Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c005” — 2007/4/16 — 21:47 — page 18 — #18
mikos: “9026_c006” — 2007/4/9 — 15:50 — page1—#1
6
Cell Migration
Gang Cheng
Kyriacos Zygourakis
Rice University
6.1 Introduction.............................................. 6-1
6.2 Characteristics of Mammalian Cell Migration ........ 6-2
Cell Movement Cycle • Persistent Random Walk •
Cell–Cell Contacts
6.3 Regulation of Cell Movement ........................... 6-4
Soluble Factors Modulate Cell Movement • ECM Proteins
and Cell–Substrate Interactions Regulate Cell Movement •
Electrical Fields Direct Cell Movement
6.4 Cell Migration Assays.................................... 6-7
Cell-Population Assays • Individual-Cell Assays
6.5 Mathematical Models for Cell Migration and Tissue
Growth.................................................... 6-10
References ....................................................... 6-12
6.1 Introduction
Cell migration is an essential component of normal development, inflammation, tissue repair, angiogen-
esis, and tumor invasion. After conception, selected cells of the developing mammalian zygote invade the
uterine wall to establish the placenta, while the intricately programmed migration of other cells within
the embryo shapes the complex form of the emerging organism [1,2]. The nervous system is another
example of large-scale cell migration during fetal development. The growth of axons and dendrites is
preceded by a phase of cell migration in which immature neurons (or neuroblasts) move from their birth-
place to settle in some other location in order to make the right connections [3]. Certain kinds of white
blood cells are able to migrate through the walls of blood vessels and into the surrounding tissues, actively
seeking and engulfing sources of decay [4]. Migrating fibroblastic and epithelial cells heal wounds, and
osteoclasts and osteoblasts are in constant movement as they remodel bone [5–7]. Tumor cell motility
is also required for invasion and metastasis. The crawling malignant tumor cells that invade and disrupt
tissue architecture account as much or more for the lethality of cancer as does uncontrolled growth [8].
Cell migration also plays a key role in determining the structure and growth rate of bioartificial tissues
built on scaffolds made from suitable biomaterials [9]. In recent years, a lot of attention has been focused
on the development of biomimetic materials capable of promoting cell functions, including migration,
by biomolecular recognition [10]. Such recognition can be achieved by surface or bulk modification
of the material with bioactive molecules such as extracellular matrix (ECM) proteins or short peptide
sequences that can induce specific interactions with cell receptors. In order to design biomimetic scaffolds
with optimal properties for each application, however, we must thoroughly understand not only the
mechanism of cell migration, but also the many factors that modulate this important process. We must
6-1

mikos: “9026_c006” — 2007/4/9 — 15:50 — page2—#2
6-2 Tissue Engineering
also develop assays to accurately characterize cell movement on various biomaterials and, ultimately, build
theoretical models that can quantitatively predict the effect of system parameters (scaffold properties,
nutrient or growth factor concentrations, pH, etc.) on tissue development. Because tissues are highly
heterogeneous systems exhibiting complicated cell population dynamics, such theoretical models must be
able to accurately describe cell–cell and cell–substrate interactions.
This review attempts to address some of these issues for anchorage-dependent mammalian cells. After
a brief description of the mechanism of cell movement, we outline the role that growth factors, substrate-
adhesion molecules, and other environmental factors play in modulating cell migration. Since accurate
measurements are essential for elucidating the effect of a specific stimulus on cell migration, we discuss
the application of several assays that may be used to characterize cell motility. Finally, the use of theoretical
models for analyzing cell population dynamics and predicting tissue growth rates is discussed.
6.2 Characteristics of Mammalian Cell Migration
6.2.1 Cell Movement Cycle
The movement of a mammalian cell on a substrate is an intricate process requiring at least three structural
elements: an ECM ligand on the substrate, its cell surface receptor, and the intracellular cytoskeleton [6].
The receptors that play key roles for cell movement belong to a large family of transmembrane proteins
called integrins [11]. Migration can be considered as a continual cycle consisting of four essential steps
[4] (1) extension of the cell’s leading margin over the substratum to form a lamellipod (i.e., a thin piece
of membrane and cytoplasm at the front of the cell); (2) attachment to the substrate; (3) pulling or
contraction using the newly formed points of adhesion as anchorage; and (4) release or detachment of
adhesions at the rear of the cell. These four steps are orchestrated by the interaction of various extracellular
and intracellular molecules. Extension of the leading margin of the cell is caused by the polymerization
of actin filaments at the lamellipodia and crucial factors involved in this process are the Arp2/3 complex,
gelsolin, and capping protein [4,12,13]. At the base of the cortical actin meshwork, cofilin promotes
the disassembly of filaments [4,14]. Integrins anchor the cell to its substratum by binding both to ECM
molecules on the outside of the cell and to the actin cytoskeleton on the inside. Cortical contractions
due to myosin molecules pull structures toward the center of the cell, causing the uropod (lamellipod’s
counterpart at the rear of the cell) to retract and unattached structures on the dorsal surface to move
backward [15–17]. The cycle is completed by a forward movement of actin and other constituents through
the cytoplasm to the leading margin of the cell [4].
6.2.2 Persistent Random Walk
Migration of individual mammalian cells in isotropic environments can be described as a persistent
random walk [18,19]. Over short time periods, cells follow a relatively straight path, showing persistence
of movement (see Figure 6.1a). If long time intervals are used to observe the cell position, however, cell
movement appears similar to Brownian motion with frequent direction changes (Figure 6.1b). At least
two parameters are needed to describe persistent random walk [20]. The first one is the speed S that is
intuitively defined as the displacement of the cell centroid per unit time. The second one is the persistence
time (usually denoted by P) that is a measure of the average time between “significant” direction changes.
The magnitude of P and S depend both on the type of the cell and on its microenvironment. Reported
values of migration speed and persistence time range from 0.5 µm/min and 4 to 5 h, respectively for
human microvessel endothelial cells and smooth muscle cells [20,21] to 20 µm/min and 4 min for
rabbit neutrophils [22]. Lauffenburger and coworkers [23] pointed out that there exists a rough inverse
relationship between S and P and that this could be understood by considering the product of these two
parameters as the cell’s analog to a “mean free-path length.” Rigorous definition of P and S necessary for
mathematical modeling and the assays to measure them will be described in Section 6.4.
mikos: “9026_c006” — 2007/4/9 — 15:50 — page3—#3
Cell Migration 6-3
200
300
400
900 1000 1100 1200 1300
Start
200
300
400
900 1000 1100 1200 1300
Start
Observation interval= 3 h
Observation interval= 0.5 h(a)
(b)
X, mm
X, mm
Y, mm
Y, mm
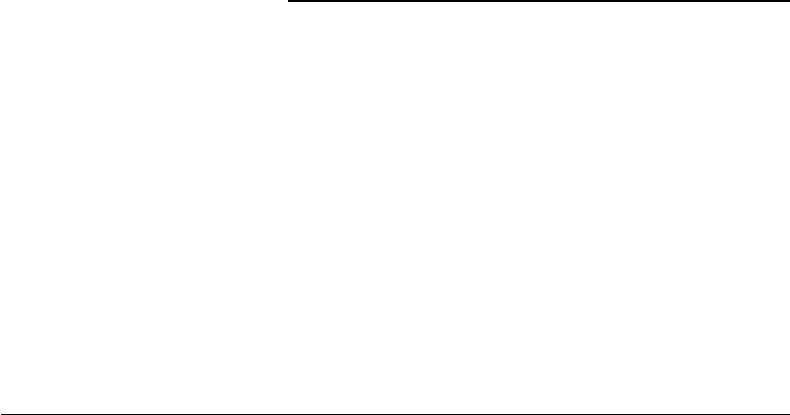
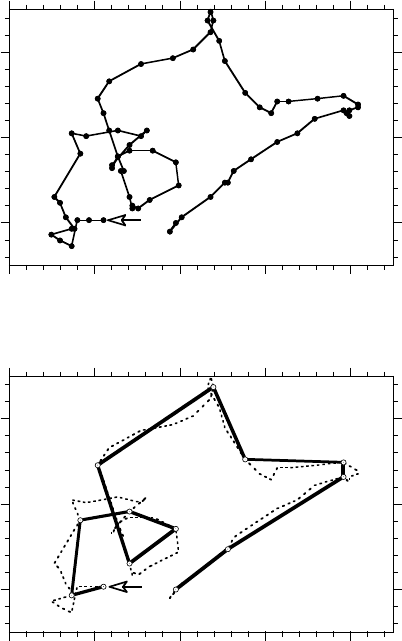
FIGURE 6.1 Typical trajectory of a bovine pulmonary artery endothelial cell migrating in a uniform environment.
Symbols represent the position of the centroid of the same cell recorded at 30 min intervals (top panel) and 3 h
intervals (bottom panel). When the observation interval is short (top panel), the cell clearly exhibits persistence in
movement direction. If a long observation interval (bottom panel) is chosen, however, the movement of the same cell
appears to be a random walk with frequent direction changes. (Adapted from Lee, Y., Mcintire, L.V., and Zygourakis,
K., Biochem. Cell Biol., 1995, 73: 461–472. With permission.)
6.2.3 Cell–Cell Contacts
In a population of migrating cells, the persistent random walk of individual cells can be interrupted by
contacts with other cells. Since cells usually stop and change the direction of their movement after such
contacts, this phenomenon is often called contact inhibition of locomotion [6]. When two fibroblasts collide
with each other, for example, ruffling of the membrane near the contact point stops to form a quiescent
region, while ruffling continues at other regions of the membrane. After about 25 min, the cells break
the adhesion and move away in new directions [6]. Cell–cell contacts have an even more profound effect
on the migration of epithelial cells. Following a collision, the leading lamellae of the epithelial cells are
gradually lost and an adhesion is formed between two cells. Sequential collisions with other cells result in
the formation of small colonies and eventually a sheet of contiguous cells [6,4]. This process is essential
for the coverage of the wounded area in wound healing [24,25]. It should be noted that cell–cell contacts
also affect cell division and the effect is also often referred to as “contact inhibition,” which actually

mikos: “9026_c006” — 2007/4/9 — 15:50 — page4—#4
6-4 Tissue Engineering
means contact inhibition of division. As is shown later, the mechanisms of these two contact inhibitions
are different, even though they are related in a rather complicated way.
6.3 Regulation of Cell Movement
6.3.1 Soluble Factors Modulate Cell Movement
Many polypeptide growth factors can upregulate cell motility. Sato and Rifkin [26] found that when a
confluent monolayer of bovine aortic endothelial cells (BAECs) was wounded with a razor blade, cells
at the edge of the wound released basic fibroblast growth factor (bFGF), which stimulated the rapid
movement of nearby cells into the denuded area. Addition of anti-bFGF IgG slowed down considerably
the cell movement and this inhibition was dose dependent. Sato and Rifkin also found that bFGF regulated
the basal level of synthesis of the protease plasminogen activator (PA) and the basal level of DNA synthesis.
These findings are consistent with earlier work demonstrating that plasmin contributed to cell migration
[27,28]. Sato and Rifkin [26] also offer the alternative explanation that bFGF acts as a motility factor
via its adhesive interactions described by Baird and coworkers [29]. Platelet-derived growth factor-BB
(PDGF-BB) has been found to be the major motility enhancing factor in human serum for human dermal
fibroblasts migrating on type I collagen, even though it is not needed for the initiation of cell movement
[30]. Transforming growth factor-α (TGF-α) enhances locomotion of cultured human keratinocytes [31].
Stimulation of MTLn3 cells (a metastatic carcinoma cell line) with epidermal growth factor (EGF) causes
rapid and transient lamellipod protrusion along with an increase in actin polymerization at the leading
edge [32]. EGF was also found to greatly enhance random dispersion of fibroblasts by increasing the
frequency of direction changes and at the same time slightly increasing the path length [33]. When
multiple types of growth factors are present, they may affect cell motility synergistically with certain
extent of specificity. For example, TGF-β1 has been found to synergistically enhance EGF-stimulated
hepatocyte motility responses on collagen-containing extracellular matrices. However, the same effect
was not achieved when TGF-β1 was added together with hepatocyte growth factor (HGF) [34]. Placental
growth factor (PlGF) can augment the migration of endothelial cell in response to vascular endothelial
growth factor (VEGF) in pathological angiogenesis [35].
Recent studies have revealed additional soluble motility-stimulating proteins, which include (a) The
scatter factor or SF (also known as HGF), a mesenchymal cell-derived protein, which causes contigu-
ous sheets of epithelium to separate into individual cells and stimulates the migration of epithelial as
well as vascular endothelial cells [36–38]; (b) the autocrine motility factor (AMF), a tumor cell-derived
protein, which stimulates migration of the producer cells [39–41]; and (c) the migration-stimulating
factor (MSF), a protein produced by fetal and cancer patient fibroblasts, which stimulates penetration of
three-dimensional collagen gels by nonproducing adult fibroblasts [42–44].
The direction of cell movement can be affected by the concentration gradient of certain growth factors,
a response called chemotaxis. Chemotaxis is very important for processes such as normal development,
inflammation, angiogenesis, and wound healing in which directed migration of specific cell types is essen-
tial. It has been found that when a wound is caused in the human body, large amounts of PDGF, EGF,
and TGF-β are secreted at different times by cells around the wound to coordinate the influx of neutro-
phils, macrophages, fibroblasts, smooth muscle cells, and endothelial cells for fast and complete healing
of the wound [45–48]. Lack of these chemotactic growth factors may impair the healing process, while
overproduction can cause excessive repair or scarring [49]. A chemotactic response is typically a func-
tion of both the absolute concentration of the attractant and the steepness of its concentration gradient.
Directional orientation bias increases with concentration gradient steepness, asymptotically approach-
ing a maximal level as steepness increases. The dependence on attractant concentration is biphasic,
increasing at low concentration to reach a maximum, then decreasing as concentration increases further
[50–52].
Several hypotheses have been proposed to explain the underlying mechanisms of these phenomena.
Lackie [6] suggested that cells decide their movement direction by receptor-mediated comparison of
mikos: “9026_c006” — 2007/4/9 — 15:50 — page5—#5
Cell Migration 6-5
the spatial difference in attractant concentration across the cell dimension. This explains the biphasic
dependence of directional orientation bias on attractant concentration for constant concentration
gradient. At low-attractant concentrations, very few receptors are bound and, thus, only a small ori-
entation bias results. At high-attractant concentrations almost all receptors are bound and, again, only
a small bias is observed. Maximum bias is found for an intermediate attractant concentration, where
the number of bound receptors is significant and most sensitive to differences in local attractant con-
centration. It should be noted that during the course of directed cell movement in chemotaxis, there
are periods during which the cell may randomly stray toward the lower concentration or any other
direction. Mechanistic models aimed at simulating chemotaxis must account for these random fluctu-
ations in the cell’s orientation even at the presence of the concentration gradient of a chemoattractant.
Tranquillo and coworkers [53,54] hypothesized that this is caused primarily by the probabilistic kinetics of
receptor/attractant binding. Their model divides a cell into two compartments along its polarization axis
and assumes that the instantaneous numbers of receptor/attractant complexes at the compartment sur-
faces are governed by a stochastic differential equation with both a deterministic and a probabilistic part,
which accounts for the random fluctuations in the binding process. The numbers of receptor/attractant
complexes are then used to calculate the concentration of motile effectors in each intracellular compart-
ment. Finally, the model postulates that the cell changes direction with an angular rate proportional to
the imbalance between the levels of motile effectors in the two compartments. Model results were shown
to provide good prediction for the chemotaxis of neutrophil leukocytes [55]. A recent extension of this
model [56] relaxes several simplifying assumptions regarding receptor dynamics in the original model
using newly obtained knowledge on transient G-protein signaling, cytoskeletal association, and receptor
internalization and recycling, including statistical fluctuations in the numbers of receptors among the
various states.
6.3.2 ECM Proteins and Cell–Substrate Interactions Regulate Cell
Movement
As described earlier, each of the four phases in cell-movement cycle involves the interaction of cell-surface
receptors with the ECM components on substratum surface. The ECM is a molecular complex whose
components include collagens, glycoproteins, hyaluronic acid, proteoglycans, glycosaminoglycans, and
elastins [57,58]. In addition, ECM harbors molecules such as growth factors, cytokines, matrix-degrading
enzymes, and their inhibitors [57]. The distribution of these molecules varies from tissue to tissue and
changes with time during tissue development, making the ECM a highly dynamic system [59,60]. The
binding of ECM molecules to the extracellular domains of integrins triggers the receptor/ligand binding,
trafficking and signaling cascade, activation of transcription factor and expression of target genes, and
eventually results in the regulation of specific cell functions, which may include adhesion, migration,
proliferation, and differentiation.
Integrin receptors are heterodimeric proteins composed of α and β subunits [11]. At least 15 α and β
subunits have been identified so far and they pair with each other in a variety of combinations, giving rise
to specific recognition on the ECM molecules with different selectivity. These combinations include the
α
5
β
1
fibronectin receptor, α
2
β
1
, α
3
β
1
, and the vitronectin receptor α
v
β
3
[61–63]. While the α
5
β
1
integrin
binds exclusively to fibronectin, α
1
β
1
can bind either to laminin or to collagen-IV and the α
v
β
3
receptor
recognizes fibrinogen, vitronectin, and probably fibronectin. The α
4
β
1
integrin has also been found to
mediate cell motility on fibronectin and vascular adhesion molecule-1 (VCAM-1) independently of the
α
5
β
1
[64].
The ability of integrin receptors to recognize and bind the short peptide sequences corresponding to
the adhesive domains of ECM proteins stimulated a lot of interest in developing biomimetic materials.
Several studies by Hubbell and coworkers [65–71] have shown that covalent immobilization of adhesive
peptides like RGD or YIGSR (which are the adhesive domains of fibronectin and laminin respectively) on
the surface of glass or polymeric substrates can promote adhesion of endothelial cells. Kouvroukoglou and
coworkers [72] found that such surface modifications significantly enhanced the migration of endothelial
mikos: “9026_c006” — 2007/4/9 — 15:50 — page6—#6
6-6 Tissue Engineering
cells, a fact that might lead to higher endothelization rates of the surfaces of implantable biomaterials.
Yang and coworkers [73] found that surface modification of poly(lactic acid) (PLA) films and poly(lactic-
co-/glycolic acid) (PLGA) porous structures with RGD peptides or fibronectin greatly promoted human
osteoprogenitor adhesion, migration, growth, and differentiation. Shin and coworkers [74] found that
bulk modification of oligo(poly[ethylene glycol] fumarate) (OPF) hydrogel with a rat osteopontin-derived
peptide (ODP) or a RGD peptide could increase the motility of marrow stromal osteoblasts and accelerate
the expansion of megacolonies of these cells on the surface of the hydrogel.
The sudden introduction of a migratory ECM ligand into the environment of a cell may provide
a key signal for the initiation of cell migration [75]. Similarly, the biosynthetic induction of such a
migration protein or of its receptors might also promote migration [64,75–79]. The driving mechanism
for migration appears to be provided by the physical interactions between specific sequences in adhesion
proteinsand their receptors [75], and by intracellular contractile proteins[4,17]. The signals involved in the
termination of cell migration include the reacquisition of cell–cell adhesion molecules (e.g., N -cadherin),
often accompanied by differentiation into the final tissue type [75].
The speed of cell movement on ECM proteins is regulated not only by the type of receptor/ligand
interactions, but also by the ligand density on the substrate and the ligand affinity for cell adhesion
receptors [80–83]. The complexity of these interactions leads to a biphasic dependence of cell speed on
substrate adhesiveness. Goodman and coworkers [84] found that the migration speed of murine myoblasts
on substrates covered with laminin or laminin fragment E8 depended in a biphasic fashion on the density
of surface ligand. Maximum cell speeds were observed for ligand densities ranging from one third to
one tenth of those required for strongest cell attachment. Low cell speeds were measured when the cells
adhered very strongly or very weakly to the surface. A slow, monotonic increase of cell speed was observed,
however, when the density of fibronectin ligands was increased. DiMilla and coworkers [22] measured the
speed of human smooth muscle cells on fibronectin and collagen-IV. They found that cell speed varied
in a biphasic fashion with increasing cell adhesion strengths for both ligands. Similar results are seen by
varying the number of receptors on the cell surface. Keely and coworkers [85] found that the strength of
adhesion of human breast carcinoma T47D cells to collagen I and IV decreased with decreasing levels of
expression of the α
2
β
1
integrin (a collagen and laminin receptor). T47D clones that exhibited intermediate
levels of adhesion to collagen had the highest motility across collagen-coated filters, suggesting that an
intermediate density of cell-surface α
2
β
1
integrin optimally supports cell motility.
The reasons for this contrasting behavior were revealed by an elegant mathematical analysis of the cell-
migration cycle by Lauffenburger [86] and DiMilla and coworkers [87]. The first key system parameter for
explaining the experimental data is the substratum adhesiveness that is proportional to the surface ligand
density and inversely proportional to the receptor/ligand equilibrium dissociation constant. Substrate
adhesiveness, however, may not only depend on the strength of receptor/ligand association. A later ana-
lysis of Ward and Hammer [88] showed that the formation of focal contacts (which were modeled as
cytoplasmic nucleation centers binding adhesion receptors) and the elastic rigidity of cytoskeletal connec-
tions may significantly affect cellular adhesive strength. The second parameter describes the asymmetry in
bond affinity as the ratio of the dissociation rate constants between the front and the rear of the migrating
cell. The model correctly predicted a biphasic dependence of cell speed on substrate adhesiveness, with cell
locomotion occurring over an intermediate and (in many cases) limited range of adhesiveness. The size of
this range was primarily governed by the asymmetry in adhesiveness between the front and the rear of the
cell. Several mechanisms can provide a front-to-rear asymmetry in cell/substrate adhesiveness including
dynamic integrin/cytoskeletal interactions [89] and polarized distributions of integrins maintained by
receptor trafficking mechanisms [90].
The substratum adhesiveness can also affect the direction of cell movement, a response called hapto-
taxis. For example, cells cultured on a surface will move onto tracks of artificial adhesive material, such as
polylysine or silicon oxide [4]. When fibroblasts are plated on a surface coated with a uniformly increasing
gradient of a charged substance, they turn and move in the direction of increasing adhesiveness [91].
Dickinson and Tranquillo [92] have developed a stochastic mathematical model based on receptor/ligand
binding and trafficking mechanism to provide a mechanistic understanding of how the magnitude and

mikos: “9026_c006” — 2007/4/9 — 15:50 — page7—#7
Cell Migration 6-7
distribution of adhesion ligands in the substratum influence cell movement. Additional sources for dir-
ectionality in movement may be simple population pressure, tissue or physical barriers [4,93], or the
three-dimensional structure of the matrix. In a process known as contact guidance, matrix fibers can be
spatially arranged as to facilitate cell movement in a preferred direction [94]. Micromachined grooves cut
into the substratum have similar effect [95].
While properties of the substratum can greatly influence cell movement, cells can also direct their
own migration by modifying the physical properties of the substratum. For example, pioneering cells
within a population of migrating neural crest cells may be laying down extracellular cues that trailing cells
recognize [96]. When a suspension of human keratinocytes is plated on a fibrin matrix, single cells invade
the matrix and progress through it by dissolving the fibrin and thereby creating tunnels [97].
6.3.3 Electrical Fields Direct Cell Movement
The migration of many mammalian cells is affected by the presence of electric fields, a phenomenon
called galvanotaxis. Nerve cells can detect electric field as weak as 10 mV/mm and turn their growth
cones to move in the direction of the negative pole [4]. Fibroblasts and cells from the neural crest also
move toward the negative pole in a steady electric field [98,99]. During wound healing in vertebrates, a
steady lateral electric field of 40 to 200 mV/mm is generated in the disrupted epithelia layers to coordinate
the directed migration of epithelial cells from the nearby regions [100]. Some experiments indicate that
when the electric field is removed, the wound healing rate is 25% slower, while nearly every clinical trial
using electric fields to stimulate healing in mammalian wounds reports a significant increase in the rate
of healing from 13 to 50% [101].
Cell’s response to electrical fields typically requires Ca
2+
influx [102], the presence of specific growth
factors [100] and intracellular kinase activity. Protein kinase C is required by neural crest cells [103]
and cAMP-dependent protein kinase is used in keratinocytes [104], while mitogen-activated protein
kinase is required by corneal epithelial cells [105]. Specifically, Zhao and coworkers [100] discovered that
corneal epithelial cells cultured in serum-free medium showed no reorientation in an electric field until
250 mV/mm and addition of EGF, bFGF, or TGF-β 1 singly or in combination significantly restored the
cathodal reorientation response at low field strengths. Interestingly, however, the directed migration of
two fibroblastic cells, NIH 3T3 and SV101, was found to be calcium independent and was, instead,
related to the lateral redistribution of plasma membrane glycoproteins involved in cell–substratum
adhesion [98].
6.4 Cell Migration Assays
6.4.1 Cell-Population Assays
The methods used to assess the locomotory capabilities and characteristics of cells fall into two major
categories. The first category includes techniques that monitor large populations of migrating cells and
analyze the number density profiles of the population after a given time period of migration. The Boyden
chamber is the most popular assay in this category [106–111]. According to this technique, cells are placed
on the upper surface of a micropore filter installed in the chamber and incubated for a sufficient period of
time to allow the cells to migrate to the lower surface of the filter. Cell motility is then quantified either by
counting the number of cells that have migrated through the filter or by measuring the distance traveled
into the filter by several of the fastest moving cells. A comparison of the number of cells that passed
through the filter or of the migration distance measured for various experimental conditions reveal if, for
example, a test substance is chemotactic for the cells under study. With appropriate modifications, the
Boyden chamber can also be used to study cell migration under flow conditions or temporal chemotactic
gradients [112,113].
Another cell-population assay measures the migration distance of endothelial cells or osteoblasts
released from growth arrest using a silicon or steel template compartmentalization technique [74,114].
mikos: “9026_c006” — 2007/4/9 — 15:50 — page8—#8
6-8 Tissue Engineering
This assay system employs tissue culture dishes that are subdivided (using, for example, stainless steel
annular rings) into two separate compartments: an inner circular core and an annular outer ring. Cells
are seeded into the inner compartment and grown to confluence. The inner ring is then removed, and
cells (released from growth arrest) start migrating from a sharp starting line. Cell motility is quantified by
measuring the distance of the migrating front from the starting line. By using a growth-arrested mono-
layer as a starting cell population for the quantification of migration, this assay system produces few or no
wounded cells at the migration front. It also allows us to evaluate the migratory response to other cell types
by coculturing them with the test cells after having seeded the effector cells into the outer compartment of
the assay system. Since migration of endothelial cells is one of the critical features of wound repair, several
researchers have also studied the movement of cells from a wound edge into a denuded area formed by
scraping a confluent monolayer with a razor blade [26,115,116]. Migration is then quantified by counting
the number of cells in successive 125-µm sections from the wound edge.
A third popular technique is the under-agarose assay where cells are allowed to migrate under a layer
of agarose gel deposited on a glass or plastic surface [117–120]. For random motility experiments, a
migration stimulus is incorporated at uniform concentration into the gel and the cell well medium.
For chemotaxis experiments, the migration stimulus is placed in a separate well from which it forms a
concentration gradient by diffusing through the gel. Typically measured quantities are again the location
of the leading front or the total number of cells migrating away from the well.
However, intrinsic cell locomotory properties are not the only factors influencing the measurements
obtained by cell-population assays. Parameters like the assay chamber geometry and size, initial cell num-
ber, or attractant diffusivity can significantly affect the population dispersal and complicate comparison
of different sets of experimental data. To alleviate these problems, a phenomenological model has been
proposed by Keller and Segel [121] and reformulated by Rivero and coworkers [122]. This mathematical
model takes the form of a partial differential equation describing the temporal evolution of the cell number
density profile and having two key parameters (a) the random motility coefficient µ and (b) a chemotaxis
coefficient χ . By comparing model predictions for cell number density profiles to experimental profiles
measured with the linear under-agarose assay [123] or the filter assay [124], the random motility µ and
the chemotaxis coefficient χ can be determined. A different approach was recently followed by Cheng
and coworkers [125] to analyze the differential effect of cell migration and proliferation on the expan-
sion of megacolonies of marrow stromal cells cultured on biomimetic hydrogels modified with RGD or
osteopontin-derived peptides [74]. This study used a discrete model based on the Markov chain approach
to simulate the cell-population dynamics of expanding megacolonies. A comparison of model predictions
to experimental data showed that surface modifications enhance the expansion rates of cell megacolonies
by upregulating the speeds of cell migration.
6.4.2 Individual-Cell Assays
Cell-population assays cannot provide detailed information on how cells move. They cannot directly
quantify important locomotory parameters (like cell speed, persistence, turn angles, etc.) or evaluate
the effects of external stimuli on these parameters. An accurate characterization of cell locomotion can
only be obtained by continuously monitoring a sufficiently large population of migrating cells using a
video microscopy system with digital or analog time-lapse capabilities. In most applications, cells migrate
on two-dimensional surfaces beneath liquid culture media or agarose gels [21,72,126–129]. Procedures
allowing tracking in three-dimensional collagen matrices have also been reported [50,130–136].
By analyzing a sequence of images obtained at fixed time intervals, the actual cell positions at the
corresponding times can be identified to reconstruct the individual cell trajectories (see Figure 6.1a). The
mean square displacement D
2
of the cells from their original positions can then be calculated and plotted
vs. time. If cell movement were a random walk, the mean square displacement D
2
would vary with time
according to
D
2
=2nµt (6.1)

mikos: “9026_c006” — 2007/4/9 — 15:50 — page9—#9
Cell Migration 6-9
where µ is the random motility coefficient (formally equivalent to a diffusion coefficient) and n is a constant
depending on the dimensionality of the random walk. For two-dimensional walks n = 2, while for three-
dimensional walks n = 3 [18,137,138]. Equation 6.1 implies that the average distance traveled by a cell is
proportional to the square root of the elapsed time.
As we mentioned in Section 6.2.2, however, mammalian cells migrating in isotropic environments
execute persistent random walks. Dunn [19] and Othmer and coworkers [139] developed the following
mathematical model to describe persistent random walks:
D
2
=nS
2
[Pt − P
2
(1 −e
−t/P
)] (6.2)
where D
2
is the mean square displacement of the tracked cells, S is the root-mean-square cell speed, P
is the persistence time, and n is the constant that gives the dimensionality of the persistent random walk
(n is 2 or 3 for 2D or 3D walks, respectively). This model assumes that S and P are time invariant. The
root-mean-square cell speed S and persistence time P can be computed by fitting the experimental D
2
vs. time data with the persistent random walk model of Equation 6.2. Nonlinear parameter estimation
algorithms [123] must be used, since graphical techniques [19] lead to results dependent on the size of
the time interval. Note that for long times (t P), Equation 6.2 reduces to the much simpler expression:
D
2
=nS
2
Pt (6.3)
Equation 6.3 implies random walk behavior and allows us to compute the random motility coefficient µ as
follows:
µ =
1
2
S
2
P (6.4)
Many investigators have successfully used the persistent random walk model to quantify cell migration
[72,140–144]. The chemotactic motion of cells in response to a chemical concentration gradient can also
be modeled by adding a directional bias to the persistent random walk process [21]. The magnitude of
the directional bias is characterized by the chemotactic responsiveness κ. By using experimentally measured
values of S, P, and κ, Stokes and Lauffenburger generated computer simulations of theoretical individual
cell paths, which were useful in elucidating the role of cell migration in physiological processes [21].
Although the persistent random walk model has been very successful in assaying and comparing the
motility of cells under various conditions, it cannot provide all the parameters that may be necessary for
an accurate quantification of the cell-migration process. Experimental observations with endothelial cells
[127] have revealed that cells slow down or even stop migrating when they divide or when they collide with
other cells. Migrating cells may also stop for a while before changing their direction of movement [127].
A detailed description of cell movement (suitable, e.g., for the computer implementation of migration-
proliferation models of tissue growth [145]) requires the following information (1) the speed of cell
locomotion (swimming speed); (2) the expected duration of cell movement in any given direction; (3)
the probability distribution of turn angles that will decide the next direction of cell movement; (4) the
frequency of cell stops; and (5) the duration of cell stops. The ultimate direction of cell movement should
also be obtained to check for spatial heterogeneities or chemotactic phenomena.
When such a detailed description of the migration process is desired, models based on Markov chain
concepts must be used to analyze the cell trajectory data [146–148]. At any time t, a migrating cell
can exist in either a directional state (if it moves in a certain direction) or the stationary state (if it has
stopped moving). Clearly, there is a change of state every time the migrating cell changes direction and
when it starts or stops moving. The central assumption here is that state changes are random and do
not depend either on the past history of the cell or on the length of time the cell spent in its current
state. Under these assumptions, the sequence of states is a stochastic Markov sequence. Details for this
analysis may be found in the monograph of Noble and Levine [147]. One usually allows only a finite
number of directional states by partitioning the set of all possible directions of movement. Four or
eight directional states are typically considered for two-dimensional walks (in addition to the stationary
