Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.


mikos: “9026_c024” — 2007/4/9 — 15:52 — page6—#6
24-6 Tissue Engineering
offers advantages for studying the effects of specific bioactive ligands or peptides presented from the
scaffold [83,84]. Studies utilizing surface modified PEGs have demonstrated that a number of cellular
functions, including adhesion [83], migration [85], and matrix production [46] can be regulated by
ligand presentation. In general, hydrogels are an appealing scaffold material because they are structurally
similar to the highly hydrated ECM of many tissues [86]. However, the use of hydrogels is often constrained
by their limited range of mechanical properties.
The elasticity provided by elastin in SM tissues has motivated the development of elastomeric scaffolds
that can similarly provide this property to engineered SM. Elastomeric polymers can recoverfrom extensive
deformation [87–89] and are designed to resemble the incompressible nature of the ECM [90]. This
property of biomaterials may be ideal to engineer functional SM tissues that require transduction of
mechanical signals from the extracellular environment in order to elicit and activate key cellular functions
[40,53,54]. This type of biomaterial resolves the limitations of lack of pliancy that limits many synthetic
polymer scaffolds (i.e., poly[lactic acid] [PLA]).
24.4.2.2 Naturally Derived Biomaterials
Type I collagen (Figure 24.3b) has been frequently used to create polymer scaffolds for engineering SM
tissues [56,73,91,92]. Naturally derived collagen is an attractive biologic material because collagen is
the primary constituent of the ECM [58], and contains adhesion ligands that facilitate cell attachment.
Although type I collagen does not require additional surface modification to promote tissue formation,
glycosaminoglycans (GAGs) [93] and growth factors [45] can be incorporated to improve mechanical
properties and to induce specific cellular functions. Type I collagen matrices used to engineer SM tissues
have demonstrated partial elasticity and are capable of withstanding cyclic stain [56]. The high tensile
strength of type I collagen can be attributed to its molecular structure, while the elasticity is conferred
by the intermolecular cross-linking. The degradation of type I collagen scaffolds is dependent on the
extent of cross-linking, pore structure and the apparent density, which are variables that can be readily
altered to meet a desired target. Although type I collagen is typically extracted from xenogeneic sources,
it is considered biocompatible and exhibits low immunogenic responses, likely due to the similarity of
this molecule between species [94]. However, naturally derived materials may suffer from batch to batch
variations.
Another collagen based biomaterial, small intestinal submucosa (SIS), has also been widely used in
tissue engineering research [95–97]. This xenogeneic matrix is harvested from the submucosal layer of
the intestine. SIS may provide functional growth factors [98]. that contribute to SM tissue formation.
In addition, SIS matrices maintain elasticity and high strength [99]. SIS has typically been obtained from
porcine sources, but isolation from rats [100] and canines [101] has also been attempted. SIS has been
used to promote regeneration of several SM tissues, in the blood vessels [102,103] and in the bladder
[99,101,104].
24.5 Engineered Smooth Muscle Tissues
A number of studies to date have utilized a combination of scaffolding technologies and cells to reconstruct
the smooth muscle component of cardiovascular, gastrointestinal, and urinary tissues. The two primary
tissue-engineering approaches used to regenerate tissues are cell transplantation and cell recruitment
from surrounding tissue. Cell transplantation requires an initial step of procuring cells, often via biopsy
from the host, followed by dissociation and expansion in vitro. The cells are then seeded onto a scaffold
and implanted as a cell–matrix construct. Alternatively, an implanted acellular matrix may be implanted
to promote the recruitment of neighboring SMCs and possibly other cell types of interest (e.g., ECs,
urothelial cells). Work to date in engineering SM tissues is briefly summarized in this section.
A great deal of research has been performed with the goal of developing blood vessel substitutes, due
to the large impact this advance would have on the millions of patients that annually suffer from diseases
of blood vessels [105]. Strategies to engineer blood vessel must provide adequate mechanical properties,
mikos: “9026_c024” — 2007/4/9 — 15:52 — page7—#7
Engineering Smooth Muscle 24-7
(a) (b)
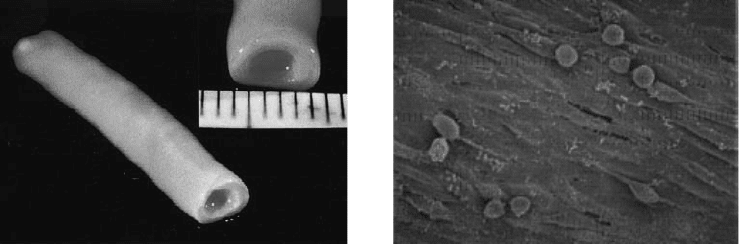
FIGURE 24.4 Engineered blood vessel substitutes. (a) Self-assembly approach to blood vessel formation relies on
the ability of sheets of cells to form their own ECM, and multiple cells sheets can subsequently be combined to form
tissues (b) Cyclic mechanical strain can play a prominent role in the development of engineered vascular tissues, as it
can lead to alignment of cells, as shown in this photomicrograph, and also leads to an increase in tissue mechanical
properties. (Photomicrograph was taken from Kim, B.S. et al., Nat. Biotechnol., 1999, 17: 979–983; L’Heureux, N. et al.,
FASEB J., 1998, 12: 47–56, and used with permission from FASEB and the Nature Publishing Group, respectively.)
to avoid catastrophic failure in this mechanically demanding site, and appropriate cellular components to
form the complex vascular wall. An early approach to engineer the blood vessels involved the culture of
different vascular cell populations in collagen gels to form three distinct layers, resembling the three layers
of native blood vessel [68]. However, this model did not lead to tissues with adequate mechanical strength.
A later approach exploited the ability of fibroblasts and SM cells to synthesize and secrete their own ECM
and form self assembled sheets. These sheets weresubsequently wrapped around a mandrel to form distinct
layers of the native vessels [65] (Figure 24.4a). This method led to tissues with much greater mechanical
strength, comparable to that of human vessels [69]. The increased mechanical strength of these tissues
my be partially attributed to paracrine effects between ECs and SMCs [35,39,51] that contribute to the
stability of nascent blood vessels by increasing matrix production. Also, implantation of a decellularized
SIS with additional type I bovine collagen into a rabbit artery led to the formation of a blood vessel
characterized by reasonable burst strength, cell and matrix organization [102].
Several groups have utilized externally applied mechanical stimulation to improve the mechanical
integrity of engineered SM tissues (Figure 24.4b). Blood vessel substitutes formed from allogeneic vascular
SMCs and ECs cultured on biodegradable PGA scaffolds were maintained under pulsatile stress, and this
resultedin an increased matrix production [57]. These engineered constructs were subsequently implanted
into swine for seven weeks and the explanted vessels exhibited adequate burst pressures and histology.
Several studies document that one can improve the properties of constructs engineered using collagen
through the use of mechanical stimulation [55,106]. The significance of mechanical stimulation was also
demonstrated by studies where synthetic SMCs cultured with ECs on collagen gels were found to undergo
a phenotypic reversion under contractile forces [5,107].
Currently, a common approach to replace or repair bladder tissue utilizes gastrointestinal segments,
but this method can result in mucus production, stone formation, and other abnormalities that may be
attributed to the different physiologic role of each tissue type. These complications have motivated invest-
igation into new methods for bladder replacement utilizing tissue engineering techniques. One common
acellular approach to engineer bladder tissue utilizes SIS membranes, as SIS membranes grafted during
partial cystectomy of canines have displayed development of all three layers of the bladder (urothelium,
SM, and serosa) [95]. Additionally, these regenerated tissues demonstrated contractile nerve regenera-
tion. Acellular biomaterial extracted from rat bladder also resulted in a well integrated construct when
implanted, but one that developed a compromised SM layer [108]. While these studies utilizing acellu-
lar scaffold implantation resulted in bladder regeneration, but function of the resultant tissues was not
reported. In contrast to this approach, PGA–PLGA scaffolds seeded with autologous canine cells led to
formation of a new tissue (Figure 24.5) that regained bladder function [12].
mikos: “9026_c024” — 2007/4/9 — 15:52 — page8—#8
24-8 Tissue Engineering
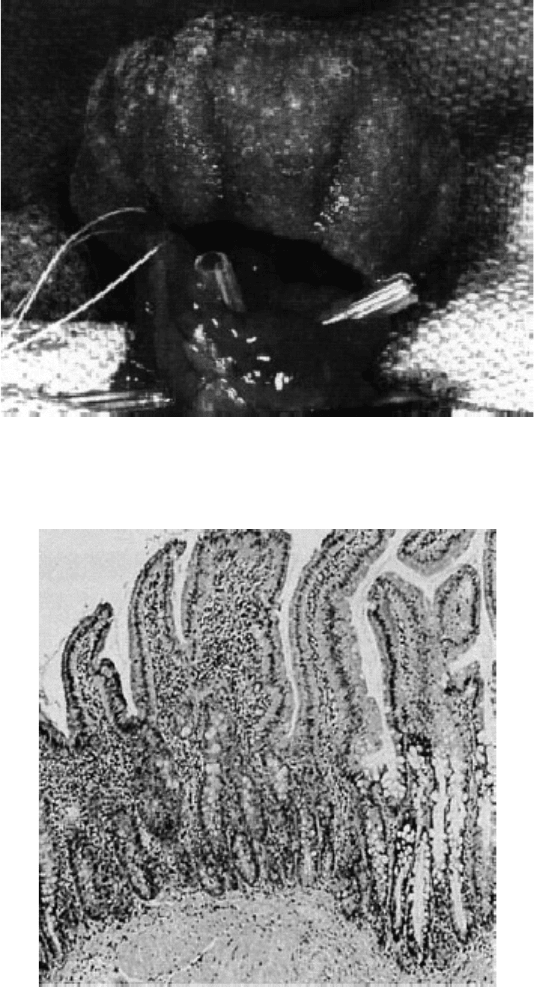
FIGURE 24.5 Engineered bladder formed from autologous cells cultured on polymeric scaffolds in vitro. The neo-
bladders can be implanted to replace lost bladder tissue. (Photomicrograph was taken from Oberpenning, F. et al.,
Nat. Biotechnol., 1999, 17: 149–155, and used with permission from the Nature Publishing Group.)
FIGURE 24.6 Photomicrographs of histologic section of engineered intestinal tissue. Epithelial organoid units were
isolated, seeded onto polymeric scaffolds, and implanted. Ultimately, the transplanted cells differentiate and form
new tissue. (Photomicrograph was taken from Vacanti, J.P., J. Gastrointest. Surg., 2003, 7: 831–835., and used with
permission from Elsevier Inc.)
A number of studies suggest it may be feasible to engineer functional gastrointestinal tissues. Isolated
crypt cells implanted on PGA tubes formed epithelial-lined tubular structures lacking in a SM component
[109] (Figure 24.6). The transplantation of intestinal organoid units, in place of individual cells on PLGA
scaffolds led to the development of a neomucosa layer [14] and SM layers [109]. However, the neomucosa

mikos: “9026_c024” — 2007/4/9 — 15:52 — page9—#9
Engineering Smooth Muscle 24-9
may have been lacking in its ability to control nutrient. SIS patch implantation, without cells, into canines
led to formation of both the epithelial and SM layer. However, a large percentageof animals died and a large
number of inflammatory cells were found in explanted SIS patches. One study utilized transplantation of
precursor cells derived from bone marrow in the place of differentiated SMCs [110], but the engineered
tissues did not regenerate a functional muscle layer, potentially due to a lack of appropriate extracellular
signals to induce the differentiation of the mesenchymal stem cells into SMCs.
24.6 Conclusion/Future Directions
Impressiveprogresshas been made to date in SM engineering, and these tissues may havesignificant clinical
impact and provide models to study basic biological processes. However our current understanding of the
complex interplay of factors that modulate the SM function are far from comprehensive, and advances
in this knowledge will likely translate to improved systems for engineering functional SM tissues. One
important issue yet to be addressed is whether approaches that successfully regenerate one type of SM tissue
will necessarily be successful for other organ systems. Physiologically, there are distinct differences between
the SM in cardiovascular, gastrointestinal, and urinary tissues. Similar approaches have been utilized to
date in most SM engineering approaches. However, inherent differences exist in the microenvironment
of different SM tissues that may have a significant effect on the developing SM tissue. The substratum to
which SMCs adhere also plays an important role in the presentation of paracrine signals and that may
regulate SMC phenotype [35,37,111]. An ideal cell source is also currently lacking, but stem cells may fill
this need. BMSCs contain a population of SM progenitors, but the difficulties in reproducibly isolating
and regulating the differentiation of these cell populations pose as an obstacle to their use. Embryonic stem
cells may provide a more homogeneous population of pluripotential cells, but these cells have not yet been
demonstrated to form functional SM tissue. In addition, for engineered SM tissues to be truly functional,
they must also be innervated and vascularized. Current research in the therapeutic angiogenesis field may
provide methods to induce formation of vascular networks [81]. However, development of fully functional
SM tissues will also require a means to transduce neural signals critical for blood vessel vasoactivity.
References
[1] Langer, R. and J.P. Vacanti, Tissue engineering. Science, 1993, 260: 920–926.
[2] Gridelli, B. and G. Remuzzi, Strategies for making more organs available for transplantation.
N.Engl.J.Med., 2000, 343: 404–410.
[3] Owens, G.K., Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev., 1995, 75:
487–517.
[4] Campbell, J.H. and G.R. Campbell, Vascular Smooth Muscle in Culture. Boca Raton, FL: CRC Press,
2 vol, 1987.
[5] Kanda, K., H. Miwa, and T. Matsuda, Phenotypic reversion of smooth muscle cells in hybrid
vascular prostheses. Cell Transplant., 1995, 4: 587–595.
[6] Nikolovski, J., B.S. Kim, and D.J. Mooney, Cyclic strain inhibits switching of smooth muscle cells
to an osteoblast-like phenotype. FASEB J., 2003, 17: 455–457.
[7] Young, B. et al., Wheater’s Functional Histology: A Text and Colour Atlas. Churchill Livingstone:
Edinburgh; New York, 2000.
[8] Stegemann, J.P. and R.M. Nerem, Altered response of vascular smooth muscle cells to exogenous
biochemical stimulation in two- and three-dimensional culture. Exp. Cell Res., 2003, 283: 146–155.
[9] McKee, J.A. et al., Human arteries engineered in vitro. EMBO Rep., 2003, 4: 633–638.
[10] Stock, U.A. et al., Tissue engineering of heart valves — current aspects. Thorac. Cardiovasc. Surg.,
2002, 50: 184–193.
[11] Shinoka, T. et al., Creation of viable pulmonary artery autografts through tissue engineering.
J. Thorac. Cardiovasc. Surg., 1998, 115: 536–545; discussion 545–546.
mikos: “9026_c024” — 2007/4/9 — 15:52 — page 10 — #10
24-10 Tissue Engineering
[12] Oberpenning, F. et al., De novo reconstitution of a functional mammalian urinary bladder by tissue
engineering. Nat. Biotechnol., 1999, 17: 149–155.
[13] Maemura, T. et al., A tissue-engineered stomach as a replacement of the native stomach.
Transplantation, 2003, 76: 61–65.
[14] Kaihara, S. et al., Long-term follow-up of tissue-engineered intestine after anastomosis to native
small bowel. Transplantation, 2000, 69: 1927–1932.
[15] Junqueira, L.C.U. and J. Carneiro, Basic Histology: Text & Atlas. 10th ed. 2003, New York: Lange
Medical Books McGraw-Hill Medical Pub. Division, p. viii, 515.
[16] Pittenger, M.F. et al., Multilineage potential of adult human mesenchymal stem cells. Science, 1999,
284: 143–147.
[17] Bianco, P. and P.G. Robey, Stem cells in tissue engineering. Nature, 2001, 414: 118–121.
[18] Bianco, P. et al., Bone marrow stromal stem cells: nature, biology, and potential applications. Stem
Cells, 2001, 19: 180–192.
[19] Krebsbach, P.H. et al., Bone marrow stromal cells: characterization and clinical application. Crit.
Rev. Oral Biol. Med., 1999, 10: 165–181.
[20] Ferrari, G. et al., Muscle regeneration by bone marrow-derived myogenic progenitors. Science,
1998, 279: 1528–1530.
[21] Ferrari, G. and F. Mavilio, Myogenic stem cells from the bone marrow: a therapeutic alternative
for muscular dystrophy? Neuromuscul. Disord., 2002, 12(Suppl 1): S7–S10.
[22] Hirschi, K.K. and M.A. Goodell, Hematopoietic, vascular and cardiac fates of bone marrow-derived
stem cells. Gene Ther., 2002, 9: 648–652.
[23] Kadner, A. et al., A new source for cardiovascular tissue engineering: human bone marrow stromal
cells. Eur. J. Cardiothorac. Surg., 2002, 21: 1055–1060.
[24] Dennis, J.E. and P. Charbord, Origin and differentiation of human and murine stroma. Stem Cells,
2002, 20: 205–214.
[25] Kinner, B., J.M. Zaleskas, and M. Spector, Regulation of smooth muscle actin expression and
contraction in adult human mesenchymal stem cells. Exp. Cell Res., 2002, 278: 72–83.
[26] Altman, G.H. et al., Cell differentiation by mechanical stress. FASEB J., 2002, 16: 270–272.
[27] Yamashita, J. et al., Flk1-positive cells derived from embryonic stem cells serve as vascular
progenitors. Nature, 2000, 408: 92–96.
[28] Koike, N. et al., Tissue engineering: creation of long-lasting blood vessels. Nature, 2004, 428:
138–139.
[29] Han, C.I., G.R. Campbell, and J.H. Campbell, Circulating bone marrow cells can contribute to
neointimal formation. J. Vasc. Res., 2001, 38: 113–119.
[30] Simper, D. et al., Smooth muscle progenitor cells in human blood. Circulation, 2002, 106:
1199–1204.
[31] Sata, M. et al., Hematopoietic stem cells differentiate into vascular cells that participate in the
pathogenesis of atherosclerosis. Nat. Med., 2002, 8: 403–409.
[32] Shimizu, K. et al., Host bone-marrow cells are a source of donor intimal smooth-muscle-like cells
in murine aortic transplant arteriopathy. Nat. Med., 2001, 7: 738–741.
[33] Majka, S.M. et al., Distinct progenitor populations in skeletal muscle are bone marrow derived
and exhibit different cell fates during vascular regeneration. J. Clin. Invest., 2003, 111: 71–79.
[34] Hirschi, K.K., S.A. Rohovsky, and P.A. D’Amore, PDGF, TGF-beta, and heterotypic cell–cell
interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation
to a smooth muscle fate. J. Cell Biol., 1998, 141: 805–814.
[35] Hirschi, K.K. et al., Endothelial cells modulate the proliferation of mural cell precursors via
platelet-derived growth factor-BB and heterotypic cell contact. Circ. Res., 1999, 84: 298–305.
[36] Darland, D.C. and P.A. D’Amore, TGF beta is required for the formation of capillary-like structures
in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis, 2001, 4: 11–20.
[37] Corti, S. et al., Chemotactic factors enhance myogenic cell migration across an endothelial
monolayer. Exp. Cell Res., 2001, 268: 36–44.
mikos: “9026_c024” — 2007/4/9 — 15:52 — page 11 — #11
Engineering Smooth Muscle 24-11
[38] Adam, R.M. et al., Signaling through PI3K/Akt mediates stretch and PDGF-BB-dependent DNA
synthesis in bladder smooth muscle cells. J. Urol., 2003, 169: 2388–2393.
[39] Iivanainen, E. et al., Angiopoietin-regulated recruitment of vascular smooth muscle cells by
endothelial-derived heparin binding EGF-like growth factor. FASEB J., 2003, 17: 1609–1621.
[40] Stegemann, J.P. and R.M. Nerem, Phenotype modulation in vascular tissue engineering using
biochemical and mechanical stimulation. Ann. Biomed. Eng., 2003, 31: 391–402.
[41] Hellstrom, M. et al., Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle
cells and pericytes during embryonic blood vessel formation in the mouse. Development, 1999,
126: 3047–3055.
[42] Stringa, E. et al., Collagen degradation and platelet-derived growth factor stimulate the migration
of vascular smooth muscle cells. J. Cell Sci., 2000, 113(Pt 11): 2055–2064.
[43] Wrenn, R.W. et al., Transforming growth factor-beta: signal transduction via protein kinase C in
cultured embryonic vascular smooth muscle cells. InVitroCellDev.Biol., 1993, 29A: 73–78.
[44] Desmouliere, A. et al., Transforming growth factor-beta 1 induces alpha-smooth muscle actin
expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts.
J. Cell Biol., 1993, 122: 103–111.
[45] Vaughan, M.B., E.W. Howard, and J.J. Tomasek, Transforming growth factor-beta1 promotes
the morphological and functional differentiation of the myofibroblast. Exp. Cell Res., 2000, 257:
180–189.
[46] Mann, B.K., R.H. Schmedlen, and J.L. West, Tethered-TGF-beta increases extracellular matrix
production of vascular smooth muscle cells. Biomaterials, 2001, 22: 439–444.
[47] Hirschi, K.K. et al., Transforming growth factor-beta induction of smooth muscle cell phenotpye
requires transcriptional and post-transcriptional control of serum response factor. J. Biol. Chem.,
2002, 277: 6287–6295.
[48] Cutroneo, K.R., Gene therapy for tissue regeneration. J. Cell Biochem., 2003, 88: 418–425.
[49] Lobov, I.B., P.C. Brooks, and R.A. Lang, Angiopoietin-2 displays VEGF-dependent modulation
of capillary structure and endothelial cell survival in vivo. Proc. Natl Acad. Sci. USA, 2002, 99:
11205–11210.
[50] Du, L. et al., Signaling molecules in nonfamilial pulmonary hypertension. N. Engl. J. Med., 2003,
348: 500–509.
[51] Nishishita, T. and P.C. Lin, Angiopoietin 1, PDGF-B, and TGF-beta gene regulation in endothelial
cell and smooth muscle cell interaction. J. Cell Biochem., 2004, 91: 584–593.
[52] Holland, T.A. et al., Transforming growth factor-beta1 release from oligo(poly(ethylene glycol)
fumarate) hydrogels in conditions that model the cartilage wound healing environment. J. Control.
Release, 2004, 94: 101–114.
[53] Kim, B.S. et al., Cyclic mechanical strain regulates the development of engineered smooth muscle
tissue. Nat. Biotechnol., 1999, 17: 979–983.
[54] Owens, G.K., Role of mechanical strain in regulation of differentiation of vascular smooth muscle
cells. Circ. Res., 1996, 79: 1054–1055.
[55] Seliktar, D. et al., Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces
remodeling in vitro. Ann. Biomed. Eng., 2000, 28: 351–362.
[56] Kim, B.S. and D.J. Mooney, Scaffolds for engineering smooth muscle under cyclic mechanical
strain conditions. J. Biomech. Eng., 2000, 122: 210–215.
[57] Niklason, L.E. et al., Functional arteries grown in vitro. Science, 1999, 284: 489–493.
[58] Alberts, B., Molecular Biology of the Cell. 4th ed. New York: Garland Science, 2002.
[59] Davis, M.J. et al., Integrins and mechanotransduction of the vascular myogenic response. Am.
J. Physiol. Heart Circ. Physiol., 2001, 280: H1427–H1433.
[60] Assoian, R.K. and M.A. Schwartz, Coordinate signaling by integrins and receptor tyrosine
kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev., 2001, 11:
48–53.
[61] Giancotti, F.G. and E. Ruoslahti, Integrin signaling. Science, 1999, 285: 1028–1032.
mikos: “9026_c024” — 2007/4/9 — 15:52 — page 12 — #12
24-12 Tissue Engineering
[62] Schwartz, M.A. and M.H. Ginsberg, Networks and crosstalk: integrin signalling spreads. Nat. Cell
Biol., 2002, 4: E65–E68.
[63] Howe, A.K., A.E. Aplin, and R.L. Juliano, Anchorage-dependent ERK signaling–mechanisms and
consequences. Curr. Opin. Genet. Dev., 2002, 12: 30–35.
[64] Cunningham, J.J., J.J. Linderman, and D.J. Mooney, Externally applied cyclic strain regulates
localization of focal contact components in cultured smooth muscle cells. Ann. Biomed. Eng.,
2002, 30: 927–935.
[65] L’Heureux, N. et al., A completely biological tissue-engineered human blood vessel. FASEB J.,
1998, 12: 47–56.
[66] Shin, H. et al., In vivo bone and soft tissue response to injectable, biodegradable oligo(poly(ethylene
glycol) fumarate) hydrogels. Biomaterials, 2003, 24: 3201–3211.
[67] Cao, Y. et al., Comparative study of the use of poly(glycolic acid), calcium alginate and pluronics
in the engineering of autologous porcine cartilage. J. Biomater. Sci. Polym. Ed., 1998, 9: 475–487.
[68] Weinberg, C.B. and E. Bell, A blood vessel model constructed from collagen and cultured vascular
cells. Science, 1986, 231: 397–400.
[69] L’Heureux, N. et al., In vitro construction of a human blood vessel from cultured vascular cells:
a morphologic study. J. Vasc. Surg., 1993, 17: 499–509.
[70] Thomson, R.C. et al., Fabrication of biodegradable polymer scaffolds to engineer trabecular bone.
J. Biomater. Sci. Polym. Ed., 1995, 7: 23–38.
[71] Wong, W. and D. Mooney, Synthesis and properties of biodegradable polymers used in synthetic
matrices for tissue engineering, In Atala, M.D., Ed., Synthetic Biodegradable Polymer Scaffolds,
Birkhäuser: Boston. pp. 51–84, 1997.
[72] Mikos, A.G. et al., Laminated three-dimensional biodegradable foams for use in tissue engineering.
Biomaterials, 1993, 14: 323–330.
[73] Kim, B.S. and D.J. Mooney, Engineering smooth muscle tissue with a predefined structure.
J. Biomed. Mater. Res., 1998, 41: 322–332.
[74] Peter, S.J. et al., Polymer concepts in tissue engineering. J. Biomed. Mater. Res., 1998, 43:
422–427.
[75] Niklason, L.E. and R.S. Langer, Advances in tissue engineering of blood vessels and other tissues.
Transpl. Immunol., 1997, 5: 303–306.
[76] Mooney, D.J. et al., Stabilized polyglycolic acid fibre-based tubes for tissue engineering.
Biomaterials, 1996, 17: 115–124.
[77] Nikolovski, J. and D.J. Mooney, Smooth muscle cell adhesion to tissue engineering scaffolds.
Biomaterials, 2000, 21: 2025–2032.
[78] Mann, B.K. et al., Modification of surfaces with cell adhesion peptides alters extracellular matrix
deposition. Biomaterials, 1999, 20: 2281–2286.
[79] Gao, J., L. Niklason, and R. Langer, Surface hydrolysis of poly(glycolic acid) meshes increases the
seeding density of vascular smooth muscle cells. J. Biomed. Mater. Res., 1998, 42: 417–424.
[80] Mooney, D.J. et al., Novel approach to fabricate porous sponges of poly(d,l-lactic-co-glycolic acid)
without the use of organic solvents. Biomaterials, 1996, 17: 1417–1422.
[81] Richardson, T.P. et al., Polymeric system for dual growth factor delivery. Nat. Biotechnol., 2001,
19: 1029–1034.
[82] Gombotz, W.R. et al., Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res.,
1991, 25: 1547–1562.
[83] Mann, B.K. and J.L. West, Cell adhesion peptides alter smooth muscle cell adhesion, proliferation,
migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J. Biomed.
Mater. Res., 2002, 60: 86–93.
[84] Tulis, D.A. et al., YC-1-mediated vascular protection through inhibition of smooth muscle cell
proliferation and platelet function. Biochem. Biophys. Res. Commun., 2002, 291: 1014–1021.
[85] Gobin, A.S. and J.L. West, Cell migration through defined, synthetic ECM analogs. FASEB J., 2002,
16: 751–753.
mikos: “9026_c024” — 2007/4/9 — 15:52 — page 13 — #13
Engineering Smooth Muscle 24-13
[86] Drury, J.L. and D.J. Mooney, Hydrogels for tissue engineering: scaffold design variables and
applications. Biomaterials, 2003, 24: 4337–4351.
[87] Guan, J. et al., Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable
poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J. Biomed. Mater. Res.,
2002, 61: 493–503.
[88] Lee, S.H. et al., Elastic biodegradable poly(glycolide-co-caprolactone) scaffold for tissue
engineering. J. Biomed. Mater. Res., 2003, 66A: 29–37.
[89] Fromstein, J.D. and K.A. Woodhouse, Elastomeric biodegradable polyurethane blends for soft
tissue applications. J. Biomater. Sci. Polym. Ed., 2002, 13: 391–406.
[90] Wang, Y. et al., A tough biodegradable elastomer. Nat. Biotechnol., 2002, 20: 602–6.
[91] Nakanishi, Y. et al., Tissue-engineered urinary bladder wall using PLGA mesh-collagen hybrid
scaffolds: a comparison study of collagen sponge and gel as a scaffold. J. Pediatr. Surg., 2003, 38:
1781–1784.
[92] Pariente, J.L., B.S. Kim, and A. Atala, In vitro biocompatibility evaluation of naturally derived
and synthetic biomaterials using normal human bladder smooth muscle cells. J. Urol., 2002, 167:
1867–1871.
[93] Cavallaro, J.F., P.D. Kemp, and K.H. Kraus, Collagen fabrics as biomaterials. Biotechnol. Bioeng.,
1994, 43: 781–791.
[94] Li, S.T., Biologic biomaterials: tissue-derived biomaterials (collagen). In Brozino, J.D., Ed. The
Biomedical Engineering Handbook. Boca Raton, FL: CRC Press, pp. 627–647, 1995.
[95] Kropp, B.P. et al., Regenerative urinary bladder augmentation using small intestinal submucosa:
urodynamic and histopathologic assessment in long-term canine bladder augmentations. J. Urol.,
1996, 155: 2098–2104.
[96] Badylak, S.F. et al., Comparison of the resistance to infection of intestinal submucosa arterial
autografts versus polytetrafluoroethylene arterial prostheses in a dog model. J. Vasc. Surg., 1994,
19: 465–472.
[97] Zhang, Y. et al., Coculture of bladder urothelial and smooth muscle cells on small intestinal
submucosa: potential applications for tissue engineering technology. J. Urol., 2000, 164: 928–934;
discussion 934–935.
[98] Voytik-Harbin, S.L. et al., Identification of extractable growth factors from small intestinal
submucosa. J. Cell Biochem., 1997, 67: 478–491.
[99] Chen, M.K. and S.F. Badylak, Small bowel tissue engineering using small intestinal submucosa as
a scaffold. J. Surg. Res., 2001, 99: 352–358.
[100] Wang, Z.Q., Y. Watanabe, and A. Toki, Experimental assessment of small intestinal submucosa as
a small bowel graft in a rat model. J. Pediatr. Surg., 2003, 38: 1596–1601.
[101] Yoo, J.J. et al., Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology,
1998, 51: 221–225.
[102] Huynh, T. et al., Remodeling of an acellular collagen graft into a physiologically responsive
neovessel. Nat. Biotechnol., 1999, 17: 1083–6.
[103] Badylak, S.F. et al., Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg.
Res., 1989, 47: 74–80.
[104] Falke, G., J. Caffaratti, and A. Atala, Tissue engineering of the bladder. WorldJ.Urol., 2000, 18:
36–43.
[105] Tu, J.V. et al., Use of cardiac procedures and outcomes in elderly patients with myocardialinfarction
in the United States and Canada. N.Engl.J.Med., 1997, 336: 1500–1505.
[106] Seliktar, D., R.M. Nerem, and Z.S. Galis, Mechanical strain-stimulated remodeling of tissue-
engineered blood vessel constructs. Tissue Eng., 2003, 9: 657–666.
[107] Reusch, P. et al., Mechanical strain increases smooth muscle and decreases nonmuscle myosin
expression in rat vascular smooth muscle cells. Circ. Res., 1996, 79: 1046–1053.
[108] Probst, M. et al., Reproductionof functional smooth muscle tissue and partial bladder replacement.
Br.J.Urol., 1997, 79: 505–515.
mikos: “9026_c024” — 2007/4/9 — 15:52 — page 14 — #14
24-14 Tissue Engineering
[109] Choi, R.S. and J.P. Vacanti, Preliminary studies of tissue-engineered intestine using isolated
epithelial organoid units on tubular synthetic biodegradable scaffolds. Transplant Proc., 1997,
29: 848–851.
[110] Hori, Y. et al., Experimental study on tissue engineering of the small intestine by mesenchymal
stem cell seeding. J. Surg. Res., 2002, 102: 156–160.
[111] Master, V.A. et al., Urothlelium facilitates the recruitment and trans-differentiation of fibroblasts
into smooth muscle in acellular matrix. J. Urol., 2003, 170: 1628–1632.
[112] Vacanti, J.P., Tissue and organ engineering: can we build intestine and vital organs? J. Gastrointest.
Surg., 2003, 7: 831–835.

mikos: “9026_c025” — 2007/4/9 — 15:52 — page1—#1
25
Esophagus: A Tissue
Engineering Challenge
B.D. Ratner
B.L. Beckstead
University of Washington
K.S. Chian
A.C. Ritchie
Nanyang Technological University
25.1 Medical Need/Clinical Problem......................... 25-1
25.2 Anatomy and Physiology of the Esophagus ............ 25-3
25.3 Criteria for a Tissue-Engineered Esophagus ........... 25-4
25.4 Scaffold Possibilities ..................................... 25-5
Background • Materials Selection • Scaffold Design
25.5 Fabrication Processes .................................... 25-7
Electrostatic Spinning • Cryogenic Molding • Rapid Freeze
Prototyping
25.6 Cell Possibilities .......................................... 25-10
Epithelial Characteristics • Epithelial Cell Source for Tissue
Engineering • Muscle Component of the Esophagus •
Engineering the Muscularis Mucosa and Externa •
Esophageal Regeneration
25.7 Bioreactors for Esophageal Tissue Engineering........ 25-15
Mechanical Conditioning of Smooth Muscle Tissue
Constructs
25.8 Conclusions and Prognostications ..................... 25-18
Acknowledgments............................................... 25-19
References ....................................................... 25-19
25.1 Medical Need/Clinical Problem
The esophagus, a muscular/mucosal tube connecting the mouth and pharynx to the stomach, is critical for
life (and good quality of life) (Figure 25.1). This seemingly simple organ is surgically challenging to repair
or replace. There are a number of conditions where surgical repair of the esophagus is indicated. These
include accident and trauma, congenital defects such as esophageal atresia (incomplete formation of the
esophagus) and tracheoesophageal fistulas and cancer. In 2003, roughly 14,000 people in the United States
were diagnosed with esophageal cancer. The prevalence of esophageal cancer in the general population
can be 10 to 100 times higher in Iran, China, Singapore, India, and South Africa. Worldwide, cancer of
the esophagus is the seventh leading cause of cancer death.
Surgical removal of a section of the esophagus and reconnection with the stomach, the most common
strategy for more advanced cancers, leads to complication rates as high as 40%. Strictures, dilation,
leakage, and infection are often observed. Attempts to use synthetics such as polyethylene, polypropylene,
teflon, or elastomers have also met with very limited success, problems being stenosis, leakage, infection,
25-1
