Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c025” — 2007/4/9 — 15:52 — page2—#2
25-2 Tissue Engineering
Esophagus
Pharynx
Superior laryn-
geal nerve
Vagus nerve
Internal
jugular vein
Trachea
Inferior
thyroid artery
Recurrent
nerve
Subclavicular
artery
Right cephalic
trunk
Vagus nerve
Azygos vein
Bronchial
artery
Right pul-
monary vein
Right lung
Inf vena cava
Diaphragm
Azygos vein
Pleura
Vagus nerve
Pleura
Thoracic duct
Left bronchus
Left lung
Left
opulmonary
artery
Pleura
Aorta
Pleura
Common
carotid
artery
Thyroid
body
Internal
carotid
artery
Superior
cervical
ganglion
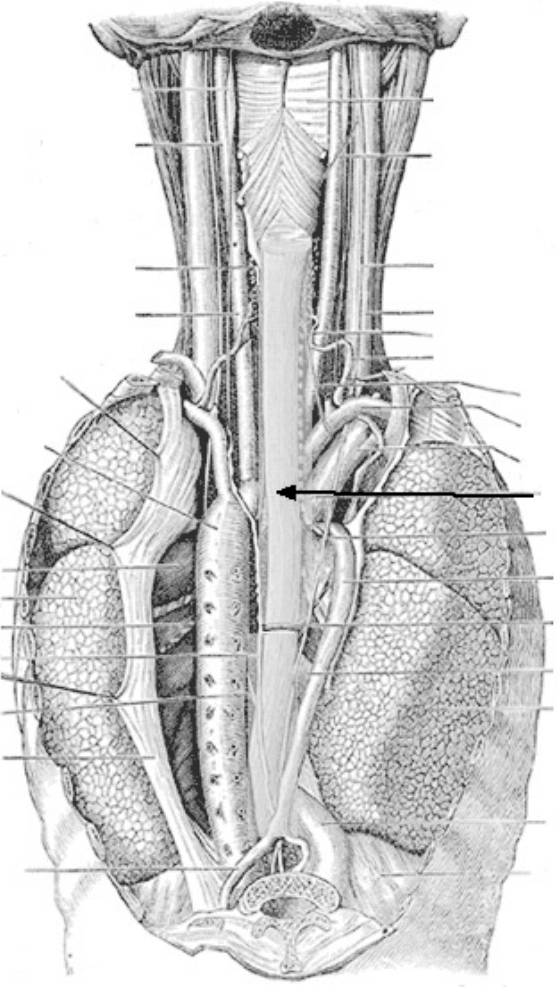
FIGURE 25.1 Diagram illustrating the anatomical location of the esophagus. (From Gray’s Anatomy.)
scarring, ulceration, and migration [Leininger et al., 1970; Watanabe and Mark, 1971; Sato et al., 1997;
Ure et al., 1998; Fuchs et al., 2001]. A living, nonimmunologic esophageal replacement could make a
significant contribution to medical practice and patient treatment.
Tissue engineering, using healthy cells supplied by the patient, offers the possibility of a normal
esophageal reconstruction after surgery, trauma, or for congenital repair. In recent years, the tissue
engineering approach has been investigated as an alternative treatment of esophageal diseases [Natsume
et al., 1993; Miki et al., 1999; Badylak et al., 2000; Yamamoto et al., 2000; Kajitani et al., 2001].
mikos: “9026_c025” — 2007/4/9 — 15:52 — page3—#3
Esophagus: A Tissue Engineering Challenge 25-3
Epithelial lining
Lamina propria
Muscularis mucosae
Submucosa
Muscularis externa
500 mm
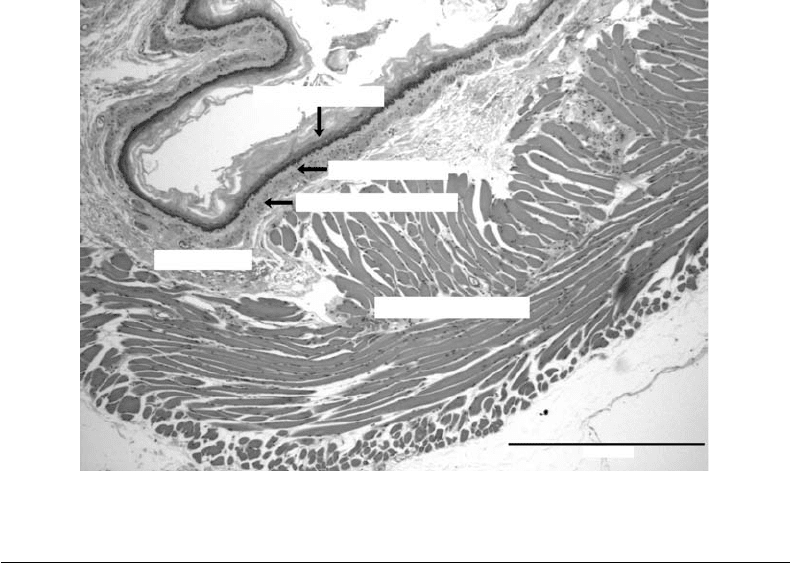
FIGURE 25.2 A cross-sectional histological section of the esophagus.
25.2 Anatomy and Physiology of the Esophagus
The esophagus is a thick-walled muscular tube extending from the pharynx to the stomach. It descends
through the posterior mediasternum, passes through the diaphragm at the esophageal hiatus, and joins the
stomachat the T9 level [Kumar, 1993]. Its length in adults is typically between 9 and 10 in. (225 to 250 mm).
It consists of three main layers, the mucosa, submucosa, and muscularis. The esophagus has no serosa
[Gray, 1995; Goyal and Sivarao, 1999; Ergun and Kahrilas, 1997; Wood, 1994].
Innervation: Sensory and motor function is supplied by branches of cranial nerves V, VII, IX, X, XI,
and XII [Wood, 1994; Ergun and Kahrilas, 1997; Goyal and Sivarao, 1999]. Neural networks lie between
the longitudinal and circular muscle layers (Auerbach’s or myenteric plexus) and the circular muscle and
submucosa (Meissner’s or submucosal plexus) [Wood, 1994; Ergun and Kahrilas, 1997]. Swallowing and
esophageal peristalsis come under the control of both the somatic and enteric nervous systems, with signal
conduction primarily through the vagus nerve.
Vasculature: Blood supply to the esophagus is via shared vasculature. Along its length, branches of larger
vessels including the thyroid artery, esophageal aortic arteries, and left gastric and splenic arteries supply
blood to the arterial network around the esophageal lumen [Gershon et al., 1994; Ergun and Kahrilas,
1997]. Capillaries within the tissues drain into deep intrinsic and adventitial veins. Blood is transported
back to the heart via the extrinsic serosal and periesophageal veins, which drain into the left gastric veins
and azygos vein [Ergun and Kahrilas, 1997].
Structure: A cross-sectional diagram of a rat esophagus is shown in Figure 25.2. The layer closest to the
lumen is typically composed of stratified squamous epithelium [Burkitt et al., 1993]. Acid reflux may cause
Barrett’s esophagus, where the stratified squamous epithelium is transformed into columnar epithelium
[Goyal and Sivarao, 1999]. Lying beneath this layer is a thin lamina propria and a thin layer of smooth
muscle, the muscularis mucosa [Burkitt et al., 1993].
The submucosa is a layer of highly vascularized and relatively loose connective tissue, which allows
distension. Within the submucosa are small mucous glands for lubrication [Burkitt et al., 1993].
The muscularis layer is normally classified as two sublayers according to the orientation of the muscle
cells. Closest to the submucosa is the circular muscle layer, where the myocytes are aligned tangentially to

mikos: “9026_c025” — 2007/4/9 — 15:52 — page4—#4
25-4 Tissue Engineering
the esophageal lumen. The next layer is the longitudinal muscle, where the myocytes are aligned parallel
to the esophageal axis [Burkitt et al., 1993; Ergun and Kahrilas, 1997].
The muscle type varies along the length of the esophagus. In the cervical esophagus, the muscularis
layer is made up, almost exclusively, of skeletal muscle, and in the distal third (closest to the stomach), the
muscularis layer consists of smooth muscle. The middle third is composed of a mixture of skeletal and
smooth muscle.
Contraction of the skeletal muscle in the cervical esophagus may be initiated voluntarily or reflexively.
The initial stage of swallowing occurs as the tongue pushes a bolus of masticated food into the oropharynx.
This initiates the involuntary pharyngeal stage of swallowing, as the bolus of food stimulates receptors
in the oropharynx, which then sends impulses to the deglutition center of the medulla oblongata and
lower pons [Ergun and Kahrilas, 1997].
The bolus of masticated food is then pushed through the esophagus by peristaltic contraction of the
muscularis layer. Peristaltic contraction may be initiated by either extrinsic or intrinsic neural pathways
[Wingate, 1993]. Longitudinal muscles ahead of the bolus contract to widen the esophagus while the
circular muscles behind the bolus of food contract to push it toward the stomach. Food normally passes
through the esophagus within 10 sec [Ergun and Kahrilas, 1997]. The junction between the esophagus
and the stomach is not a true sphincter and in certain conditions, matter may pass from the stomach into
the esophagus [Kumar, 1993].
25.3 Criteria for a Tissue-Engineered Esophagus
Based upon the above anatomical description, and from an engineering and biology standpoint, what
are the criteria that must be met before a practical and functional surgical alternative through tissue
engineering is in place? The following demands challenge us in the development of a tissue-engineered
living prosthesis:
Radially elastic/longitudinally rigid: The normal biomechanical behavior of the esophagus must be
duplicated in a surgical replacement. First, this mechanically strong and compliant tube must expand
radially to permit ingestion of a bolus of food or liquid, yet it should exhibit relatively little elasticity
longitudinally. The organization of collagen and elastin within the structure, along with its convoluted
luminal cross section can account for these mechanical properties.
Muscular: The esophagus is a highly muscular organ. Smooth muscle cells and skeletal muscle cells
are present with proportions varying along the length of the tube. Muscle laminae in the walls of the
esophagus are organized orthogonally to one another. The act of swallowing produces a peristaltic pulse
along the esophagus to drive contents to the stomach. This peristaltic process must be replicated in a
tissue-engineered construct.
Mucosal lining: The epithelial cell lining of the lumen of the esophagus generates a mucosal exterior
layer that lubricates and also protects the air–tissue interface. This lining enables the esophagus able to deal
with the range of “chemicals” and insults it must tolerate including hot foods, strong alcoholic beverages,
abrasive foods, and acidic foods.
Innervation: A nerve network in the esophagus coordinates the peristaltic action.
Angiogenesis: The esophageal wall is a relatively thick tissue (>1 mm) and requires its own blood vessel
network to sustain the core muscle tissue and remove cell wastes.
Cell sources: Since we do not know how to deal with the immunological issues associated with allogeneic
cell sources, a practical surgical prosthesis will probably be comprised of autologous cells. Cell harvesting
from a biopsy should not be a problem. However, sterility issues, cell separation, expansion, and seeding
on a scaffold are all challenges to address.
Scaffolds: A scaffold will be used to give anatomical shape and biological signals to the growing cells
forming the new tissue. The criteria for scaffolds are many. Of course, it should be nontoxic (nominally
“biocompatible”). The esophageal scaffold should have a shape and form similar to the organ to be
replaced (including the puckered or invaginated form of the lumen). It should separate epithelial and

mikos: “9026_c025” — 2007/4/9 — 15:52 — page5—#5
Esophagus: A Tissue Engineering Challenge 25-5
muscular lamina, but allow biological communication between them. It should give cells the proper
signals for attachment, growth, and orientation. This scaffold should be elastomeric as opposed to stiff.
It should allow or even encourage angiogenesis and integration into the anatomical site. Finally, it should
biodegrade without a trace after the living tissue has gained sufficient strength to support itself and
function anatomically.
Bioreactor issues: The seeded cells will be cultured in vitro until the evolving tissue is adequate for trans-
plantation into the patient. A bioreactor must sustain the cells with oxygen and nutrients, remove wastes,
provide an appropriate mechanical environment to condition the cells, provide a sterile environment, and
also create the air–tissue interface necessary for proper development of the epithelial cell–mucosal layer.
Surgical issues: Finally, the growing tissue engineered construct must be taken from the bioreactor and
implanted. What is the optimal period of in vitro development before in vivo implantation? How should
it be sutured? How should it be implanted to optimize angiogenesis? What patient management issues are
needed pre and post surgery?
The following sections illustrate research efforts underway at the Nanyang Technological University
and the University of Washington to take an engineering systems approach to the esophageal replacement
problem and address the demanding criteria for a tissue-engineered esophagus.
25.4 Scaffold Possibilities
25.4.1 Background
Currently two main approaches to tissue/organ regeneration are in vivo and in vitro tissue engineering.
In vivo tissue engineering uses noncell-seeded biomaterials, which include decellularized tissues such
as the acellular small intestinal submucosa, amniotic membranes, and pig-heart valves [Badylak et al.,
1995; Khan et al., 2001]. In contrast, in vitro approaches involve the manipulation of cells on biomaterial
scaffolds in vitro prior to implantation. Despite these obvious differences, both approaches involve the
use of biomaterial scaffold and rely on the body’s ability to regenerate.
The scaffold plays a crucial role in tissue engineering of the esophagus. The growth of the anchorage-
dependant esophageal cells requires a suitable scaffold for attachment in order to proliferate and function.
These scaffolds are three-dimensional biodegradable structures that provide spatial cellular signaling
environment necessary for the regenerative processes. These signaling processes are responsible for
triggering the expression or repression of genes that regulate cell division, production of extracellular
matrix (ECM), differentiation, proliferation, migration, and even apoptosis [Peters and Mooney, 1997;
Bottaro and Heidaran, 2001]. In essence, a scaffold is a temporary biodegradable structure containing
the appropriate cells that, through various biological remodeling processes, will eventually form vital
tissues/organs.
A suitable tissue engineered scaffold for esophageal replacement must closely mimic the host tissue
it replaces with respect to mechanical, surface, structural, and biological properties. Some of these
considerations necessary for esophageal tissue engineering include (i) materials selection, (ii) scaffold
design, and (iii) choice of fabrication techniques.
25.4.2 Materials Selection
The esophagus is a highly elastic and muscular organ, and one of the main considerations is to identify
materials that are mechanically compatible. This criterion limits the choice to only polymeric biomaterials.
These polymers must be biocompatible, biodegradable, mechanically compliant, and have suitable surface
chemistry. In addition, the polymers must be amenable to fabrication and sterilization techniques without
altering their biocompatibility and properties. Some of these biodegradable polymers used in tissue
engineering applications have been comprehensively reviewed elsewhere [Pachence and Kohn, 2000;
Langer and Tirrell, 2004].
mikos: “9026_c025” — 2007/4/9 — 15:52 — page6—#6
25-6 Tissue Engineering
The three groups of polymeric biomaterials commonly used in tissue engineering applications include
(1) naturally derived polymers, that is, alginates, chitosan, hyaluronic acid; (2) biologically derived
materials such as the decellularized tissues, that is, collagens, small intestinal submucosa, urinary bladder
matrix, and amniotic membranes; and (3) synthetic polymers, that is, poly(lactic acid), poly(glycolic
acid), and poly(lactic-co-glycolic acid), poly(hydroxybutyrate-valerate). Some of the recent developments
in biomaterials for tissue engineering applications include self-assembly nanofibers [Huang et al., 2000;
Hartgerink et al., 2002], and elastic protein-based polymer systems [Urry et al., 1991; McMillan and
Conticello, 2000].
Many materials have been evaluated for esophagus repair and reconstruction. These include collagens
[Natsume et al., 1993], poly(glycolic acid) [Shinhar et al., 1998, Miki et al., 1999], urinary bladder matrix
[Badylak, et al. 2000]; elastin biomaterials obtained from porcine aorta [Kajitani et al., 2001], and Allo-
derm®[Isch et al., 2001]. All the above materials showed promise, especially the collagen and acellular
matrices, but the problem of stenosis remained. The acellular matrices appear to show better cell–matrix
interactions than synthetic ones. This may be due to the fact that these acellular matrices, being the
ECM materials, contain a complex mixture of structural and functional proteins, glycoproteins, and pro-
teoglycans arranged in a unique, tissue-specific three-dimensional ultrastructure [Badylak, 2002]. How-
ever, cell adhesion to degradable synthetic polymers can be improved by modifying the surfaces with RGD
peptide for cell surface adhesion receptors [Glass et al., 1994; Cook et al., 1997; Schmedlen et al., 2002].
Our research group is currently evaluating the interactions between the esophageal epithelial and
smooth muscle cells on various materials including chitosan, various blends of biodegradable poly-
mers with chitosan, collagens; and decellularized porcine matrices such as urinary bladder matrix, small
intestinal submucosa, and esophagus.
25.4.3 Scaffold Design
With the exception of the acellular matrices, all other scaffolds using synthetic polymers or pure collagen
must be fabricated. As such, important structural features of the scaffold design must be considered.
The ideal scaffold should direct the biological process of tissue formation and regeneration. One of
the principal objectives in tissue engineering is to mimic the ECM in terms of their surface chemistry,
mechanical properties and structure. In addition to the choice of biomaterials, to provide suitable surfaces
for cell attachment and recognition, the physical structure of the scaffold plays an equally important
role. The effects of pore size, morphology, microgeometry, and scaffold thickness are known to influence
cellular adhesion, tissue organization, angiogenesis, and matrix deposition [Wake et al., 1994; Brauker
et al., 1995; Zeltinger et al., 2001; Ward et al., 2002; Rosengren and Bjursten, 2003].
Pore size and total porosity, for example, are also known to influence fibrovascular tissue invasion and
extent of fibrosis [Mikos et al., 1993]. In the case of the esophagus, fibrosis reaction must be minimized in
order to maintain its mechanical performance. Conceptually, the scaffold for esophageal tissue should
have a range of pore sizes. On the outer surface of the scaffold, the pore size should be large (ranging
from 50 to 200 µm) to facilitate cell seeding, and transport of nutrients and waste. There should also
be smaller pores (ranging from 35 to 70 µm) necessary to promote angiogenesis [Marshall et al., 2004].
The luminal surface, in order to mimic the basal membrane in the esophagus, should be dense (in the
range of several microns in size). This barrier layer is to facilitate diffusion of signaling molecules and
nutrients but prevents cell migration across the surface. An example of such a scaffold structure with
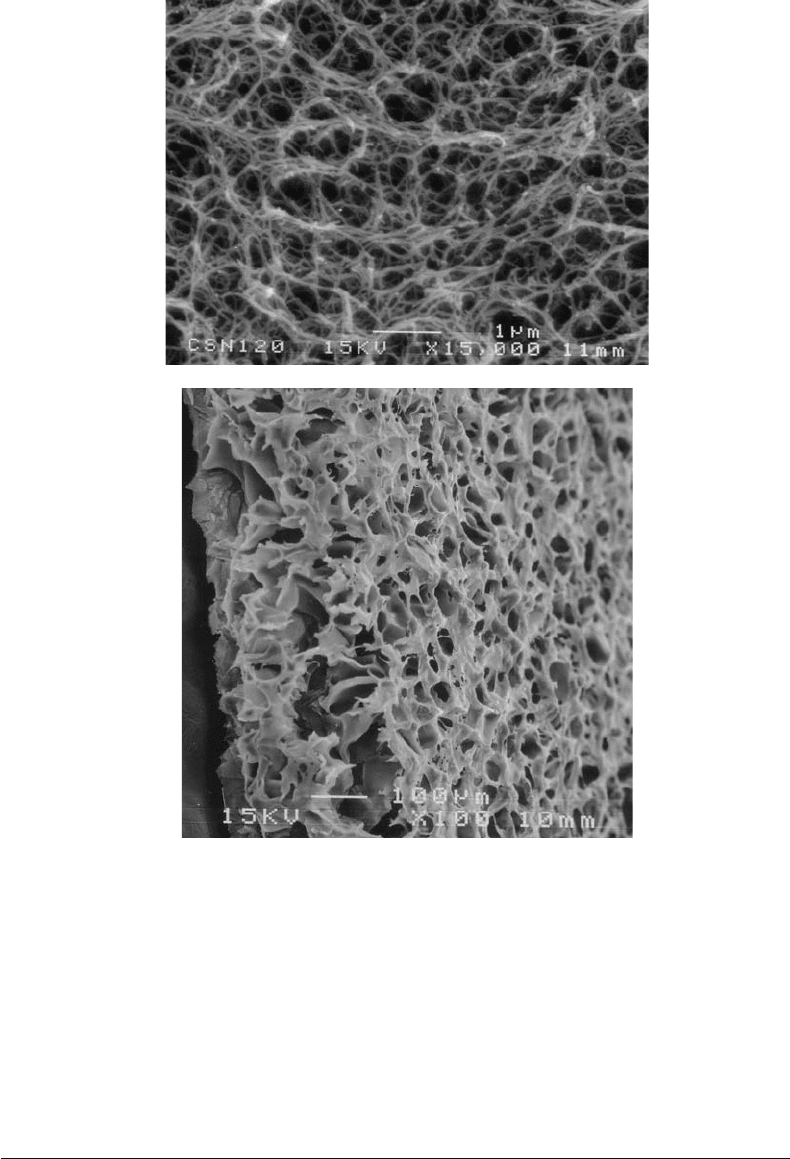
varying pore sizes is shown in Figure 25.3a,b [Chian, 2003].
Another important aspect of the esophageal scaffold is the need for pores with specific orientation. The
muscularis mucosa of the esophagus consists of a single layer of longitudinally oriented smooth muscle
fibers, whereas the muscularis externa has an inner circular and outer longitudinal muscle layers. It is
therefore advantageous to have channels in the scaffold that can provide directional guidance for these
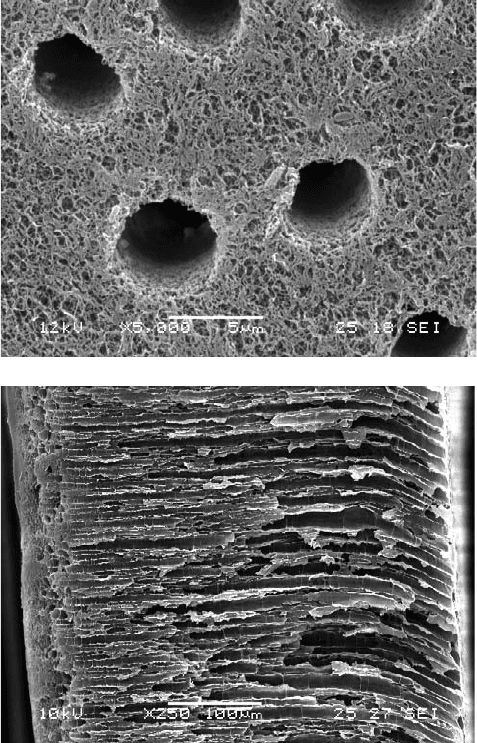
muscular tissues. Examples of such scaffolds with porous channels are shown in Figures 25.4a,b [Chian,
2003]. Our research effort is currently underway to study if these channels in the scaffold are effective in
guiding these smooth muscle cells in culture.
mikos: “9026_c025” — 2007/4/9 — 15:52 — page7—#7
Esophagus: A Tissue Engineering Challenge 25-7
(a)
(b)
FIGURE 25.3 Chitosan scaffold showing a graded pore structure that may be suitable for replicating features found
in the natural esophagus (a) Scaffold showing a dense basement membrane-like surface. (b) Scaffold showing highly
interconnecting porous structure.
However, it must be noted that the features designed into the scaffold are only important in the initial
stages of cell attachment and proliferation. As these scaffolds are biodegradable, the porous features and
mechanical strengths are only transient. In an ideal situation, it is hoped that as the implanted cells interact
suitably with the scaffold material, ECM produced by the cells will be laid down to replace the scaffold
materials as they degrade. Therefore it is very important to select a suitable biomaterial as scaffold material
that has a degradation timescale similar to the tissue forming process, which ranges from seconds to weeks.
25.5 Fabrication Processes
The methods of producing porous scaffolds for tissue engineering are well reviewed elsewhere [Thomson
et al., 2000; Atala and Lanza, 2002]. Many of these processes used in scaffold fabrication are adapted from
mikos: “9026_c025” — 2007/4/9 — 15:52 — page8—#8
25-8 Tissue Engineering
(a)
(b)
FIGURE 25.4 Examples of chitosan scaffolds with channel structures that may be useful for aligning muscle cells.
(a) Porous surface of scaffold. (b) Cross section of a chitosan scaffold.
textile [Summanasinghe and King, 2003] and membrane technologies. Some of the common scaffold
fabrication process that have been widely evaluated and reviewed [Sachlos and Czernuszka, 2003] include
the following: Fiber bonding [Mooney et al., 1996], solvent casting and particulate leaching [Mikos et al.,
1994; Wake et al., 1996], membrane lamination [Mikos et al., 1993], melt molding [Thomson et al., 1995],
extrusion [Widmer et al., 1998], solid free-form methods [Giordano et al., 1996; Park et al., 1998], gas
forming [Mooney et al., 1996], freeze drying [Whang et al., 1995], and phase inversion [Lo et al., 1995].
In this chapter, we will highlight some of the newer methods that are currently being explored for
fabricating tissue engineering of tubular scaffolds. These include (i) electrostatic spinning, (ii) cryogenic
molding, and (iii) rapid freeze prototyping.
25.5.1 Electrostatic Spinning
Electrostatic spinning is a well-established method for producing porous materials [Formhals, 1934;
Amato, 1972; Bornat, 1982]. More recently, this technique has been adapted for producing biodegradable
scaffolds from a range of polymers and collagens [Stitzel et al. 2000; Bowland et al., 2001; Matthews et al.,
2002; Li et al., 2002; Wnek et al., 2003; Chu et al., 2004]. In an electrostatic spinning process, a high-voltage
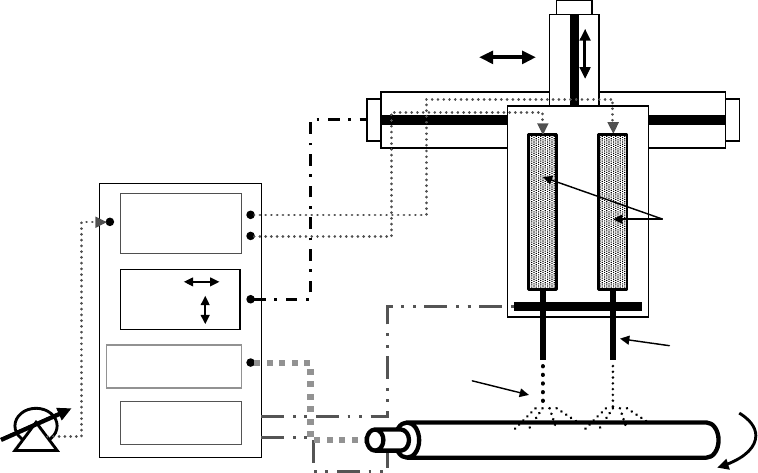
mikos: “9026_c025” — 2007/4/9 — 15:52 — page9—#9
Esophagus: A Tissue Engineering Challenge 25-9
Pneumatic
pressure
control
High-voltage
control
+
–
X–Y
axis
control
Mandrel rotation
control
Polymer
Needle
Spray
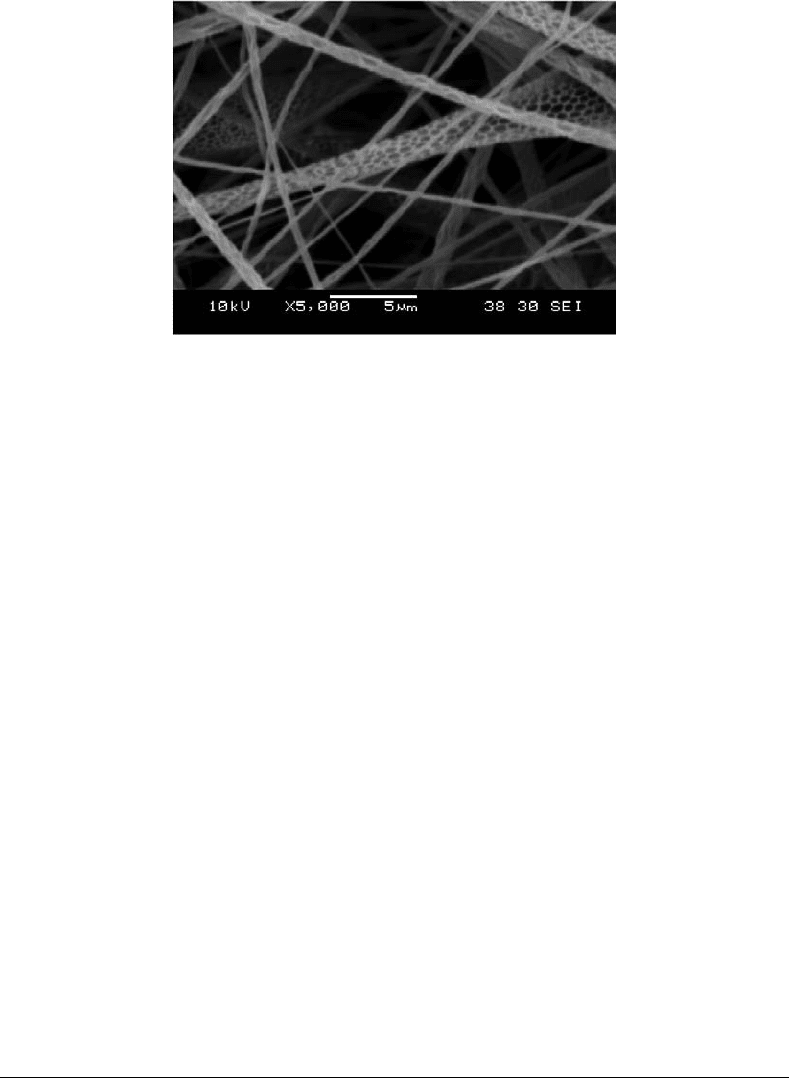
FIGURE 25.5 An electrostatic spinning system (a) apparatus for electrostatic spinning. (b) Porous nanofibers
of PLA.
field is created between the polymer solution/melt and a collector. The polymer solution/melt is usually
contained in a syringe and the needle is connected to an electrode. The oppositely charged electrode is
connected to a collector, which can be either a stationary plate or a rotating mandrel. Typically, a high-
voltage source of up to 30 kV is required for this process. Figure 25.5a shows a schematic diagram of a
typical electrostatic spinning system. In order to form the electrostatic spray, the electric field between
the end of the needle and the collector must increase until the mutual charge repulsion overcomes the
surface tension of the polymer solution [Doshi and Reneker, 1995]. Increasing the electric field results
in a charged stream of polymer fluid ejecting from the tip of the Taylor cone [Yarin et al., 2001]. The
ejecting solution undergoes a whipping process [Shin et al., 2001], wherein the solvent evaporates leaving
a charged polymer fiber randomly laid onto the grounded collector. The electrostatic spinning system
offers many advantages over conventional methods of scaffold manufacture, and these include (i) the
ability to produce varying fiber size, from nanometer to micron size, (ii) fabricating composite scaffolds,
(iii) good porosity control, and (iv) the process is amenable to a wide range of synthetic and biological
polymers. Figure 25.5b shows this use of this technique to produce nanofibers of polylactic acid with
porous surfaces [Leong et al., 2004]. We are exploring further the potential of this method to fabricate
scaffolds with various biodegradable polymers.
25.5.2 Cryogenic Molding
Another method for forming the esophageal scaffold that we are currently evaluating is the cryogenic
molding process. In this process, the polymer solution is injected into a metal mould and allowed to freeze
completely. The mould is then opened and the frozen polymer removed for freeze-drying or coagulated
immediately. We have used this method successfully to produce a tubular scaffold made from chitosan
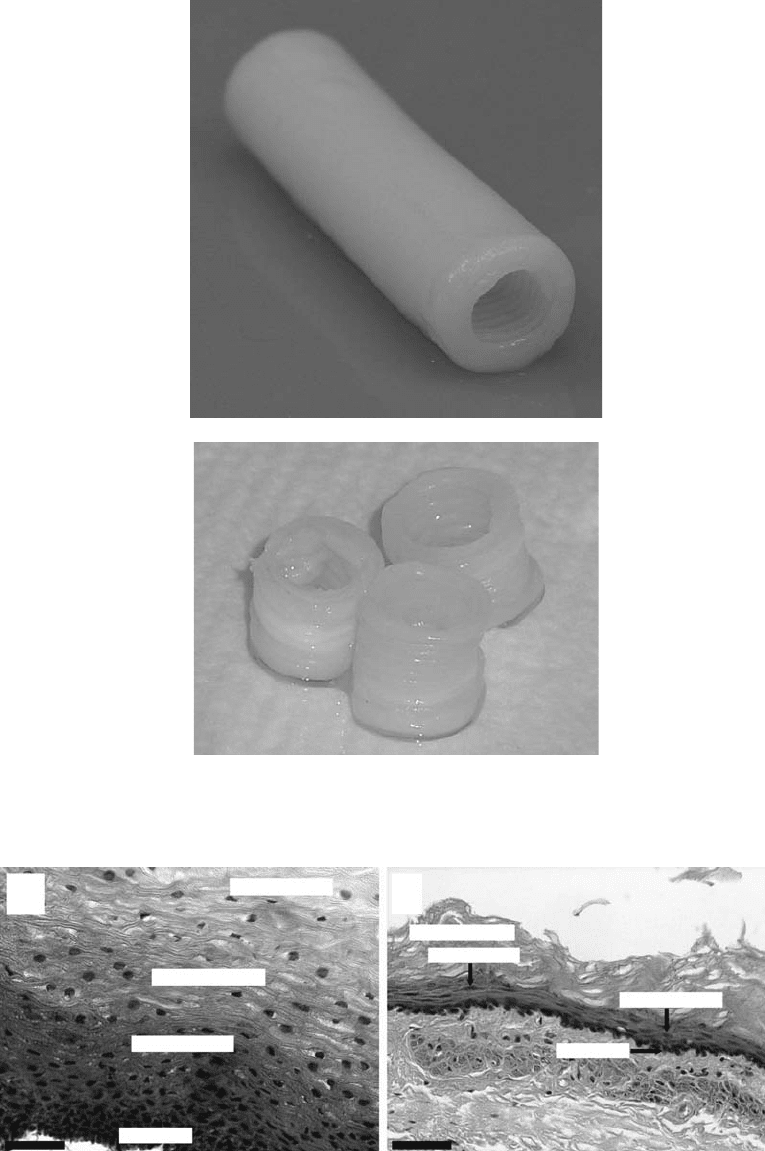
solution. Figure 25.6 shows esophageal scaffolds that were made using this cryogenic molding process.
This process offers the advantages of (i) reproducible scaffolds, (ii) it is amenable to a wide range of
polymers, (iii) low cost, and (iv) it provides good porosity control, comparable to other phase separation
methods commonly used in forming tissue engineering scaffolds.
mikos: “9026_c025” — 2007/4/9 — 15:52 — page 10 — #10
25-10 Tissue Engineering
FIGURE 25.6 A cryogenically molded tubular chitosan scaffold.
25.5.3 Rapid Freeze Prototyping
The various forms of rapid prototyping techniques have been successfully used in producing
three-dimensional scaffolds for hard tissue implants [Giordano et al., 1996; Levy et al., 1997; Mat-
suda and Mizutani, 2002]. Attempts to produce scaffolds using the rapid prototyping technique for soft
tissue engineering applications from agar hydrogels [Landers et al., 2002], fibrin hydrogels [Landers et al.,
2002], chitosan, or chitosan-hydroxyapatite [Ang et al., 2002] have also been reported.
A new method was recently developed by our research group for producing scaffolds for soft tissue
engineering application that is suitable for a wide range of polymers and biological materials. The method
is adapted from the rapid freeze prototyping process that uses water to build ice prototypes. This process
is capable of and has been successfully used to generate three-dimensional ice objects by depositing and
rapidly freezing water layer by layer [Zhang et al., 2001; Chao et al., 2002]. However, in our adapted
system, we used a robotic dispensing system to dispense chitosan solution onto a cold stage where it
is allowed to freeze. The layers are built by repeatedly dispensing chitosan solution onto the previously
frozen structure. When the required frozen structure is formed, it can be either freeze-dried or coagulated
in alkaline solution to form the porous scaffold. Figure 25.7 shows samples of chitosan scaffolds fabricated
using the adapted rapid freeze prototyping process.
The challenges in scaffold technology are many. The combination of selecting or developing a suitable
material and utilizing a suitable fabrication method is often difficult. As cells have specific interactions
with a substrate, a synthetic scaffold may eventually need to be a structure made from different materials,
and with different pore size and surface chemistry. As we learn more about cell–material interactions, the
closer we get to understanding, and enhancing our ability to mimic, the complex scaffold structure that
nature can provide so readily. More research needs to be done in understanding the biological processes
involved in tissue regeneration, and to develop novel and ingenious methods for fabricating scaffolds that
replicate nature’s ECM structures.
25.6 Cell Possibilities
25.6.1 Epithelial Characteristics
The epithelial lining of the esophagus is composed of stratified, squamous epithelial cells. In the
human esophagus, these cells are nonkeratinizing, whereas in the rat they form a stratum corneum
(see Figure 25.8) [Leeson and Leeson, 1981]. This epithelium is organized into distinct cellular layers, dis-
tinguished by appearance and protein expression. As the epithelial cells advance from the basal layer to the
mikos: “9026_c025” — 2007/4/9 — 15:52 — page 11 — #11
Esophagus: A Tissue Engineering Challenge 25-11
(a)
(b)
FIGURE 25.7 Samples of chitosan scaffolds fabricated using the rapid freeze prototyping process.
Superficial layer
Intermediate layer
Prickle-cell layer
Basal layer
Keratinized layer
Granular layer
Basal layer
Prickle-cell layer
(a) (b)
FIGURE 25.8 Esophageal epithelial morphology. (a) Nonkeratinizing human esophageal epithelium. (b) Keratiniz-
ing rat esophageal epithelium (H&E, bar = 50 µm).
