Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

5.2 Helical Polymer-Based 1-D and 2-D Architectures 165
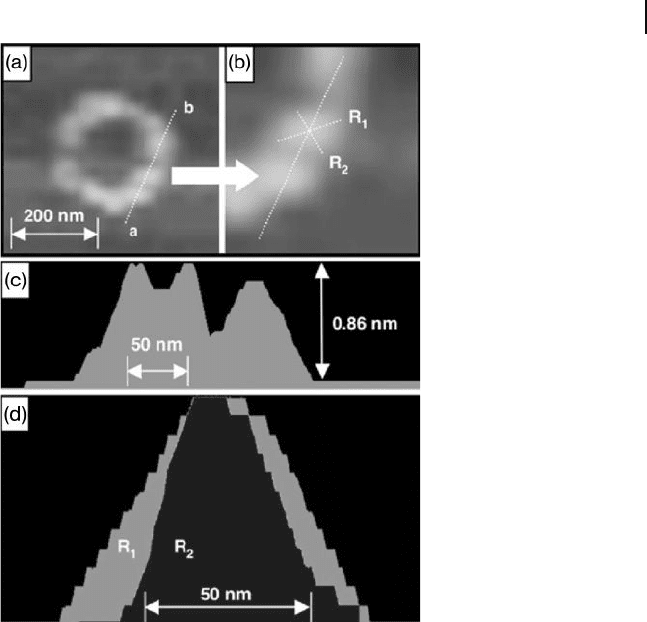
Figure 5.4 High - resolution AFM image of 1 .
(a) Circle structure; (b) Highly zoomed image
of (a); (c) Cross - section profi le of line a – b in
(a); (d) Cross - sectional profi les of segments
R
1
and R
2
. The greater width of R
1
in the
profi le indicates that the polymer chain
possesses a right - handed helical structure.
Each bright spot and its cross - sectional profi le
clearly indicate that the polymer chain has a
helical structure. From the cross - sectional
profi le, it can be seen that the local
conformation of R
2
is fl at on the bottom,
but R
1
is bulged on the top [33] .
chains might be required to confi gure the long - period helicity. In several AFM
images and their cross - section profi les, the height and width were frequently dif-
ferent at the right and left or up and down points in the circle structure (e.g.,
Figure 5.1 b).
5.2.1.2 Driving Force for the Formation of 1 - D Architectures
The chain dynamics were realized on the surfaces, and not in the solution system.
Although these structures cannot be formed in the solution system due to thermal
fl uctuation, the mechanism of topology switching of single polymer chains is
different from that of the associated forms. The dominant driving force of
topology switching would originate from a degree of freedom, as the topology
of a single polymer chain on the surface depends heavily on the chain length.
166 5 Helical Polymer-Based Supramolecular Films
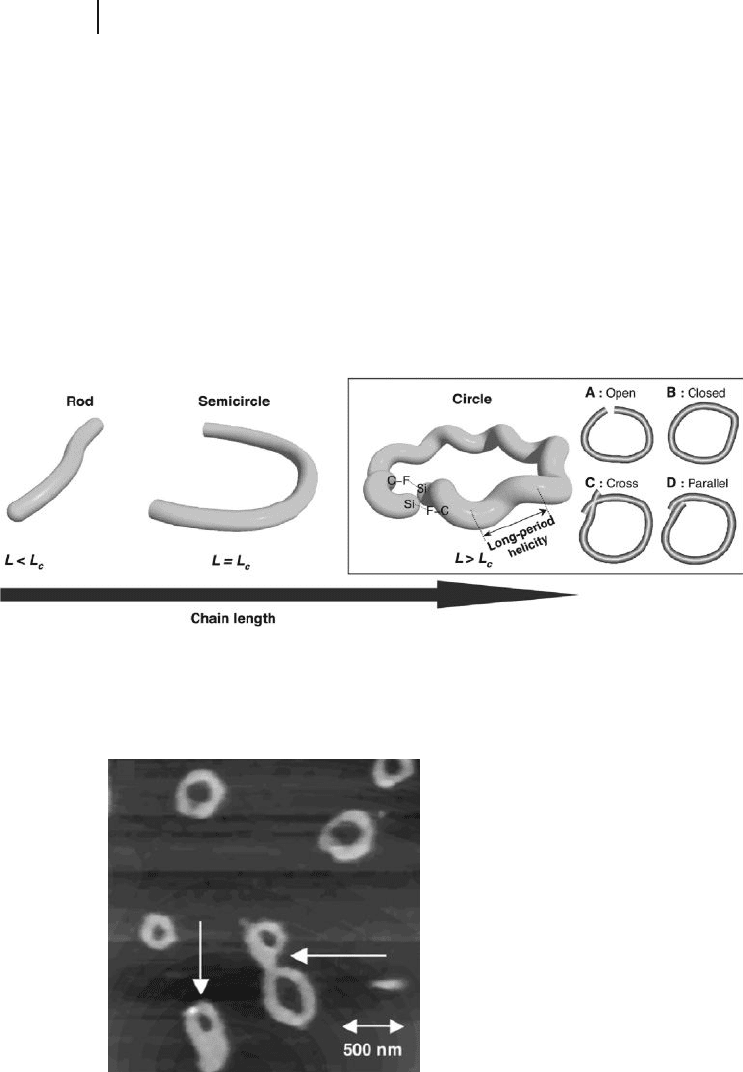
The proposed structural models regarding the circle structure are shown in
Figure 5.5 . Imperfect open - circle structures with a narrow end - to - end gap were
frequently observed, as illustrated in Figure 5.5 (open circle A). In the case of B,
it is hypothesized that the intramolecular CF/Si interaction allows it to form and
stabilize a perfect closed - circle structure, because the fl uorine atoms on the side
chains may attach to the Si atoms on the main chain, close to the end groups.
Besides this effect, a Si − O − Si bond may be formed between the end - termini via
hydrolysis of the SiH end group by water adsorbed onto the mica surfaces [28] . In
the case of circle C, the end - termini of the polymer chain are crossing each other,
while in circle D the end - termini are not crossing but are parallel to each other.
These structural models can be the precursors of toroidal structures.
As expected, much longer polymer chains frequently formed toroid - like struc-
tures, as shown in Figure 5.6 . These circle and toroid - like architectures may be
Figure 5.5 Schematic representation of
morphology switching of single polysilane
chain 1 with chain length dependence based
on AFM observations. Here, L
c
indicates the
critical length of the semi - circle structure as
an intermediate state between the rod and
circle structures. In the circle structure, (a),
(b), (c), and (d) indicate the proposed models
(open, closed, cross, and parallel,
respectively).
Figure 5.6 Typical AFM image of toroid - like structures
adsorbed onto mica surfaces.
5.2 Helical Polymer-Based 1-D and 2-D Architectures 167
useful in device applications because a nanoscaled circle architecture composed
of organic/inorganic materials has been expected to provide a superior device
performance due to the lack of defects in the end - terminus part, which strongly
affects energy loss [29] .
5.2.2
Formation of Mesoscopic 2 - D Hierarchical Superhelical Assemblies
Optically active polysilanes, such as poly[( S ) - 3,7 - dimethyloctyl - 3 - methylbutylsilane]
( 2 ) and poly[( R ) - 3,7 - dimethyloctyl - 3 - methylbutylsilane] ( 3 ), which are able to
undergo the helix – helix transition [30] at − 20 ° C in iso - octane (as shown in Scheme
5.2 ) were used in this study. Notably, we present here both the construction of
mesoscopic highly ordered and hierarchical – superhelical assemblies based on
only weak homochiral intermolecular interactions.
5.2.2.1 Direct Visualization of a Single Polymer Chain
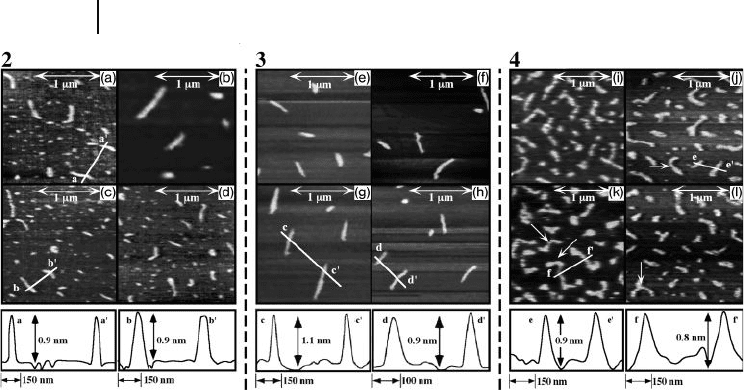
Figure 5.7 shows the typical AFM images of single polymer chains of polymers
2 , 3 , and racemic 4 , deposited onto the freshly cleaved mica surfaces and their
section analysis. The samples were prepared by casting a dilute iso - octane solution
of polymer sample (approximate range of 5 ∼ 10 μ g ml
− 1
), after which the strands
Scheme 5.2 Chemical structures of optically
active poly{( S ) - 3,7 - dimethyloctyl - 3 - methyl
butylsilane} 2 , poly{( R ) - 3,7 - dimethyloctyl - 3 -
methyl butylsilane} 3 , which can undergo a
helix – helix transition at − 20 ° C in iso - octane,
poly{( rac ) - 3,7 - dimethyl - octyl - 3 -
methylbutylsilane} 4 , and poly{ n - decyl - ( S ) - 2 -
methylbutylsilane} 5 .
168 5 Helical Polymer-Based Supramolecular Films
corresponding to the single polymer chains were randomly and separately adsorbed
onto the mica surface. Both, the 2 and 3 polymer chains typically possessed an
isolated and stretched conformation. Although the information on the helical
conformation, such as the helicity and helical pitch, could not be obtained due to
a lack of resolution, these AFM images demonstrate the rod - like conformation,
which is expected from the empirical relationship between the molar extinction
coeffi cient ε , the viscosity index α ( 2 : 1.47, 3 : 1.32 in toluene at 70 ° C), and the rela-
tively long persistence length ( ∼ 60 nm) [31] . A different polysilane, poly{ n - decyl -
( S ) - 2 - methylbutylsilane} ( 5 ), which is a stiff polymer with a persistence length of
70 nm, was observed by means of AFM as a rod - like chain onto the sapphire sur-
faces [23b] . Therefore, these results were consistent with the previous report.
However, slightly bent chains were frequently observed when the polymer
chains of the racemic polymer 4 ( M
w
: 5.2 × 1 0
5
, M
w
/ M
n
: 5.39), which is a random
copolymer system composed of equal amounts of R - comonomer and S - comono-
mer, were compared to those of 2 and 3 (marked by arrows in Figure 5.7 j, k, and
l). Additionally, the molar absorption coeffi cient ε was lower than that of 2 and 3
(2.8 × 1 0
4
(Si repeat unit)
− 1
) in iso - octane at 20 ° C, and the α value, which indicates
the rigidity of the polymer chain, was also relatively lower than that of 2 and 3
(1.15 in tetrahydrofuran at 40 ° C), as shown in Figure 5.8 . In addition to the empiri-
cal relationship between ε and the viscosity index of polysilanes [14b] , the present
AFM observations suggest that the topology of 4 is more fl exible than that of 2
and 3 .
Figure 5.7 Typical AFM images of single
polymer chains of 2 , 3 , and 4 , and their
cross - sectional profi les [lines a – a ′ in (c) and
b – b ′ in (d) of 2 , lines c – c ′ in (g) and d – d ′ in
(h) of 3 , and lines e – e ′ in (j) and f – f ′ in (k) of
4 ] onto the mica surfaces. 2 : M
w
= 2.9 × 1 0
5
,
M
w
/ M
n
= 3.43. 3 : M
w
= 2.6 × 1 0
5
,
M
w
/ M
n
= 3.53. 4 : M
w
= 3.2 × 1 0
5
,
M
w
/ M
n
= 5.39. The concentrations of casting
solution were ca. 7 μ g ml
− 1
for 2 , ca. 5 μ g ml
− 1
for 3 , and ca. 10 μ g ml
− 1
for 4 .
5.2 Helical Polymer-Based 1-D and 2-D Architectures 169
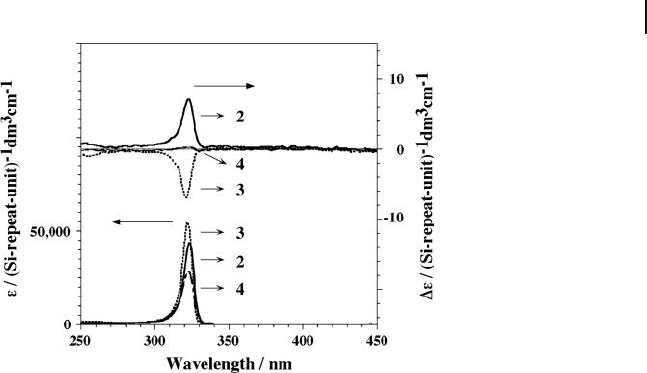
Figure 5.8 Circular dichroism and UV absorption spectra in
homogeneous iso - octane solutions of 2 (at 20 ° C, solid line),
3 (at − 5 ° C, dotted line) and 4 (at 20 ° C, broken line). Either
the ε or Δ ε value was normalized (Si repeat unit)
− 1
.
5.2.2.2 Formation of Superhelical Assemblies by Homochiral
Intermolecular Interactions
The polymer chain, which is much longer than the persistence length of 2
( M
w
: 3.9 × 1 0
5
, M
w
/ M
n
: 3.02), formed a unique aggregated structure. Figure 5.9
shows the typical AFM images of the superhelical assemblies of 2 . The highly
oriented assembled polymer chains were observed on the mica surfaces (Figure
5.9 a), which might be attributed to a dewetting property and evaporation process
of iso - octane on the mica surfaces. In the case of a fast evaporation process at
80 ° C, no uniform superhelical assemblies were found.
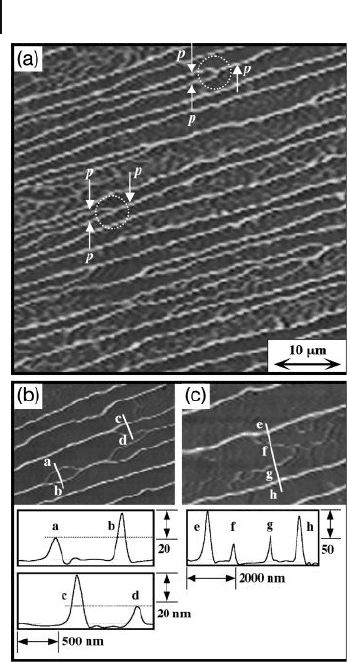
Figures 5.9 b and c show the assembled polymer chains and cross - section analy-
sis in the narrow area. The lines a – b and c – d in Figure 5.9 b indicate the height
and width, respectively, of the branching points in the main assembled polymer
chain. The height of two different polymer chains splitting from the branching
points was approximately 17 nm at the lower part and 39 nm at the higher part.
The lines e – h in Figure 5.9 c indicate the height and width of the main assembled
polymer chain. The height of the main assembled polymer chain, corresponding
to approximately 70 nm, is higher than the total of the branching chain (17 + 39 nm),
and shows that the main assembled coil is formed by a twisting of branches. These
results reveal that the assembled polymer chain is composed of several twined
polymer chains.
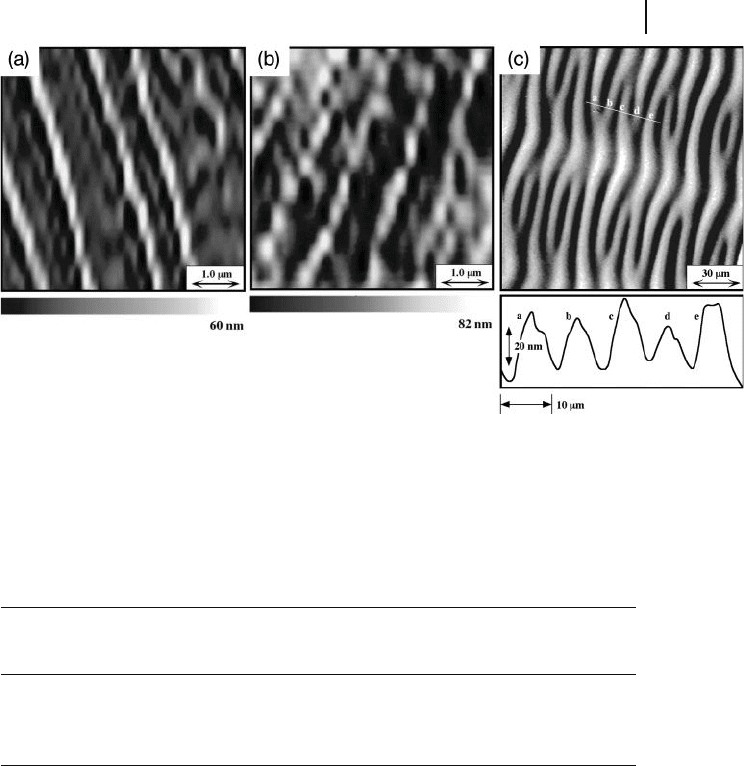
Figure 5.10 a shows the high - resolution AFM image of coiled - coil assemblies of
polymer 2 . Right - handed superhelical polymer chains, which possess a long helical
pitch, were clearly observed. In the case of the coiled - coil forms of polymer 3 ( M
w
:
7.9 × 1 0
5
, M
w
/ M
n
: 4.78), superhelical polymer assemblies were also observed,
which have the opposite handedness (left - handed) as polymer 2, as shown in
170 5 Helical Polymer-Based Supramolecular Films
Figure 5.9 AFM images and cross - sectional profi les [lines a – b
and c – d in (b) and e – f – g – h in (c)] of highly ordered
superhelical assemblies of 2 onto the mica surfaces.
M
w
=
3.9 × 1 0
5
, M
w
/ M
n
= 3.02. The concentration of the
casting solution was ca. 500 μ g ml
− 1
.
Figure 5.10 b. It should be noted that each helical ( S or R ) polysilane was confi g-
ured in its corresponding unidirectional superhelical structure. It has been sug-
gested that the 2 and 3 chains possess the P (right - handed) and M (left - handed)
helical screw - senses, respectively. In our case, large - scaled, right - handed and left -
handed superhelical fi bers are composed of right - handed 2 and left - handed 3 ,
respectively [32] . In Figure 5.9 a, here again, it was found that the branched chains
(branching points are denoted by a dotted circle), which are components of the
main assembled chain, also possessed a right - handed helical structure (marked
by arrows). Each branched chain and its small aggregate maintained the same
helicity, leading to the formation of uniform and hierarchical superhelical assem-
blies with the same helicity as the components. It has been reported that, in both
natural and artifi cial systems, heretofore, the helicities of the components were
5.2 Helical Polymer-Based 1-D and 2-D Architectures 171
Figure 5.10 AFM images of superhelical structures of 2 , 3 ,
and 4 polymer chains onto mica surfaces. (a) 2
( M
w
= 3.9 × 1 0
5
, M
w
/ M
n
= 3.02); (b) 3 ( M
w
= 7.9 × 1 0
5
,
M
w
/ M
n
= 4.78); (c) 4 ( M
w
= 3.2 × 1 0
5
, M
w
/ M
n
= 5.39).
different from that of the hierarchical superstructure that eventually formed; for
example, the right - handed superhelix of collagen is composed of three left - handed
helices [2a, 5k, 33] . Given that the same helicity was maintained during the process
of association without reference to the state of the aggregate (number of polymer
chains), homochiral intermolecular interactions would be a dominant driving
force for the formation of uniform and hierarchical superhelical assemblies. The
data reporting on the height, helical pitch and handedness of the coiled - coil chains
are listed in Table 5.1 .
A comparison of the helical fi bers constructed from 2 and 3 showed, surpris-
ingly, almost the same value for the heights and helical pitches, but a difference
in the handedness. A similar phenomenon has been reported in a biological
system, where Larson and coworkers observed the left - handed superhelical struc-
ture composed of RecA and double - stranded DNA by means of AFM [33] . In that
Table 5.1 Numerical data of assembled fi bers of 2, 3 , and 4
polymer on the mica surfaces (see Scheme 5.2 ) .
Polymer
M
w
× 1 0
5
M
w
/M
n
Height of
aggregate (nm)
Helical pitch
(nm)
Screw - sense of
fi bers
2 3.9 3.02
65 ± 12 646 ± 82
Right - handed
3 7.9 4.78
49 ± 10 636 ± 77
Left - handed
4 3.2 5.39
51 ± 9.5
– –

172 5 Helical Polymer-Based Supramolecular Films
case, the pitches of the superhelices of the complexes (RecA and DNA fi lament)
were very similar to each other, regardless of the number of component fi laments.
Moreover, from observations of the heights of the helical fi bers of 2 and 3 , it is
believed that the formation of the highly ordered structure in the large scale is
achieved by the assembly of several polymer chains. This observation is an example
of mesoscopic large - scaled, highly ordered superhelical assemblies that possess
both of the helical senses constructed by a synthetic helical polymer. Most of the
synthetic helical polymers have a polar functional group in the main or side chain,
and therefore an associated structure is formed by the combination of various
strong intermolecular interactions, such as hydrogen bonding, electrostatic inter-
actions, coordination bonding, and π - π stacking. In the present case, only weak
intermolecular interactions can account for the polymer association, because the
polysilanes 2 and 3 are composed of nonpolar groups with side chains. It is note-
worthy that no assembled superhelical structures were observed with 4 . As for the
morphology of the observed structure of 4 , although large - scaled stripe patterns
were formed on the substrate, the structures possessed no helicity. As discussed
above, there was no major difference between 2 or 3 and 4 in the conformation
of a single polymer chain. These results suggest that the homochirality of the
single helical polymer chain rather than the chain conformation structure contrib-
utes to the formation of the large - scaled, highly ordered superhelical assembly [34] .
Similar phenomena have been observed in the 2 - D crystallization of small chiral
organic molecules on the surfaces. For example, homochiral enantioselective crys-
tallization from racemic mixture has been reported [34a] . K ü hnle and coworkers
have shown that cysteine molecules formed homochiral pairs ( d - d and l - l ) from
racemic mixture using STM. Chiral transfer from single molecules into self -
assembled monolayers on the metal surfaces has also reported by Fasel et al . [34b] ;
here, enantiopure P and M chiral single molecules on the surfaces formed the
enantiopure self - assembled monolayers with clockwise and anticlockwise formats,
respectively. In addition to the enantiopure self - assembly, it has been reported that
the enhancement of chiral interactions in two dimensions was observed in the
enantioseparation of chiral molecules, using STM [34c] . These results also sug-
gested that homochiral intermolecular interaction would be an important factor
to form the 2 - D chiral surfaces.
5.2.3
Formation of 2 - D Crystallization of Poly( γ -
L - Glutamates) on Surfaces
Poly( γ - l - glutamate) ( 6 ) is a polypeptide that is well known for forming a stable
α - helical conformation, even when the substituted side chains are varied, as shown
in Scheme 5.3 . Polymers of 6 have longer alkyl side chains and can form 2 - D self -
organized arrays on highly oriented pyrolytic graphite ( HOPG ) [20c] .
In the following section, the formation of 2 - D epitaxial arrays on surfaces and
a comparison of structures between 2 - D epitaxial arrays and 3 - D bulk phases will
be described, based on the results of AFM and wide - angle X - ray diffraction ( XRD )
studies.
5.2 Helical Polymer-Based 1-D and 2-D Architectures 173
5.2.3.1 Direct Visualization of 2 - D Self - Organized Array by AFM
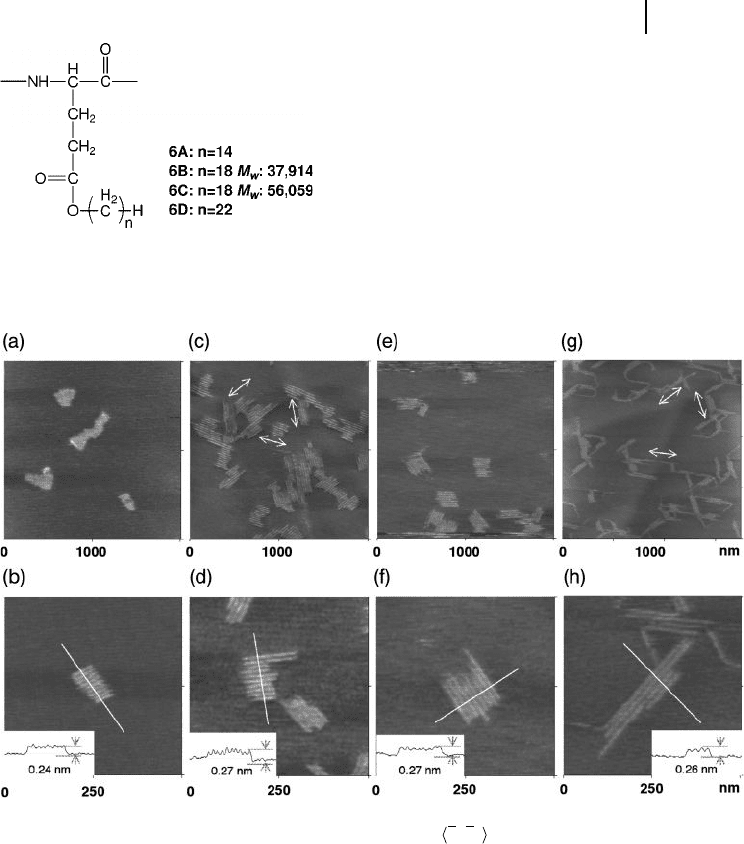
Figure 5.11 shows a collection of high - resolution island images for the 6 series,
which all revealed a similar island structure. The island - like arrays consisted of
rods, which possessed an entirely straight conformation in a parallel arrangement.
A single rod, which was separated from the islands, was not found at all. It is
Figure 5.11 Typical AFM images and
cross - sectional profi les (insets) of polymers 6
adsorbed onto HOPG substrate. (a, b) 6A ;
(c, d) 6B ; (e, f) 6C ; (g, h) 6D . The Z - scale of
the images is constant at 4 nm. Cross -
sectional profi les of the island structures are
shown in the insets of the images. The arrows
indicated the running directions of the rods in
the 2 - D array, and the directions
corresponded to 1210 directions of the
graphite basal plane. In whole images, the
entire surface is uniformly covered by bright
islands, representing the polymer adsorbates,
and the dark portion surrounding islands is
bare graphite surface.
Scheme 5.3 Chemical structure of poly( γ - L - glutamate)
derivatives 6 , where n is defi ned as the number of methylene
units in the alkyl group.
174 5 Helical Polymer-Based Supramolecular Films
important to emphasize here that the corrugations of the islands were constant
for each of the members within the 6 series; this suggests that the alkyl chains on
6 were fl at and contacted graphite in the same manner. It also indicates that the
polymers adsorbed on the HOPG were not in an aggregated form, but rather in a
monolayer form. The stripped patterns in the arrays were triangular in arrange-
ment, with the running stripes directly rotated 60 ° from each other (see Figure
5.11 c and g, where the marked arrows indicate the running direction of the stripes
in the islands). This clearly indicated that the formation of the island structures
is by epitaxial adsorption.
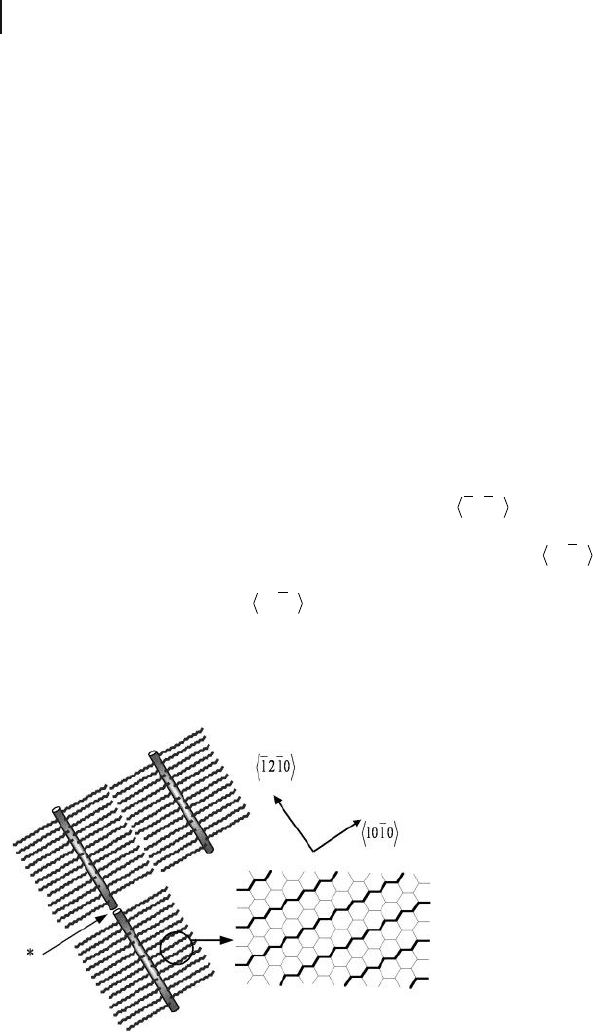
5.2.3.2 Orientation in 2 - D Self - Organized Array
The orientation of the adsorbed structure was estimated by the relationship
between the alignment of the alkyl chain and the graphite substrate. Figure 5.12
shows the schematic representation of the structural model proposed for 6 . In the
array model, the intervals of the rods correspond to intermolecular distances
between fl at - oriented adjacent polymers. Extended alkyl side chains with the all -
trans conformation are divided to both sides of the helical main chain, and align
perpendicular to the main chain. The driving force for the formation of the array
is predominantly an epitaxial interaction between the alkyl side chains and the
graphite surface. The rods of the 6 polymers run in the
1210 direction. When
the rod model was superimposed on a graphite lattice, the alkyl side chains, which
were angled at 90 ° to the direction of the rods, were aligned in the
1010 direc-
tion. It is well known that alkyl chains adsorb epitaxially onto HOPG, and that the
alkyl chains are aligned in the
1010 direction [35] , clearly showing the accuracy
of the proposed model and indicating that the formation of the array is predomi-
nantly due to epitaxial adsorption of the side groups.
Figure 5.12 Schematic representation of the proposed
structural models for the 2 - D array, displaying the rod
structure and the epitaxial alignment of alkyl side chains on
the HOPG substrate of 6 .
