Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


Synthesis of Inorganic Nanotubes
C.N.R. Rao and Achutharao Govindaraj
195
Advanced Nanomaterials. Edited by Kurt E. Geckeler and Hiroyuki Nishide
Copyright © 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
ISBN: 978-3-527-31794-3
6
6.1
Introduction
Zero - dimensional nanoparticles and one - dimensional (1D) nanowires and nano-
tubes are important classes of nanomaterials [1, 2] . The fi rst family of nanotubes
is that of carbon nanotubes described by Iijima [3] . Nanotubes are, however, no
longer confi ned to carbon but encompass a variety of inorganic materials [4, 5] ,
and peptides [6] . In this article, our concern is with nanotubes of inorganic materi-
als excluding carbon.
The early examples of inorganic nanotubes synthesized in the laboratory are
those of molybdenum and tungsten sulfi des by Tenne and coworkers [7] . These
layered sulfi des form fullerene - type structures and hence also nanotubes. Several
methods to prepare nanotubes of Mo and W sulfi des and of the analogous
selenides have been reported in the last few years [1, 2] . The synthesis of BN
nanotubes has also received considerable attention because of the similarity of
the structure of BN to graphite. In the last few years, nanotubes of several
inorganic materials including binary oxides, nitrides, halides as well as metals
and non - metallic elemental materials have been prepared and characterized [1,
2, 4, 5] . Besides nanotubes of binary compounds, those of complex materials
such as perovskite titanates and spinels have also been reported. Composites
that involve nanotubes, nanowires, and nanoparticles are also known. In this
article, we shall provide a status report on the synthesis of inorganic nanotubes.
In doing so, we shall cover the synthetic strategies employed for the different
classes of inorganic nanotubes. In view of the vast literature that has emerged
in the last 2 – 3 years, we were unable to cite all the papers in this area and
have restricted ourselves to representative ones. We apologize for any oversight
or error in judgement.

196 6 Synthesis of Inorganic Nanotubes
6.2
General Synthetic Strategies
Several strategies have been employed for the synthesis of inorganic nanotubes.
In the case of molybdenum and tungsten sulfi des and such layered chalcogenides,
decomposition of precursor compounds such as the trisulfi des (e.g., MoS
3
or WS
3
)
and ammonium thiometallate or selenometallate has been successful. An impor-
tant method, used particularly in the case of oxide nanotubes, is the hydrothermal
and solvothermal route, carried out in the presence of surfactants or other addi-
tives in certain instances. Electric arcing and laser ablation have been used to
synthesize nanotubes of BN and other materials. Sol – gel chemistry is useful for
the synthesis of nanotubes, especially of oxides. Chemical vapor deposition (CVD)
is commonly used for the synthesis of some of the nanotubes.
A popular method of synthesis in the last few years has employed templates.
The templates can be porous membranes of alumina or polycarbonate. The pores
are used to deposit the relevant materials or these precursors, followed by anneal-
ing and removal of the template. Deposition of the material in the porous channels
is carried out by the sol – gel method or by an electrochemical procedure. Electro-
chemical anodization is commonly used for the synthesis of nanotubes of TiO
2
,
ZnO, and such oxides. The porous membrane method has emerged to be a general
means of preparing a large variety of inorganic nanotubes and nanowires. Carbon
nanotubes, surfactants, polymer gels, and liquid crystals have all been used as
templates, wherein the precursor material is covered over the templates, followed
by annealing and removal (burning or dissolution) of the template. In what follows,
we shall discuss the synthesis of various inorganic nanomaterials where we will
indicate the method and give the most essential aspects of the procedure. In order
for the reader to obtain greater details, we have provided a large list of
references.
6.3
Nanotubes of Metals and other Elemental Materials
Synthesis of gold nanotubes was reported by Martin and co - workers [8, 9] in the
1990s. They prepared Au tubules with lengths of up to a few micrometers and
diameters of a few hundred nanometers by electrochemically depositing gold into
the pores of a microporous alumina membrane (AM). To obtain gold tubules,
initially the AM pore walls were chemically derivatized by attaching a molecular
anchor such as a cyanosilane, so that the electrodeposited metal preferentially
deposits on the pore wall, which leads to tubule formation.
Electrodeposition in membrane pores is an important method for the synthesis
of metal nanotubes. Thus, Au nanotube arrays have been prepared by direct elec-
trodeposition in the nanochannels of alumina templates. The nanochannel
alumina templates with pore diameters of about 105 nm and 45 nm were used to
synthesize nanotubes with average outer diameters respectively of 105 nm and

6.3 Nanotubes of Metals and other Elemental Materials 197
45 nm and a wall thickness of 15 nm. The lengths went up to several micrometers.
The alumina templates are readily removed by treatment with NaOH. The nano-
tube arrays so obtained have a well - controlled microstructure and are polycrystal-
line with a face - centered cubic structure [10] . Au nanotubes have also been
prepared by electroless deposition in the pores of track - etched polycarbonate mem-
branes that contain 10 μ m thick and 220 nm diameter pores [11] . The inner sur-
faces of the polycarbonate membranes were fi rst sensitized with a Sn
2+
salt and
then activated by forming a layer of Ag, before depositing Au for a period of 2 h.
The gold nanotubes were cleaned with 25% HNO
3
solution for 15 h. Hydrophobic
or hydrophilic self - assembled monolayers on gold nanotubes can be formed by
rinsing the samples in ethanol for 20 min, followed by immersion in a solution of
ethanol that contains HS(CH
2
)
15
CH
3
or HS(CH
2
)
15
COOH. The Au nanotubules
are polycrystalline and have lengths of up to 6 μ m and inner diameters of ∼ 1 nm.
By controlling the Au deposition time, Au nanotubules of effective inside diam-
eters of molecular dimensions ( < 1 nm) can be prepared. They found electroless
deposition allows for more uniform gold deposition in a short duration of time.
Since the electroless plating method used to deposit Au nanotubes in polymeric
templates does not work in AMs, Martin and coworkers [12] have developed a
modifi ed electroless plating strategy that can be used to deposit high - quality Au
nanotubes within the pores of alumina templates.
Three - dimensional (3D) Au nanotube arrays with smooth as well as nanoporous
walls have been obtained by using anodic alumina and conducting polyaniline
nanorod templates [13] . In this procedure, polyaniline nanorods were predeposited
electrochemically in the interior of a porous alumina membrane, which was then
used as a template for the formation of vertically aligned Au nanotubes. For the
synthesis of Au nanotube arrays with nanoporous walls, gold/silver alloy nanow-
alls were electrodeposited from cyanide solutions that contain gold/silver ions
(mole ratio, Au
+
/Ag
+
= 1 : 3). The nanoporous walls were generated by de - alloying
(selective dissolution of the less noble metal) the gold/silver alloy shells with con-
centrated nitric acid, which also dissolves the polyaniline nanorods. The porous
architecture is formed because of an intrinsic dynamic pattern formation process,
in which the more noble metal (Au) atoms tend to aggregate into two - dimensional
clusters through a phase separation process at the solid – acid interface. The length,
average inner diameter, and wall thickness of the Au nanotubes with smooth walls
was ∼ 4 μ m, ∼ 196 nm, and 62 ( ± 18 nm), respectively. The Au nanotubes with
smooth nanoporous walls (nanopore diameter of ∼ 8 nm) had similar physical
dimensions as the nanotubes.
Gold nanotubes embedded within the pores of the polycarbonate template mem-
branes were subjected to reactive ion etching (RIE) using an oxygen plasma to
selectively etch approximately 2.3 mm of the polycarbonate, leaving the polycrystal-
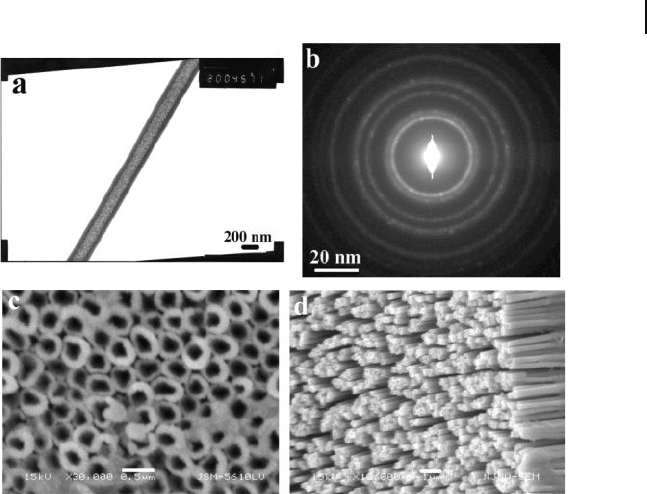
line gold nanotubes intact. Figure 6.1 shows fi eld - emission scanning electron
microscopy (FESEM) images of the top surface of a template membrane after
electroless deposition of gold followed by RIE [14] . Single crystalline and bamboo -
like Au nanotube arrays growing in the [111] direction, with a diameter of 100 –
150 nm and a length of 10 μ m, and standing perpendicular to the Ti metal foil
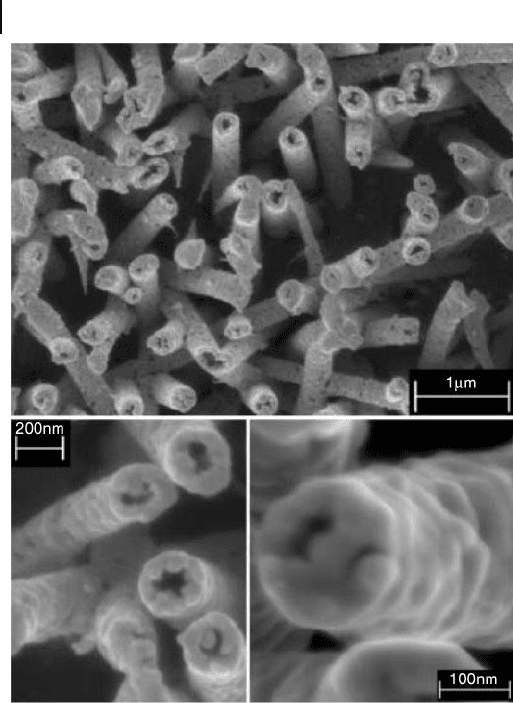
198 6 Synthesis of Inorganic Nanotubes
substrates are obtained by using a radiation track - etched hydrophilic polycarbonate
membrane [15] . By using water - dissolvable Na
2
SO
4
nanowires as templates, Au
nanoparticle tubes have been obtained by the self - assembly of Au nanoparticles
[16] . The as - synthesized Au nanoparticle tubes were further calcined at 300 ° C
(5 ° C min
− 1
) for 30 min to transform them into polycrystalline Au nanotubes. At
this temperature, the Au nanoparticles (3 – 5 nm diameter) melt and form nano-
tubes of ∼ 1 μ m length and ∼ 80 nm diameter. Using an electroless deposition
procedure, continuous, polycrystalline Au nanotubes with controllable shape, size
(tens of nanometer in diameter), shell thickness ( ∼ 5 nm), and length (up to 5 μ m)
can be grown by using Co nanoparticles as sacrifi cial templates [17] . Here, the
alignment of Co nanoparticles into a 1D structure is induced by manipulation of
Figure 6.1 Field - emission SEM images of gold nanotubes at
different magnifi cations. Micrographs were taken on the
etched side of the membrane. Reproduced with permission
from [14] . Copyright 2004, Wiley - VCH.

6.3 Nanotubes of Metals and other Elemental Materials 199
the magnetic fi eld. In this reaction, Au
3+
is reduced to Au
0
by the Co
0
nanoparticles
as given by the following reaction:
32 3 2
03 2 0
Co Au Co Au+→+
++
(6.1)
Goethite (FeOOH) nanorods have been used as templates to grow Au nanotubes
with a length of a few hundred nanometers and an aspect ratio between 3 and 4
[18] . The uniform growth of gold nanoshells on goethite nanorods was achieved
by SiO
2
- mediated assembly/attachment of Au nanoparticles/seeds on these rods,
followed by a one - step seeded growth by the catalyzed reduction of HAuCl
4
using
formaldehyde. The successful attachment of small Au seeds on goethite nanorods
requires modifi cation of the nanorod surface, which was carried out by depositing
a thin layer of silica using tetraethoxysilane (TEOS), followed by silanization using
(3 - aminopropyl)trimethoxysilane (APS). Gold nanoparticles ( ∼ 3 nm in diameter)
are prepared by the reduction with tetrakishydroxymethylphosphonium chloride
(THPC), which were used for the self - assembly of Au seeds on the surface of
goethite rods. The growth of Au shells on the goethite surface was accomplished
by the selective reduction of HAuCl
4
with a weak reducing agent such as formal-
dehyde, catalyzed by the Au seeds. In a typical synthesis, 100 mL of the dispersion
of goethite was added to 48 g of poly(vinyl pyrrolidone) (PVP) and the mixture
stirred for 24 h before transferring to ethanol (100 mL). Twelve millilitres of the
goethite – PVP dispersion was diluted with 200 mL of ethanol that contained 20 mL
of ammonium hydroxide and 0.75 mL of TEOS, under mechanical stirring. The
goethite nanorods coated with silica were washed several times with ethanol.
Silanization was carried out by adding 0.6 mL of pure APS to 10 mL of the
goethite@silica dispersion under magnetic stirring, followed by additional washing
with ethanol to remove excess APS. For the assembly of Au nanoparticles on the
nanorod surface, 5 mL of the solution of silanized goethite particles was centri-
fuged and re - dispersed in 5 mL of the Au colloid solution in an ultrasound bath
for 1 to 2 min. This step was repeated until no further adsorption of gold on the
surface of the particles was observed. Further growth of the Au coating on the
nanorods was carried out as follows. A mixture of 0.425 mL of 0.01
M HAuCl
4
and
10 mL of 1.8 × 1 0
− 3
m K
2
CO
3
was aged in the dark for a day so that Au
III
ions were
reduced to Au
I
ions. This solution (5 mL) was mixed with 0.01 – 0.03 mL of the Au -
covered goethite solution and 0.01 mL of formaldehyde solution. The thickness
and surface roughness of the obtained shells could be adjusted by simply varying
the concentration ratio between the seeds (modifi ed goethite rods) and the growth
reagents (HAuCl
4
and formaldehyde).
Platinum and PtPd alloy nanotubes with polycrystalline walls and a face - cen-
tered cubic structure (50 nm in diameter, 5 – 20 μ m long, and 4 – 7 nm wall thick-
ness) can be synthesized by the galvanic replacement reaction of Ag nanowires
[19] . In this procedure, Ag nanowires are refl uxed for 10 min with platinum acetate
in aqueous solution. When an aqueous platinum acetate solution is mixed with a
dispersion of Ag nanowires, the galvanic replacement reaction generates a tubular
sheath whose morphology is complementary to that of the Ag nanowire (step 1 in
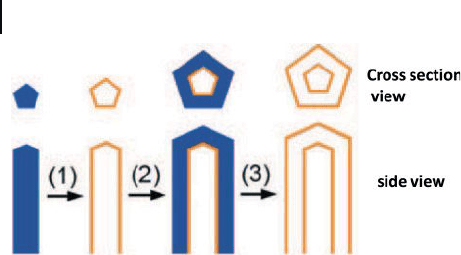
200 6 Synthesis of Inorganic Nanotubes
Scheme 6.1 ). Following the same scheme for multiple - walled nanoshells, coaxial
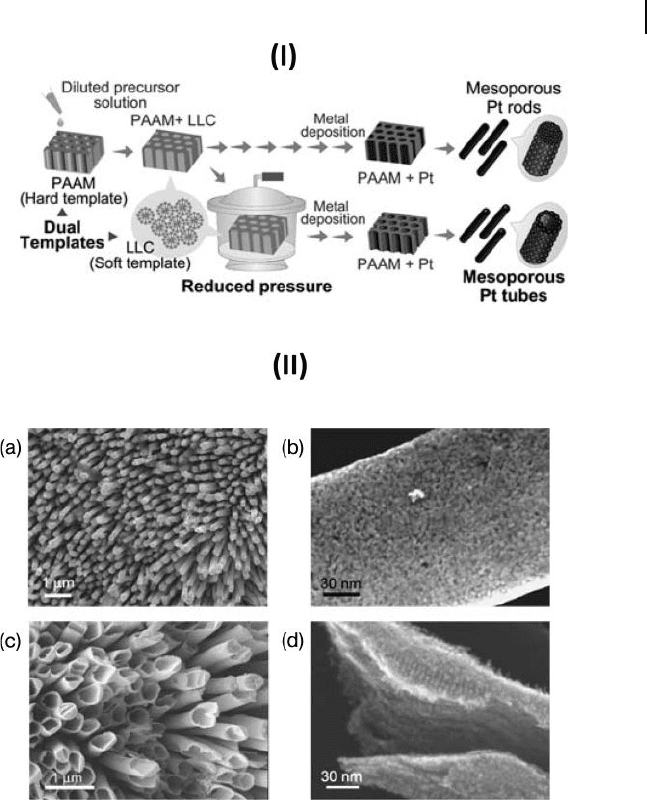
nanotubes with more than two walls can be prepared. Mesoporous Pt nanotubes
have been prepared by incorporating lyotropic liquid crystals in the pores of an
AM before depositing the metal (Fig. 6.2 ) [20] . By using a mixed non - ionic – cationic
liquid crystalline surfactant (nonaethylene glycol monododecyl ether (C
12
EO
9
),
polyoxyethylene (20) sorbitan monostearate (Tween 60) and water), polycrystalline
Pt nanotubes (with a face - centered cubic structure) with small inner (3 – 4 nm) and
outer (6 – 7 nm) diameters have been obtained [21] . In a typical synthesis, the liquid
crystalline phase that contains hexachloroplatinic acid (H
2
PtCl
6
), C
12
EO
9
, Tween
60, and water at a molar ratio of 1 : 1 : 1 : 60 was treated with hydrazine to cause the
reduction of the metal salts confi ned in the lyotropic mixed crystals of the two
different surfactants to yield the metal nanotubes. The resulting solid was sepa-
rated, and washed with water and ethanol prior to drying in air. The same proce-
dure has been used to prepare nanotubes of other metals such as Pd and Ag.
Hierarchical assemblies of hollow Pd nanostructures have been grown by using
Co nanoparticles as self - sacrifi cial templates [22] . Assemblies of hollow Pd nanos-
tructures (from 80 nm Pd nanoparticles) are obtained with raspberry - like or nan-
otube - like geometries, by the replacement reaction between H
2
PdCl
4
and Co
nanoparticles, which occurs rapidly, wherein the reduced Pd atoms nucleate and
grow into very small particles, and eventually evolve into a thin shell around the
cobalt nanoparticles. The replacement reaction is given by:
Co PdCl Pd Co C+→++
−+−
4
221
4
(6.2)
The polycrystalline 1D Pd nanostructures have a face - centered cubic structure with
lengths that run to several micrometers and diameters up to ∼ 60 nm.
Ni and Co microtubules were prepared by Han et al. [23] . by the pyrolysis of
composite fi bers consisting of a poly(ethylene terephthalate) (PET) core fi ber with
the electroless - plated metal at the exterior. Ni microtubules prepared by this
method were single - crystalline, but the Cu microtubules were polycrystalline.
Electrodeposition in the pores of AMs has been carried out by Bao et al. [24] . to
obtain ordered arrays of Ni nanotubules. The pore walls were modifi ed by these
Scheme 6.1 Schematic representation for the fabrication
of multiple - walled nanotubes composed of Pt/Ag alloys.
6.3 Nanotubes of Metals and other Elemental Materials 201
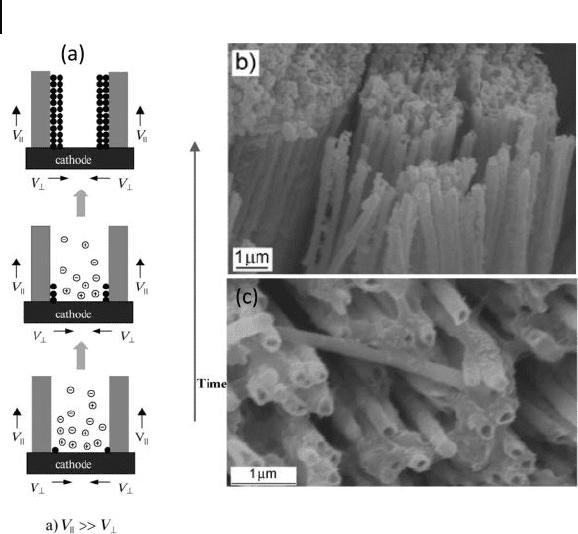
workers with an organic amine to assist the formation of nanotubes (Fig. 6.3 ). In
the absence of the amine, nickel nanowires were obtained. Nickel, when elec-
trodeposited in the pores, binds preferentially to the pore walls because of its
strong affi nity towards the amine. The AM is removed by treatment with NaOH.
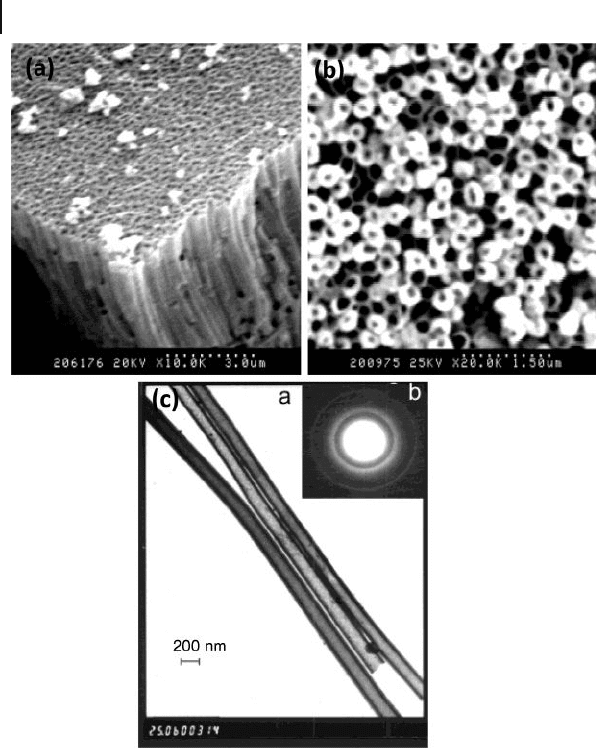
The top - view SEM image in Figure 6.3 b shows open - ends of the Ni nanotubules
after the removal of the top layer of the AM with NaOH solution. The electron
Figure 6.2 I) Schematic view of the preparative procedure for
mesoporous Pt nanorods and nanotubes. II) SEM images of:
a,b) mesoporous Pt nanorods, and c,d) mesoporous Pt
nanotubes. Figures (b) and (d) are highly magnifi ed images of
(a) and (c), respectively. Reproduced with permission from
[20] . Copyright 2008, RSC.
202 6 Synthesis of Inorganic Nanotubes
Figure 6.3 SEM images of a) Ni nanotubules
after dissolution of the alumina template,
b) showing the top - view of an array of Ni
nanotubules after partial removal of the
template, and c) transmission electron
microscopy (TEM) image of the Ni tubules
after dissolution of the alumina template.
Inset shows electron diffraction pattern of the
Ni nanotubules. Reproduced with permission
from [24] . Copyright 2001, Wiley - VCH.
diffraction pattern of the Ni nanotubules in the inset of Figure 6.3 c shows diffuse
rings, which indicates that the Ni nanotubules are polycrystalline and have a face -
centered cubic structure. The transmission electron microscopy (TEM) image of
the Ni nanotubules in Figure 6.3 c shows that the diameter of the Ni nanotubules
are uniform with average outer diameter of 160 ± 20 nm. The deposition follows
a bottom - up approach. Thus, the growth of nanotubules starts at the Au cathode
at the bottom of the pores. The length and the wall thickness of the Ni nanotubules
depend on experimental conditions, such as pore - wall modifying agent, and elec-
trodeposition parameters. A low current density appears to be a key factor. For
6.3 Nanotubes of Metals and other Elemental Materials 203
instance, with a current density of 0.3 mA cm
− 2
and an electrodeposition time of
24 h, Ni nanotubules of 20 μ m in length and 30 nm in wall thickness were obtained,
while with electrodeposition for 48 h, Ni nanotubules grow up to 35 μ m in length
and 60 nm in wall thickness. Ordered magnetic Ni nanotubes have been prepared
by employing electrodeposition by adding an amphiphilic triblock co - polymer
(Pluronic P123) to the electrodeposition solution [25] . By adjusting experimental
parameters such as current density and electrodeposition time, the wall thickness
and length of the nanotube are controlled (Fig. 6.4 ). The Ni nanotubes prepared
at a current density of 0.13 mA cm
− 2
with an electrodeposition solution that con-
tains 37 g L
− 1
of P123 and a deposition time of 48 h yielded uniform tubes of about
50 nm wall thickness, 60 μ m length, and an outer diameter of ∼ 250 nm, which
corresponds to the pore diameter of the alumina template. Weak, diffuse rings
in the selected - area electron diffraction (SAED) pattern of the nanotubes showed
they were polycrystalline.
The preparation and growth mechanism of Ni, Co, and Fe nanotubes have been
reported by Cao et al. [26] . wherein the nanotubes are actually constructed from
non - layered materials of the metal, and the tubular growth is directed by the
current in the template - based electrodeposition process (Fig. 6.5 ). Typically, the
length of the as - synthesized tubular structures can reach about 60 μ m, which
Figure 6.4 a) TEM image and b) SAED
pattern of a Ni nanotube after removing
the alumina template. c) Top - view and
d) side - view SEM images of an ordered array
of Ni nanotubes after partial removal of the
template. The experimental parameters are:
current density, 0.13 mA cm
− 2
, concentration
of P123 of 37 g L
− 1
, and deposition time of
48 h. Reproduced with permission from [25] .
Copyright 2006, Wiley - VCH.
204 6 Synthesis of Inorganic Nanotubes
corresponds to the thickness of the AM. Most of them have an outer diameter of
50 – 100 nm, which corresponds to the pore diameter of the AM, and an inner
diameter of about 30 – 50 nm. The electron diffraction patterns reveal that the nano-
tubes are single - crystalline and have a body - centered cubic structure for iron, a
face - centered cubic structure for nickel, and a hexagonal close packed structure
for cobalt. Employing AMs, aligned Fe nanotubes have been grown electrochemi-
cally [27] . The wall thickness of the nanotubes could be controlled by changing the
deposition parameters. Co nanotubes have also been grown by electrodeposition
in an AM [28] Electrodeposition in a rotating electric fi eld produces dense arrays
of single crystalline Cu nanotubes [29] . The applied rotating fi eld makes the ions
graze the surface of the pores in helical paths and makes the deposition occur
selectively in the region near the wall of the nanopores. The wall thickness of the
metal nanotubes so obtained are in the range of 15 – 20 nm and can be controlled
by changing the amplitude of the rotating fi eld. The nanotubes had a diameter of
∼ 230 nm with lengths going up to several micrometers.
Electrochemical deposition methods have also been used for the synthesis of
nanotubes of many other metals including Zn, Sn, and Ag having polycrystalline
structures [30] . In order to fabricate these metal nanotube arrays, a thin layer of
Figure 6.5 a) Schematic diagram of the steps in the growth of
metal nanotubes by template - based electrochemical
deposition method. b,c) SEM images of cobalt and iron
nanotube arrays, respectively. Reproduced with permission
from [26] . Copyright 2006, Wiley - VCH.
