107] .
6.5.3
Z n O , C d O , and A l
2
O
3
Nanotubes
ZnO nanotubes have been prepared by CVD and thermal evaporation, as well as
by hydrothermal and solution methods. For example, large - scale ZnO nanotube
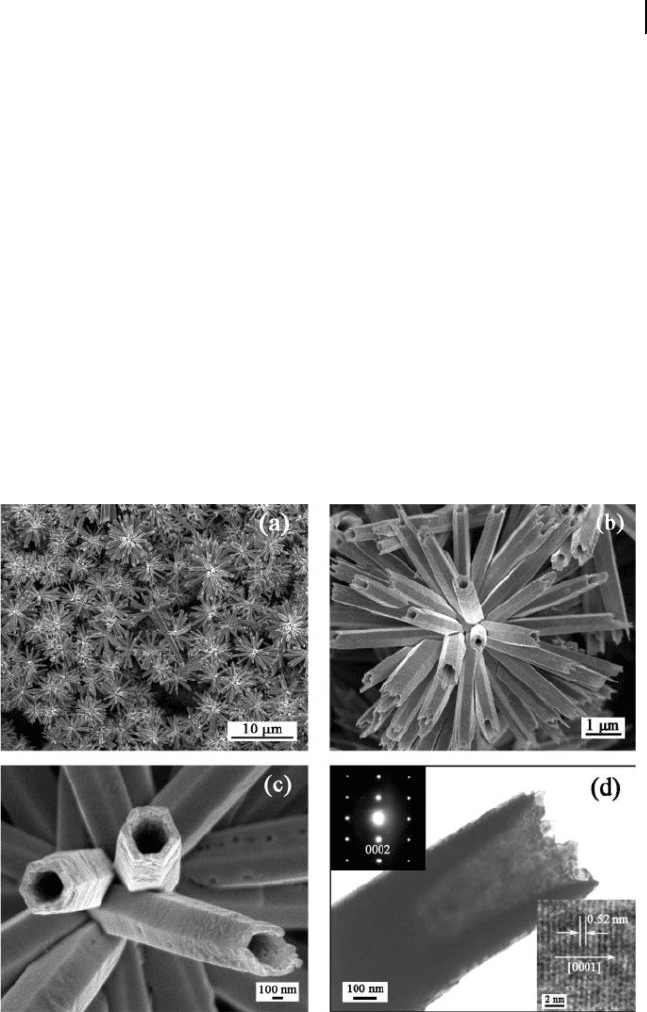
bundles have been synthesized by a simple wet chemical approach (Fig. 6.13 ) [108] .
In this method, an aqueous solution of Zn(NO
3
)
2
and hexamethylenetetramine is
stirred over long periods and heated at 90 ° C. This method yields nanotubes with
an inner diameter of ∼ 350 nm and a wall thickness of ∼ 60 nm, and forms radiating
Figure 6.13 Morphological and structural
characterization of ZnO nanotube bundles:
a,b) Low - magnifi cation FE - SEM images.
c) High - magnifi cation FE - SEM image. d) TEM
image. The upper left and lower right inset
images of (d) are the SAED pattern and the
high - resolution transmission electron
microscopy (HRTEM) image of a single
nanotube, respectively. Reproduced with
permission from [108] . Copyright 2007, ACS.

222 6 Synthesis of Inorganic Nanotubes
structures. These ZnO nanotubes are single crystalline in nature, having the wur-
tzite structure, and preferentially grow along the [0001] direction. High aspect ratio
nanotubes are obtained by a three - step low temperature process that involves ionic
layer absorption, deposition of the ZnO seed layer, followed by hydrothermal
annealing of the seed layer and deposition of the 1D ZnO nanostructures [109] .
The uniform ZnO nanotubes have a single - crystalline wurtzite structure with
lengths that exceed 10 μ m and diameters of around 27 nm. By hydrothermal
annealing, ZnO nanotubes grown along the < 001 > direction have been obtained
[109] . Nanotube - based paint - brush structures of ZnO have been prepared on Zn
foils by solvothermal means by adjusting the pH of the solution [110] .
Electrochemical deposition from aqueous solution into porous alumina mem-
branes can be employed to prepare ZnO nanotube arrays [111] . Single - crystalline
ZnO nanotube arrays with an hexagonal wurtzite structure have been generated
on glass substrates by a two - step solution approach [112] . The method involves the
electrodeposition of oriented ZnO nanorods and subsequent coordination - assisted
selective dissolution along the c - axis to form tubular structures caused by the
preferential adsorption of ethylenediamine (EDA) and OH
−
on different crystal
faces. After dissolution in aqueous EDA solution for 10 – 15 h, the inner/outer wall
surfaces of the ZnO nanotubes become smooth with a wall thickness of
∼ 10 – 30 nm.
Reaction of Zn(NO
3
)
2
with methenamine in aqueous medium under hydrother-
mal conditions gives rise to ZnO nanotubes [113] . These nanotubes are hollow
with rough surfaces, which indicates a layer - stack structure. The ZnO nanotubes
have an hexagonal wurtzite structure with lengths in the range of 1 – 3 μ m and wall
thickness in the 50 – 100 nm range. The cross section is hexagonally faceted, which
provides strong evidence that the single nanotube grows along the c - axis direction.
ZnO nanotube structures have been obtained by the hydrothermal self - assembly
of zinc ions at the interface of surfactants, such as cetyltrimethylammonium
bromide (CTAB) and the triblock copolymer of poly(ethylene oxide) – poly(propylene
oxide) – poly(ethylene oxide) (P123) [114] . These molecules are suitable for the
assembly of layered zinc species – surfactant hybrids that can be exfoliated into
intermediate single sheets that roll to form tubular ZnO nanostructures because
of the heat stress and the crystallization of ZnO sheets. The size of the tubular
ZnO is determined by the type of surfactants, the concentration of the surfactant,
and the Zn species/surfactant molar ratio. Tubular products can be obtained at a
relatively high Zn
2+
/surfactant molar ratio as the concentrations of CTAB and P123
are higher than their critical micelle concentrations. The hydrophobic side of each
surfactant layer is connected through a weak bond, which gives rise to an organic
layer, while the hydrophilic side of each surfactant layer adsorbs metal ions or
hydrates, to form an inorganic layer. Dehydration and crystallization of amorphous
inorganic layers cause the volume to shrink and results in stress in the inorganic
layers, and breaks the equilibrium between the organic and the inorganic layers.
Crystallography of these ZnO nanotubes is determined by how the nanosheets
exfoliate and roll. The large cylindrical ZnO nanotubes have diameters ranging
from 200 to 400 nm (wall thickness, 80 nm) with lengths going up to 14 μ m. The

6.5 Metal Oxide Nanotubes 223
nanotubes are single - crystalline and possess a wurtzite structure. With an increase
in the amount of Zn(CH
3
COO)
2
· 2H
2
O, middle - size ZnO nanotubes with diame-
ters in the range from 40 to 70 nm and lengths up to 550 nm are obtained. The
inner diameter of these nanotubes varies from 14 to 30 nm.
ZnO nanotubes with nanometer - scaled holes on the side - walls are obtained by
a low - temperature hydrothermal procedure based on a preferential etching strat-
egy [115] . The procedure is as follows. By a two - step heating of a solution mixture
of ZnCl
2
and ammonia hydrothermally, nanotubes of nearly homogeneous size
with ∼ 250 nm diameter, 40 nm wall thickness, and 500 nm length are formed. The
fi rst stage involves the transformation of the precursor Zn NH
3
4
2
()
+
to ZnO
through hydrothermal decomposition at 95 ° C, which leads to the formation of
single crystal ZnO nanorods. At the same time, ZnO dissolves as the equilibrium
moves to the left. The growth of ZnO rods becomes dominant since the high
concentration of Zn NH
3
4
2
()
+
favors the precipitation of ZnO. As the temperature
goes down (95 to 75 ° C), along with the partial consumption of the Zn NH
3
4
2
()
+
,
etching of the ZnO nanorods occurs. Formation of porous ZnO nanotubes with
nanoholes, which are single crystalline with a wurtzite structure, result from the
preferential etching along the c - axis and slow etching along the radial directions.
Nanoholes are created on the side - walls of the tubular structure. ZnO nanotubes
have been assembled into microsphere superstructures by employing a mixture
of poly(ethylene glycol) (PEG), ethanol, and water [116] . Addition of metallic zinc
species into the solution mixture leads to aggregation of the PEG polymer coils to
Zn
II
/PEG globules with a diameter of ∼ 500 nm. The globules turn into tube - like -
structured ZnO – PEG microsphere assemblies ( ∼ 2 mm) after ultrasonic
pretreatment.
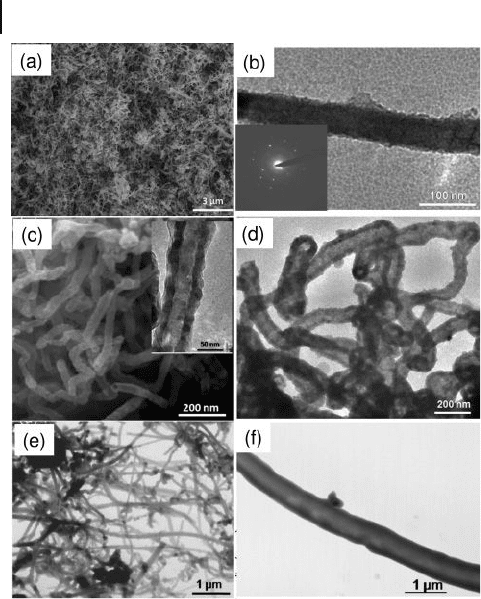
ZnO nanotubes are also produced by the oxidation of Zn nanowires because of
the Kirkendall effect. This effect has also been used to produce nanotubes of SiO
2
,
Co
3
O
4
, and ZnCr
2
O
4
as well as of ZnS, CdS, and CdSe (Fig. 6.14 ) [117] . It is
observed that there is a distribution in the diameters of the nanotubes just as in
the case of the diameters of the starting nanowires. The Zn nanowires possess a
smooth surface with an average diameter of 50 nm and lengths of several tens of
micrometers with a zig - zag morphology. The ZnO nanotubes formed from the
thermal oxidation of the Zn nanowires have an outer diameter that goes up to
90 nm with lengths that vary from 400 nm to a few micrometers. The ZnO nano-
tubes have a wall thickness of around 20 nm and an inner diameter close to the
diameter of the starting metal nanowires.
CdO nanotubes with a mean diameter of 50 nm have been obtained by the
thermal evaporation of Cd powder without using any catalyst or template [118] .
The CdO nanotubes are single - crystalline with a cubic structure and have diam-
eters of around 40 – 65 nm, a wall thickness of around 15 nm, and lengths of over
a few tens of micrometers. Some of the nanotubes appear straight in morphology,
while others are twisted with some straight parts.
Well - defi ned uniform Al
2
O
3
nanotubes are obtained by the pulse anodization of
aluminium in H
2
SO
4
solution [119] . Periodic galvanic pulses were employed to
achieve mild and hard anodization (HA) conditions, where the pulse duration for
224 6 Synthesis of Inorganic Nanotubes
HA determines the length of nanotubes. By properly choosing the pulse param-
eters, continuous tailoring of the pore structure of the resulting nanoporous
anodic alumina (i.e., periodic modulation of pore diameters along the pore axis)
is achieved. This also enables weakening of the junction strength between cells,
thereby helping in the separation of individual alumina nanotubes from the
porous anodic alumina. The average inner and outer diameter of the nanotubes
is estimated to be 80 and 95 nm, respectively, with lengths of up to several tens of
micrometers. The inner diameter of the alumina nanotubes can be controlled by
varying the etching time of the pore walls by using an appropriate etching solution
(e.g., H
3
PO
4
). Aluminium oxide nanotubes have been fabricated by using tris(8 -
hydroxyquinoline) gallium organic nanowires (GaQ
3
) as soft templates to coat
alumina using ALD [120] . By dissolving the template in toluene or by heat treat-
ment, alumina nanotubes are prepared, where the alumina shell thickness is
controlled by the number of precursor/purge cycles (Fig. 6.15 ).
Figure 6.14 Nanowire – nanotube
transformation as a result of the Kirkendall
effect: a) Low - magnifi cation FESEM images of
Zn nanowires. b) TEM image of a Zn
nanowire (the inset is the SAED pattern).
c) FESEM image of ZnO nanotubes (inset
shows the TEM image of a ZnO nanotube).
d) TEM image of ZnS nanotubes. e,f)
Scanning transmission electron microscope
images of Si nanowires and SiO
2
nanotubes,
respectively. Reproduced with permission
from [117] . Copyright 2008, ACS.
