FTA (изд-во). Flexography: Principles And Practices. Vol.1-6
Подождите немного. Документ загружается.

186 FLEXOGRAPHY: PRINCIPLES & PRACTICES
based inks), or pH and viscosity (water-
based inks), must be maintained.
When doctor blades or chamber blade sys-
tems are used, high viscosity inks will cause
ink starvation by not reloading the anilox
cells. The print will not be consistent and the
color variations in the product will not be
acceptable to the customer. If the ink is run-
ning at a lower viscosity than the viscosity of
the color match, the color will get lighter. If
the viscosity gets too close to the minimum
running viscosity for that ink, printing
defects may occur.
Controlling Ink Viscosity and pH. Viscosity
checks should be completed every 10 to 15
minutes when using solvent-based inks.
When using water-based inks, pH and vis-
cosity should be checked every 30 minutes.
It is important to check pH first, followed by
viscosity, when using water-based inks
because the viscosity is affected by the pH
level. Solvent inks require more frequent
A checklist approval
form ensures that all
areas of setup have
been carried out
properly.
SUPERVISOROPERATOR
I HAVE MADE ALL THE APPLICABLE CHECKS AND APPROVE THIS ORDER FOR START-UP.
COMMENTS:
BOXES
LABELING
STANDARD
STANDARD
NEXT
OPERATION
INK
SPECIAL SIZE
SPECIAL
REWINDER
SHRINKWRAPPING
SHIPPING FROM PRESS
FANFOLDING
PADDING
TRIMMER
OTHER
TIPPING
WRITE ON
PRIMER
FACE COPY—WATER BASED
FACE COPY—SOLvENT BASED
BACK COPY—WATER BASED
BACK COPY—SOLVENT BASED
1)
1)
1)
1)
2)
2)
2)
2)
COPY REGISTRATION REWIND
READ
QUALITY LEVEL
COLOR SEPARATIONS
IMPRESSION
2
NO SPLICES SIZE OF FOLD
PINFEED HOLE ALIGNMENT
UV CURING
VARNISH
TAPE TEST RUB TEST
LIST PMS COLORS
POSITION
1
QUALITY
3
MISC. MACHINE COUNT:
% OVERRUN
FANFOLD FOLDING STRAIGHT
CUTTING CLEAN
POSITION OF FOLD
PERF TEARS CORRECTLY (NOT TOO HARD/NOT TOO EASY)
HINGE FOLDING CORRECTLY
SPLICES REQUIRED
DIE CUTS HAND APPLIED
REGISTRATION
PERFED
CUTTING CLEAN
SINGLES LAY FLAT
MACHINE APPLIED
DIE CUT
SCORED
LINER TEST
JOG EVEN SIZE
MULTIWEB
OR COUPON
CONSTRUCTION TYPE
BASE STOCK
PROPER RELEASE (NOT TOO TIGHT/NOT TOO EASY)
GLUE
TOP STOCK
SIZE JACKET
MOCK-UP
DIE NO.
LINER SIZE
CARD CURL
BUTT CUT
SLITS
HOLES
SHEETED
MARGINAL PUNCHES
TEAR TAB
SCRATCH OFF
UPC SCAN
CUSTOMER
DATE
STOCK
JOB NO.
PRESS NO.
INV. NO.
PRESS APPROVAL START-UP SHEET
ITEM NO.
SIZE
3)
3)
3)
3)
4)
4)
4)
4)
5)
5)
5)
5)
6)
6)
6)
6)
7)
7)
7)
7)

checks of viscosity since the solvent evapo-
rates quickly and is more unstable than the
water in water-based inks.
To control an ink’s viscosity, small am-
ounts of a reducer should be added. The vis-
cosity level should never get so high that it
takes more than a pint of reducer to return the
viscosity to the correct level. Careful control
of ink viscosity (pH and viscosity for water
inks) results in consistent color and uniform
ink flow throughout the production run.
To control solvent-based ink viscosity, sol-
vent reducer is added during the pressrun.
The make-up solvent should have a slightly
higher percentage of the fast solvent than the
solvent in the ink system. For example, if the
ink system is 10% normal propyl acetate and
90% normal propyl alcohol, the acetate is the
fast solvent and will tend to evaporate faster
than the alcohol, which is the slow solvent in
the blend. The make-up solvent should be a
slightly higher concentration of acetate to
keep the ink stable, as well as maintain the
viscosity during longer runs.
To control viscosity in water-based ink sys-
tems, water and amine are used. A stabilizing
varnish may also be used. Stabilizing varnish
is water and amine that have been premixed
to the correct percentages for the ink system
being used. An addition of stabilizing varnish
should lower the ink viscosity while maintain-
ing the pH of the ink. Water alone will reduce
the viscosity, but it may also reduce pH, which
causes other print problems.
Many presses that are used for long runs are
equipped with automatic viscosity controllers.
Viscosity controllers dispense a predeter-
mined amount of solvent into the ink system
to keep the ink stable throughout the press-
run. It is important, however, to check the vis-
cosity manually when using these controllers
to ensure that they are working properly.
Water-based inks should be run in a specific
pH range to keep them working efficiently.
The pH range for a given ink varies by manu-
facturer and the requirements of the job.
Generally, water-based inks are in the pH
range of 8.0 to 9.3. When the pH of a water-
based ink falls too low, the ink will begin to
body and thicken, eventually causing rewet-
ting problems and dirty printing. If the pH is
too high, the viscosity of the ink will be too
low, causing drying or blocking problems.
Color variations and print defects caused by
pH levels can be eliminated by maintaining pH
within its recommended range.
A pH meter is the instrument used to read
pH levels. These instruments should be cali-
brated to a buffer solution with a known pH
on a regular basis. Similar to viscosity con-
trollers, many presses have automatic pH
controllers that check pH and add predeter-
mined amounts of stabilizer or amine to main-
tain the proper pH level in the ink. Operators
should double-check these devices by read-
ing the pH manually to guarantee they are
working properly during the run.
Adding Ink to the Fountain
During longer runs, the press operator may
find more ink is needed. This requires specif-
ic attention to detail. The text below details a
typical ink addition procedure:
1. Double-check the ink to be added for
correct color. Do not assume that an
ink container is marked correctly.
2. Adjust viscosity (and pH for water-
based inks), of the ink to be added.
Solvent-based ink to be added to the
fountain should be thinned to a vis-
cosity that is two to four seconds high-
er than the ink in the fountain.
Viscosity of water-based inks should
be five to 10 seconds higher than the
ink in the fountain. Agitation of the ink
will cause a viscosity drop of three to
five seconds.
3. Add only enough ink to bring the level
back to the optimum level.
The optimum level should be determined
before the production run. It is based on the
PRESSROOM PRACTICES 187
188 FLEXOGRAPHY: PRINCIPLES & PRACTICES
amount of ink coverage for a given color,
which depends on the ink coverage for the
job being printed. The big print areas that use
more ink will require the ink pans to be filled
more often than those that use little ink. Add
fresh ink each hour to a low coverage area
rather than filling up the fountain and adding
only solvent or stabilizer for the whole shift.
Fresh ink helps maintain the correct balance
between colorant, resin and solvent (water
and amine for water-based inks).
Ink loses printability and flow characteris-
tics if only solvent (water and amine for
water-based inks) is added over a period of
time. This loss is due to the depletion of the
colorant and the resin in the ink. For the ink
to work properly, and to avoid printability
problems, press operators must know the
following about the inks they are using:
• upper and lower working viscosity
limits;
• solvents and additives to be used;
• range of pH for water inks; and
• maximum pigment load the ink can
handle.
By maintaining the proper percentages of
ink components, the color will be more con-
sistent and fewer print problems will occur.
Inspection and Quality Checks
During a pressrun, the operator should
make ongoing inspections of the press and
the job being printed. The following items
are typical pressrun checks:
• Listen to the press for any unusual noises
that may indicate a mechanical problem.
• Double-check the ink level in the foun-
tain or pumping unit. When using a
pump, check the ink flow to the pan.
• Ensure that the low roll indicator is on.
Prepare for roll changes and splices.
• Monitor print quality during the press-
run with the use of a strobe light, video
monitor or the naked eye.
During the pressrun, print registration
may change. This change in registration
could be due to any of the following:
• incorrect web tension between in-feed
and out-feed ends of the press;
• rewind roll tension is too high;
• web splices offsetting the web;
• rolls of stock varying from roll to roll;
• baggy edges on the rolls of stock mov-
ing the web to one side; and
• web thickness and adhesive coating
vary on rolls.
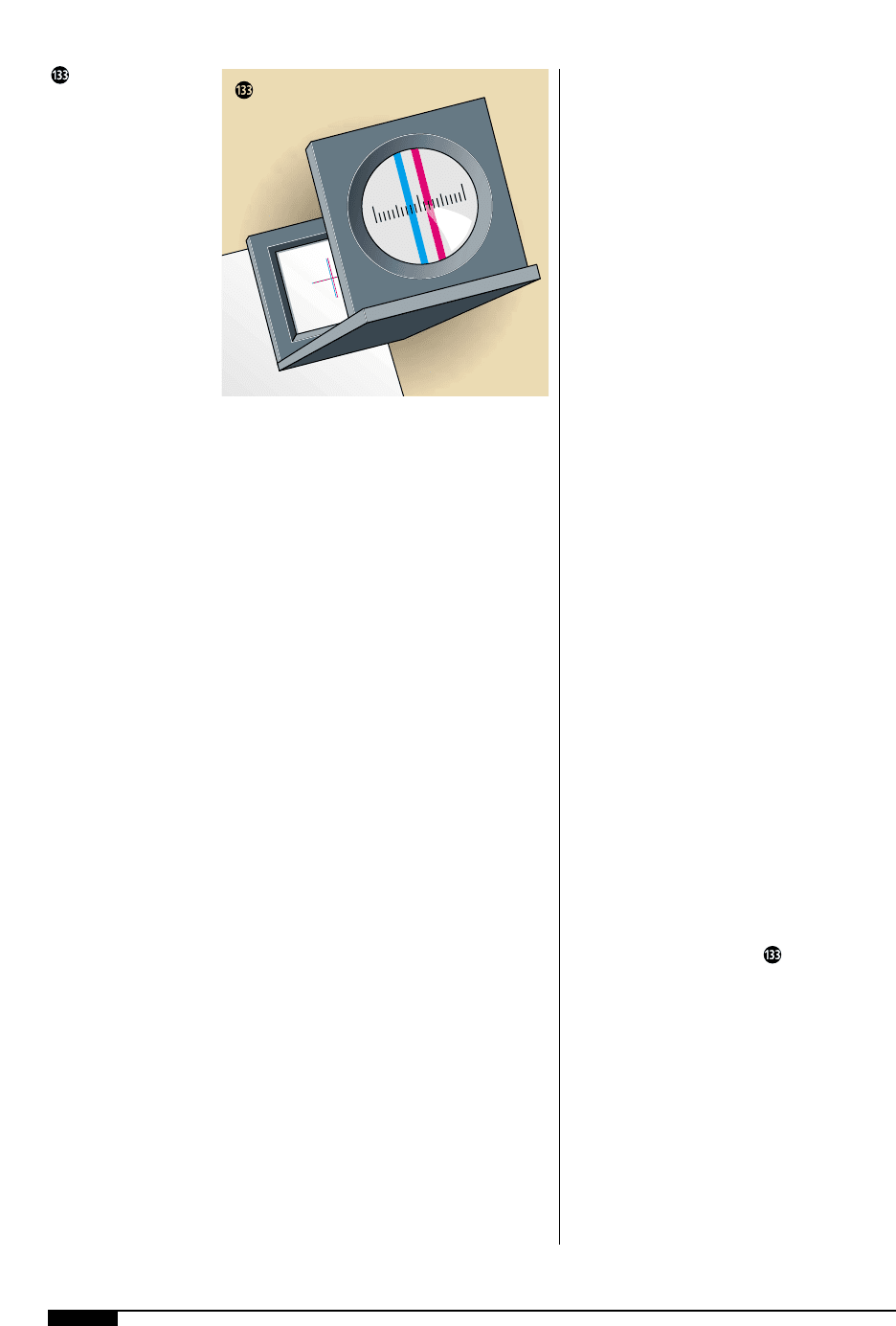
Inspecting a print sample is the most accu-
rate check of print quality. Operators should
not rely on checking print quality on the
moving web alone. Samples of the printed
product must be checked visually with the
aid of a magnifier for poor registration, poor
ink lay, color drifts, color match and ink
adhesion or cure (Figure ).
During the inspection the operator should
be looking for variations in:
• the cleanliness and sharpness of the
print;
• ink or impression misses;
• ink picking;
• flaws due to plate lift, lint or other
causes; and
• print quality.
Printed samples should be checked against
A magnifier, or loupe,
should be used to aid
in visual print checks.
the color standard on an ongoing basis to
ensure consistency throughout the run. If a
densitometer or spectrophotometer is being
used when the job is set up, and during the
initial color match, it should be used during
the pressrun. Samples from each printed roll
should be evaluated.
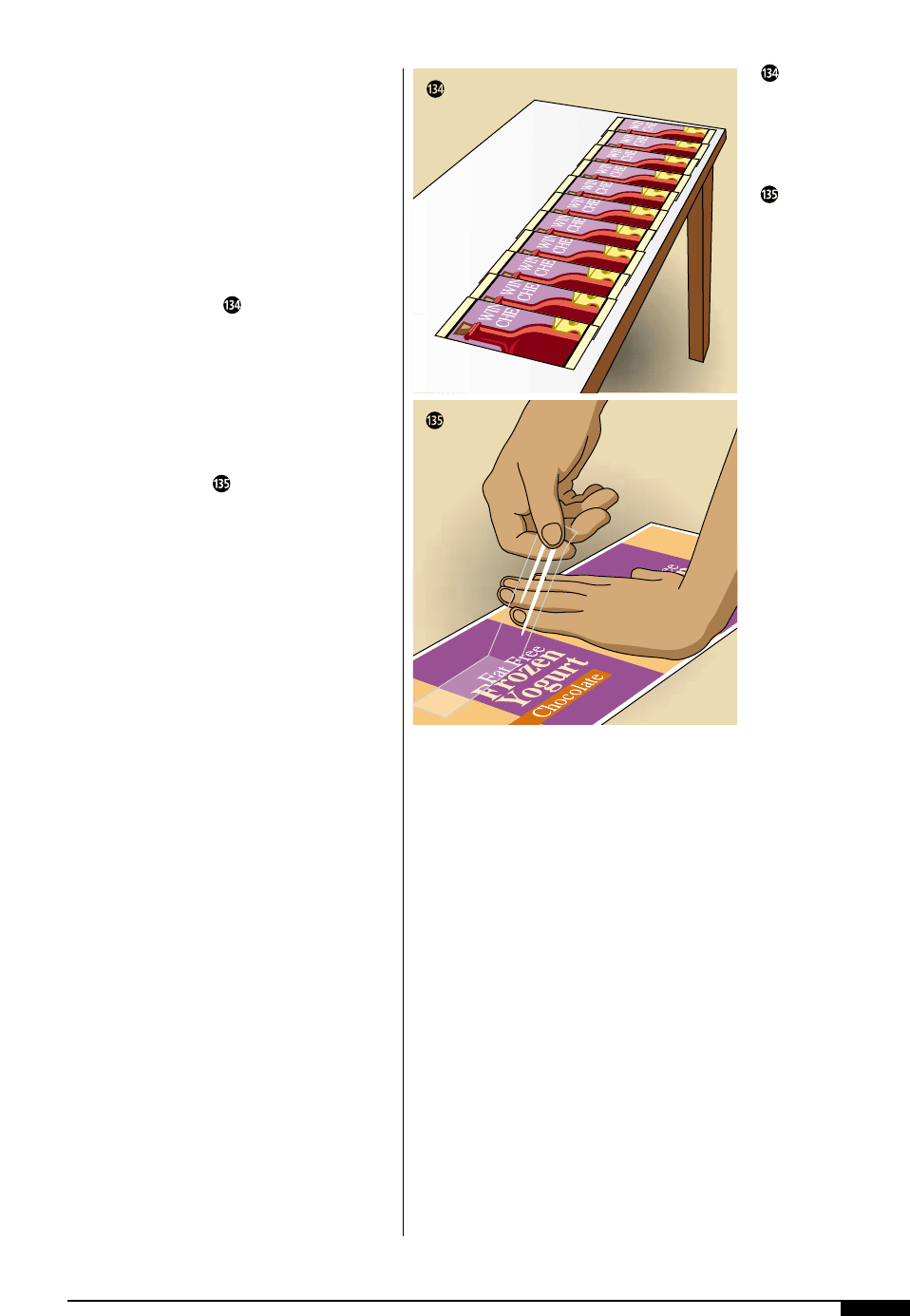
Color-drift checks are performed by laying
consecutive samples in a row on a white
background (Figure ).
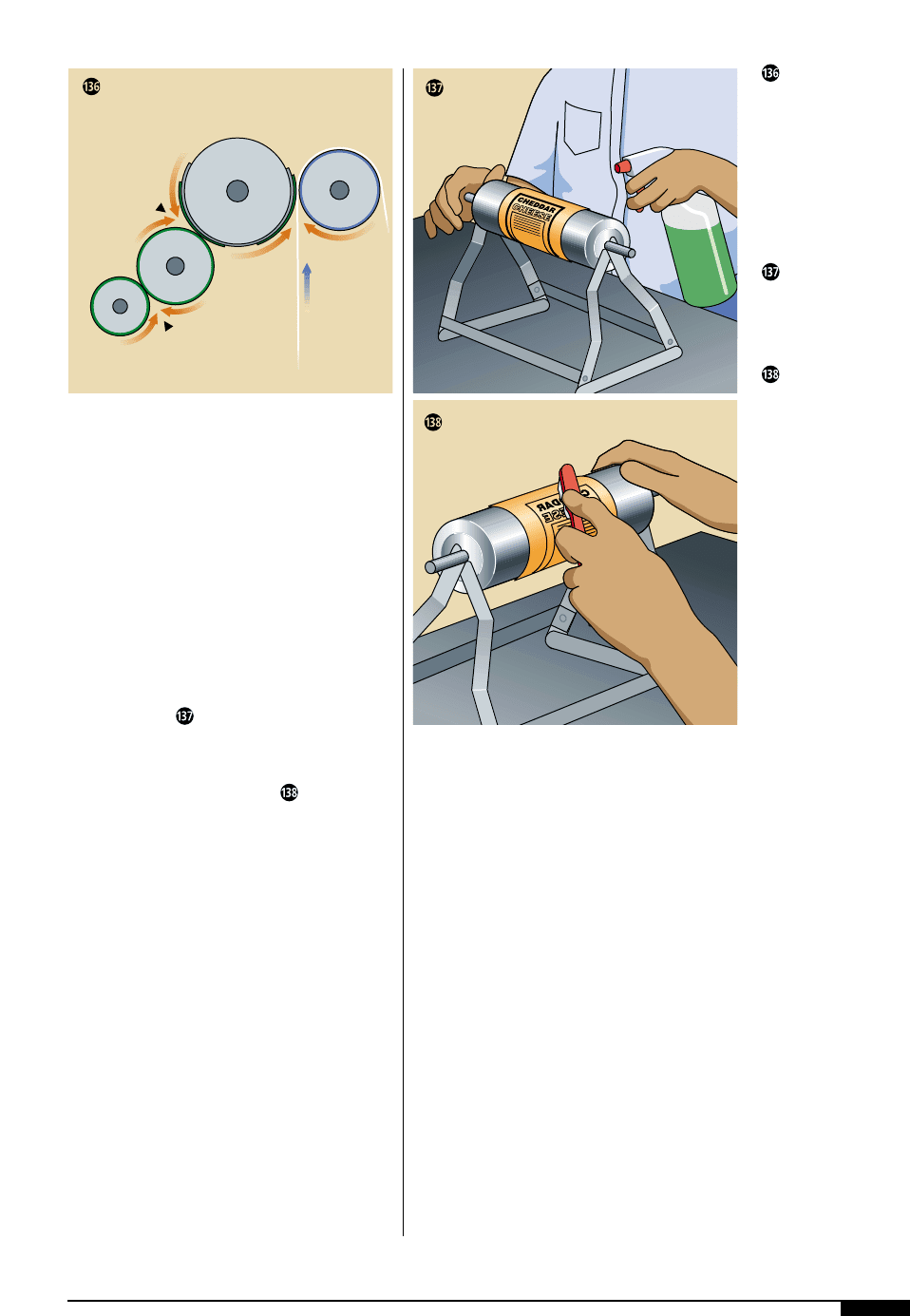
Ink-adhesion tests are performed by put-
ting cellophane tape firmly onto the printed
image and pulling upward from the image
slowly for half of the length of the tape. The
remaining tape is pulled quickly off the other
half. The tape is then examined to see if it is
free of ink (Figure ).
Rewind tension must be monitored regular-
ly. Too much or too little tension will cause the
roll to telescope or collapse. Variances in the
tension may also cause registration or block-
ing problems. Blocking occurs when the ink
or varnish sticks to the back of the liner.
Die-cutting and stripping quality must be
checked throughout the pressrun to ensure
consistency from label to label and roll to roll.
One cause of die-cutting changes during a run
is heating of the die and the anvil roll. As a
result, the running pressure between these
two rolls increases. The operator must make
periodic adjustments to offset that change.
Quality Awareness
The flexographic printing process requires
consistent and careful monitoring during the
pressrun. There are no assurances that a job
printing well presently will be doing so in five
or 10 minutes. Proper pressrun procedures
are the mainstream of top quality finished
products. Press operators must learn and per-
form all pressrun procedures well. Quality
checks should be performed regularly and the
results should be documented. These results
should be kept for a period no less than the
life of the product produced. Quality is the
result of conscious effort by the operator.
Shipping Preparation
Shipping specifications are typically on
the job jacket for each printing order. The
product labels should be checked closely.
Personnel must ensure that the customer is
provided the proper information for weight,
footage, number of impressions or a combi-
nation of these items. Some customers may
also require densitometer or spectropho-
tometer readings from the finished rolls.
Preparing for the Next Job
Preparing for an upcoming job is com-
monly done while the current job is running.
Preparation generally requires assembly of
the necessary plate rolls, substrates, inks,
cores and tooling. The press operator and
assistants should perform the following
PRESSROOM PRACTICES 189
Check for color-drift of
the printed piece by
laying consecutive
samples in a row on a
white background.
Testing for ink-adhe-
sion requires putting a
strip of cellophane tape
firmly onto the printed
image and pulling
upward from the image
slowly for half of the
length of the tape. Then
pull the remaining tape
quickly off the other
half. The tape is exam-
ined; it should be free
of ink.

190 FLEXOGRAPHY: PRINCIPLES & PRACTICES
activities in preparation for the next job:
• review information in the next
job jacket;
• plan print station wash-ups;
• note required inks and colors;
• note colors and anilox rolls in current
use that may be needed; and
• gather all materials and supplies.
CLEANUP PROCEDURES
Most jobs require some degree of cleanup
after a pressrun because inks for the next run
are often different. Since the press is prof-
itable only when it is in production, an effi-
cient cleanup program is needed. A correct
wash-up procedure minimizes downtime
between jobs and maintains the press in good
working condition. Cleanup procedures vary
by company and brand of equipment.
Before beginning the actual cleanup, the
operator should double-check the next order
to determine if any inks can remain for the
next job.
Cleanup Steps
The following details typical cleanup pro-
cedures:
1. Turn off all heaters and blowers so the
web is not damaged or burnt.
2. Remove all pressure from the dies, nip
rolls and fountain roll.
3. Remove the plate cylinder.
4. If using a pump, turn off the pump and
disconnect the power supply to stop the
flow of ink.
5. Remove the ink supply hose (if using a
pump).
6. Remove the stand pipe in the fountain
(if using a pump) and drain the ink back
into the ink container. Use a scraper or
card to push the remaining ink down
the drain hole.
7. If not using a pump, place the drain
hose from the fountain into the ink con-
tainer and remove the drain plug to
drain the ink. Use a card or scraper to
push the remaining ink down the drain
and into the container.
8. Plug the drain hole again.
9. Pull back on the doctor blade pressure
so it is not resting on the anilox.
10. Run the fountain roll into the anilox and
flood the rolls with cleanup solution.
11. Quickly remove the ink pan and doctor
blade assembly and place in a cleanup
container.
12. Clean the face and sides of the rubber
roll and anilox, and any other areas
where ink is built up, using a shop rag
and cleanup solution. Do not allow the
rolls to dry.
13. Wipe each roll with normal propyl alco-
hol to remove any residue from cleanup
soaps that may be used in a water-based
ink cleanup.
14. Clean the fountain pan and drain hose,
concentrating on areas where ink
buildup exists. Use a dry rag to give the
pan a final shine.
15. Clean the doctor blade assembly. Use
caution when cleaning the assembly.
Remove ink collected on all sides of the
assembly.
16. If using submersible pumps, place the
pump in a bucket of cleanup solution
and flush it out. This procedure cleans
the pump and the hoses. After flushing
with cleanup solution, flush with fresh
solvent or water.
17. Repeat the above steps for each print-
ing station.
18. Clean any excess ink or adhesive from
all impression cylinders, idlers and nip
rolls.
The print station of a two-roll system con-
tains pinch points between various rolls.
These pinch points are referred to as inward
nips (Figure ). Use extreme care in this
area because it is easy to catch a rag or fin-
ger in the nip.
Clean the Plates
The plates may be removed from the
plate cylinders for cleaning, or they may be
cleaned on the cylinders before demount-
ing. When cleaning solvent-based ink off of
the plates, the solvent blend for the ink sys-
tem should be used. Excessive amounts of
acetate should be avoided, as it will dam-
age the plates. When cleaning water-based
ink off plates, a cleanup soap should be
used (Figure ).
A soft-bristled brush may be used to scrub
the plate clean after the solvent or soap is
applied to the plate (Figure ). The plates
should be blotted dry with a rag or absorbent
towel. Avoid rubbing the plate as this may
damage the plate image.
Remove and Clean the Cutting Die
After the press stations and plates have
been cleaned, the die should be removed
and stored. Lay the die on a clean rag. A soft
fiber brush, wooden paint stick, or other
pointed instrument be used to remove any
adhesive or ink buildup in the areas between
the cavities of the raised engraving.
With the die cavities clean, the die should
be inspected for damage and stored back in
its shipping box or another protective stor-
age container.
Label Ink Containers
Ink containers used during the run should
be marked with the correct ink color and/or
number. Any additives, bases or solvents that
were added to the ink, and their quantities,
should be noted on the containers. Dirty
cleaning solution must be removed from the
press and poured into a waste disposal con-
tainer. The waste container should be recy-
cled or disposed of as hazardous waste.
Remove Unprinted Stock
Unprinted stock should be labeled and
returned to its assigned storage location.
Clean Tools and Press Area
All pH meters, viscosity cups and other
tools used during the pressrun should be
PRESSROOM PRACTICES 191
Pinch points between
various rolls are
referred to as inward
nips. The person clean-
ing the print station
must use extreme care
in this area because it is
easy to catch a rag or
finger in the nip.
A cleanup soap should
be used to remove
water-based inks from
the plate.
A soft-bristled brush
may be used to scrub
the plate clean after the
solvent or soap is
applied to the plate.
Impression
Cylinder
Substrate
Plate
Cylinder
Anilox Roll
Fountain
Roll
Nip Roll
Danger Zone
Nip Roll
Danger Zone

192 FLEXOGRAPHY: PRINCIPLES & PRACTICES
cleaned and prepared for use on the next
run. All spills and splashes around the
machine should be wiped up not only for the
sake of appearance, but for safety as well.
Clean Ultraviolet Curing Units
When using ultraviolet (UV) curing sta-
tions for inks or varnishes, the curing unit
must be cleaned on a regular basis for it to
function properly and create the needed
radiation to set the ink or coating.
Use isopropyl alcohol to clean UV lamps
and reflectors. Cotton gloves supplied with
the lamp should be worn when installing or
handling the light source to keep skin oils
from coming into contact with the bulb. This
is important because oil from the skin will
affect the lamp’s performance.
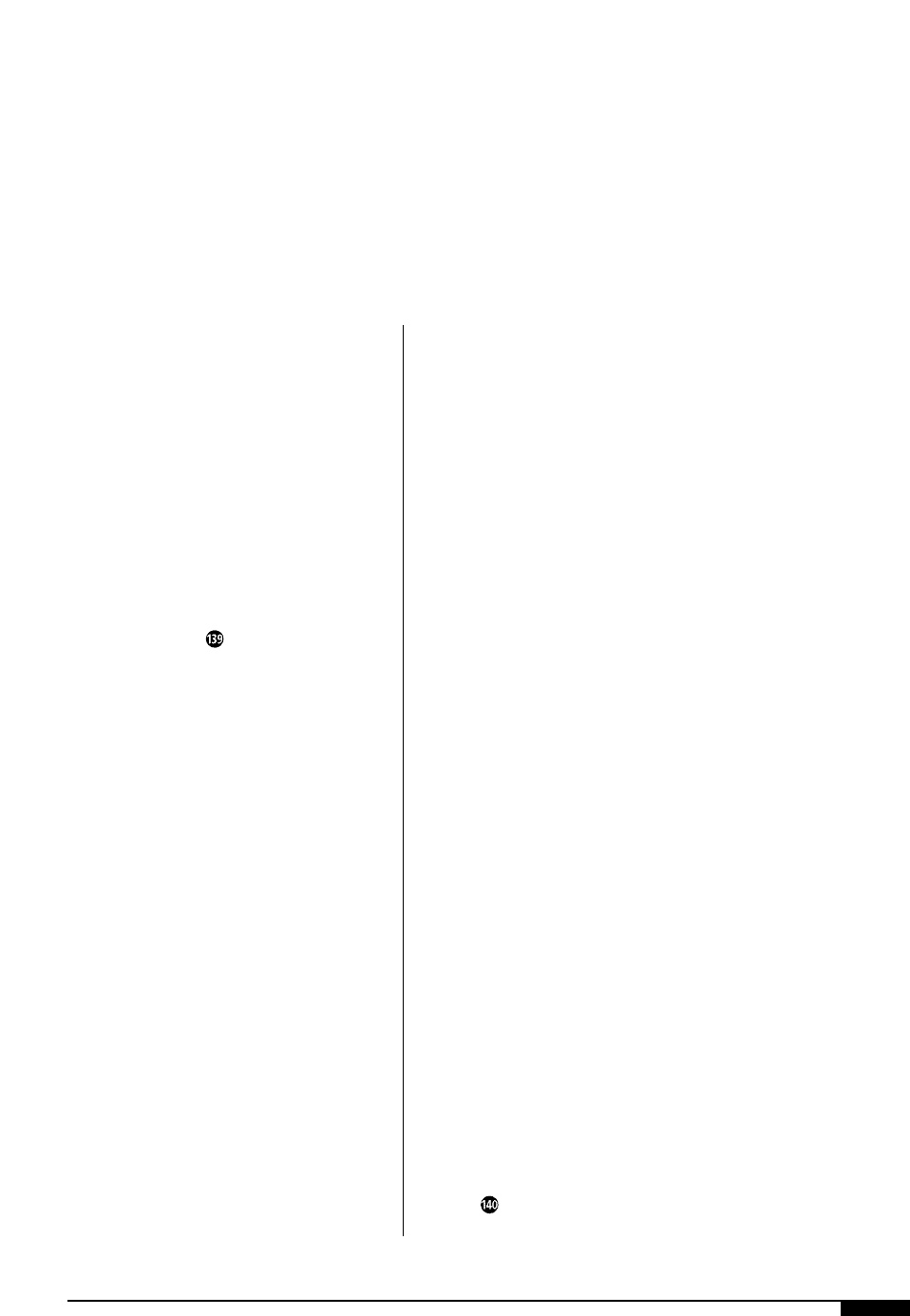
S
etting up a wide-web press
varies from machine to machine.
This variation is due to the wide
variety of presses used in the
market today. For this reason,
this section focuses on the
basics of setup that are common to most
wide-web printers.
PRESS SETUP
The first step in press setup is to review the
job jacket (Figure ), job history sheet and
any supporting material such as prepress
proofs and previously printed samples of the
job. From this information, the press opera-
tor and press assistants must visualize how
the job must be set up to meet the customer's
requirements.
In many cases, a mounter proof is sup-
plied. This should be inspected to ensure
that the printing plates are not defective, reg-
istration between colors is correct, and
makeready is not needed on the print cylin-
ders to compensate for plate height varia-
tions.
Select the Print Stations
From the information contained in the job
jacket, mounter proof, and prepress proof or
sample, the operator must select the appro-
priate printing station for each print cylin-
der. A primary consideration when choosing
print stations is whether the job is a surface
print (design prints on the outside of the
substrate) or reverse print (design prints on
the inside of the substrate). For example, a
potato chip bag is surface printed to keep
the ink from coming into contact with the
chips; bathroom tissue is reverse printed so
that after packaging the ink will be against
the tissue to help protect the ink and
enhance the gloss of the package.
Surface-printed colors are placed in the
press so that the lightest colors are printed
first, and the darkest colors last. Reverse
print orders are just the opposite. This rever-
sal is to facilitate trapping the lighter colors
with the darker colors. All print stations may
not be required for a given job. The operator
must determine which stations to use based
on existing colors in the press, colors need-
ed for upcoming jobs, anilox rolls that are in
each printing station, and the drying capaci-
ty needed between print stations. Large ink
coverages may need to pass through several
between-deck dryers to dry the ink properly
before the next ink color is printed.
The print cylinders must be inspected for
damage to the plates, and to ensure that the
design on the plates matches the information
on the job jacket and supporting material.
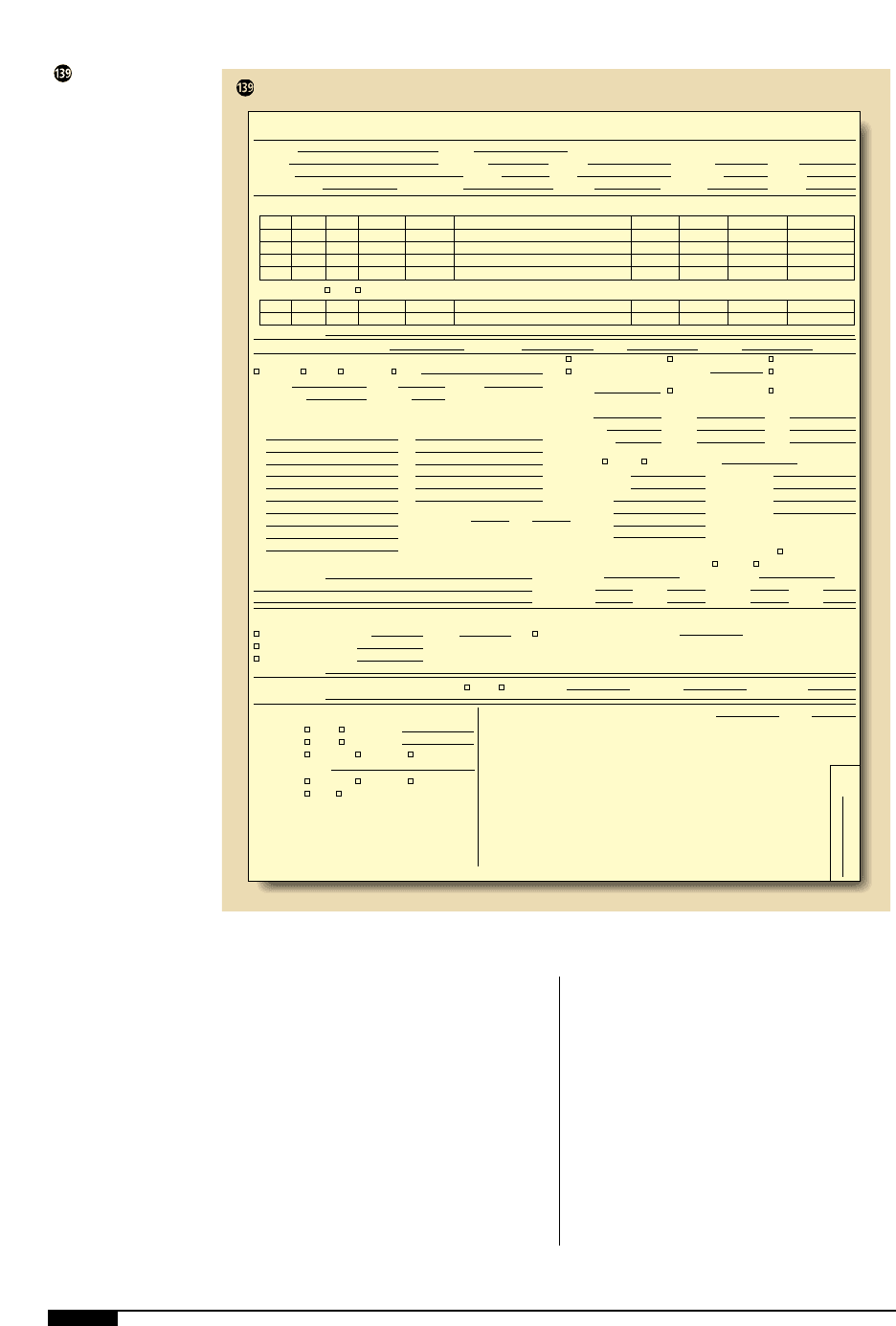
Determine the Substrate Wind
Before cylinders can be shafted and
geared for the pressrun, it is essential to
determine the required unwind and rewind
for the job’s end use. A rewind number that
corresponds to the position of the copy on
the printed roll is assigned to the design
(Figure ). This rewind number deter-
mines the end of the plate cylinder on which
PRESSROOM PRACTICES 193
Wide-Web
Press Procedures
194 FLEXOGRAPHY: PRINCIPLES & PRACTICES
the gears must be placed to provide the cus-
tomer with the correct wind for end-use
applications. In some cases, gears are not
interchangeable and the correct gear posi-
tion is determined when the job is mounted.
In this case, the operator needs to check the
gear and rewind numbers.
Install Cylinder Hardware
After the gear side of the plate cylinders is
determined, the necessary plate-cylinder
hardware must be installed in the correct
sequence. At this point in the setup
sequence, the operator or assistant should
place the bearers, gears, bushing and regis-
ter control mechanisms on the plate cylin-
ders. Some cylinder hardware setup should
be performed during the previous pressrun
to help reduce downtime.
Change Anilox Rolls
In many cases, anilox rolls must be
SHIPPING
SHIPPING
OTHER OPERATIONS
PRESSROOM
PLATEROOM
STOCK
JOB JACKET
SAMPLES TO:
PACKING SLIP
SHIP VIA:
BILL/LADING
OLS
OLS
REGULAR
PLAIN
PLAIN
OTHER
OTHER
SPECIAL INSTRUCTIONS:
SHIP TO:
NEXT DAY 2ND DAY
REWINDING
BURSTING
TRIMMING
COLLECT
OTHERSHIP FROM:
PREPAID
OLS
COD
SPECIAL INSTRUCTIONS:
SPECIAL INSTRUCTIONS:
CARRIER
AMOUNT PER ROLL
FINISHED SIZE
FINISHED SIZE
CORE SIZE
CYLINDER SIZE
NO. ACROSS
NO. AROUND
DIE SPECS
GRAPHICS SPECS
MARG. LEFT
MARG. RIGHT
HORZ.
HORZ.
VERT.
VERT.
PERFS:
FANFOLD ATPINFEED:
NO. PER SHEET
REWIND
POSITION
HORZ.
HORZ.
VERT.
VERT.
SLITS:
DIE NO(S)
PERF/SHEETER
PRINT CYL(S).SIZE
ORDERED
ORDERED
ORDERED
REC'D
REC'D
REC'D
SPACING
SPACING
CYLINDER SIZE
NO. ACROSS
NO. AROUND
SPACING
SPACING
DATE SENT
CARTON SIZEOTHER
AMOUNT PER PACKAGE
SPECIAL INSTRUCTIONS:
AMT. PER CTN.
INITIALS
PLAINOLSSHIPPING LABEL
SHRINKWRAPPING
FLEXO
LEFT RIGHT
LETTERPRESS
SAME
MACHINEHAND
BUTT CUTDIE CUT
SHEETEDOTHERCARRIERCARDLABEL
FRONT
ROLLS
CONTINUOUS
SIZE ACROSS
CORNER RADIUS
CUSTOMER NO.
CUSTOMER
DESCRIPTION
REQUESTED SHIP DATE
RUSH NO.
SALES PERSON
PREP’D BY
ACTUAL SHIP DATE % OVERRUN % UNDERRUN
QUOTE NO.
PO NO.
REPEAT NO.
DATE ORDERED
JOB NO.
TOTAL QTY.
PRESS NO.
1)
2)
3)
4)
5)
6)
7)
8)
9)
10)
BACK
1)
2)
3)
4)
5)
6)
AROUND
PRESS DRAW
LINER SIZE
COLORS
NO. OF NEW PLATES
HERE
VARNISH: OVERALL SPOT
SPECIAL INSTRUCTIONS:
YESOVERLAMINATE NO
NO. OF REMARKS MADE BY MOUNTED BY
JOB NO.
A job jacket is a
folder or envelope
that stores valuable
information about
the job to be run.
PRESSROOM PRACTICES 195
A rewind number
corresponds to the
position of the copy
on the printed roll.
The rewind number is
used to determine the
unwind and rewind
requirements for the
job.
changed in the press during the setup of a
job to acheive the correct ink density for the
design. Changing anilox rolls is often more
complicated than changing plate cylinders
since more disassembly of the press is
required. An anilox that is removed or
installed in a print station should be covered
to protect against surface damage.
Anilox Roll Selection Guidelines
Anilox roll selection varies with the print-
ing application, substrate, plate material and
the ink system being used. The best selec-
tion is an anilox roll that will provide the ink
transfer needed to meet the customer’s color
standard while providing consistent inking
of the images on the plates. This selection
rule is especially true when printing tone or
process work.
When selecting laser-engraved anilox rolls,
consider the following general guidelines:
• 60° engravings are generally used for
most applications. Although laser en-
graved anilox rolls can be manufactured
with 30°and 45° configurations, 60° rolls
have been shown to provide advantages
in ink release.
• Line-count selection is determined by
the copy to be reproduced on the press.
In applying coatings and varnishes, rolls
with line counts of between 85 and 200
are commonly used. For printing solids
(large ink coverage areas), line counts
of between 200 and 400 are usually used.
When printing screened areas (tone and
process images), the general rule is to
use an anilox roll with a minimum of a
4:1 ratio between the line count of the
screened area on the plates and the line
count of the anilox roll. In other words,
to print a 100-line process job, an anilox
with a minimum of 400 lines should be
selected. In this example, the higher
line-count roll can be used to provide
finer inking of the plate images as long
as the density of the print can meet the
color standards for the job. This 4:1 ratio
is only a general starting point.
• Cell-volume selection is determined by
the job to be produced on the press. A
volume range of 1.6 BCM (billion cubic
microns) to 4 BCM is generally used for
reproducing screen and process work.
Rolls with a BCM between 3 and 7
should be selected for printing line
work. Volumes used for printing solids
are generally in the 5 to 8 BCM range.
Higher BCM rolls should be used to
apply coatings, adhesives and varnishes.
Load Cylinders into
the Print Stations
After the anilox rolls have been selected
for the job and are loaded into the press, the
print cylinders should be loaded into the
Printing
Reads
This Way
P
rin
tin
g
R
e
a
d
s
T
h
is
W
a
y
Printing
Reads
This Way
P
rin
tin
g
R
e
a
d
s
T
h
is
W
a
y
Printing
Reads
This Way
P
rin
tin
g
R
e
a
d
s
T
h
is
W
a
y
Printing
Reads
This Way
P
rin
tin
g
R
e
a
d
s
T
h
is
W
a
y
1. 2.
3. 4.
5. 6.
7. 8.
