Faulon J.L., Bender A. Handbook of Chemoinformatics Algorithms
Подождите немного. Документ загружается.

228 Handbook of Chemoinformatics Algorithms
0
1
2
3
4
5
6
7
8
02468
Observed activities
Predicted activities
y = 0.91x + 0.62
R
2
= 0.80
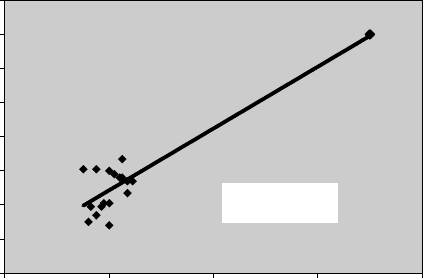
FIGURE 7.3 A typical example when one outlier increases the prediction accuracy. Without
the outlier, R
2
= 0.13.
obtained (see Section 6.4) and each descriptor matrix is processed by some algorithms
(depending on the user’s decision and types of descriptors and optimization proce-
dures selected) described in Section 6.5 and possibly other procedures not mentioned
here. If necessary, a diverse subset of compounds is selected from the entire modeling
set (see Section 6.6). Possible structural and activity outliers are removed from the
modeling set (see Section 6.7). Additional procedures may be required for classifica-
tion and category QSAR modeling, especially, if a dataset is imbalanced or large (see
Section 6.8). Each dataset should be divided into modeling and external evaluation
sets (see Sections 6.9 and 6.10). For each descriptor set, splitting a modeling set into
multiple training and test sets is carried out using a sphere-exclusion algorithm (see
Section 6.10). Then the models are developed (see Section 7.1) for all combinations
of pairs (descriptor collection, method); appropriate target function and criteria of
predictivity are applied (see Section 7.2) based on the nature of the response variable
(continuous, classification, or category). The models with acceptable statistics for
both training and test sets (for the test set prediction, the respective ADs are used—
see Section 7.5), if any, should be rigorously validated by procedures described in
Section 6.9 and Sections 7.3 and 7.4, and some other procedures and their ADs should
be established (see Section 7.5). Finally, the results of QSAR modeling will be ready
to be used for chemical database or virtual library mining.
With more than 40 years of history behind it, QSAR modeling is a well-established
research field that (as perhaps with any scientific area) had its ups and downs. There
were several recent publications that criticized the current state of the field. Thus, a
recent editorial published by the leading chemoinformatics Journal of Chemical Infor-
mation and Modeling (JCIM; also reproduced by the Journal of Medicinal Chemistry)
introduced severe limitations on the level and quality of QSAR papers to be considered
acceptable [37]. Another recent editorial opinion by G. Maggiora [38] outlined lim-
itations and some reasons for failures of QSAR modeling that relate to the so-called
activity cliffs. In another recent important paper, T. Stouch addressed the question as
Predictive Quantitative Structure–Activity Relationships Modeling 229
to why in silico ADME/Tox models fail [39]. These examples naturally lead to an
important and perhaps critical question as to whether there is any room for further
advancement of the field via innovative methodologies and important applications.
Our previous and ongoing research in the area of QSAR suggests that the answer
is a resounding “yes.” We believe strongly that many examples of low impact QSAR
research are due to frequent exploration of datasets of limited size with little attention
paid to model external validation. This limitation leads to models having questionable
“mechanistic” explanatory power but perhaps little if any forecasting ability outside
of the training sets used for model development. We believe that the latter ability
along with the capabilities of QSAR models to explore chemically diverse datasets
with complex biological properties should become the chief focus of QSAR studies.
This focus requires the re-evaluation of the success criteria for the modeling as well
as the development of novel chemical data mining algorithms and model validation
approaches. In fact, we think that the most interesting era in QSAR modeling is just
beginning with the rapid growth of the experimental SAR data space [40].
In the last 15 years, innovative technologies that enable rapid synthesis and high-
throughput screening of large libraries of compounds have been adopted in almost
all major pharmaceutical and biotech companies. As a result, there has been a huge
increase in the number of compounds available on a routine basis to quickly screen for
novel drug candidates against new targets or pathways. In contrast, such technologies
have rarely become available to the academic research community, thus limiting its
ability to conduct large-scale chemical genetics or chemical genomics research. The
NIH Molecular Libraries Roadmap Initiative has changed this situation by forming
the national Molecular Library Screening Centers Network (MLSCN) [41] with the
results of screening assays made publicly available via PubChem [42]. These efforts
have already led to the unprecedented growth of available databases of biologically
tested compounds (cf. our recent review where we list about 20 available databases
of compounds with known bioactivity [40]). This growth creates new challenges for
QSAR modeling such as developing novel approaches for the analysis and visual-
ization of large databases of screening data, novel biologically relevant chemical
diversity or similarity measures, and novel tools for virtual screening of compound
libraries to ensure high expected hit rates. Due to the significant increase in recent
years of the number of publicly available datasets of biologically active compounds
and the critical need to improve the hit rate of experimental compound screening,
there is a strong need in developing widely accessible and reliable computational
QSAR modeling techniques and specific end-point predictors.
REFERENCES
1. Kovatcheva,A., Golbraikh,A., Oloff,S., Xiao,Y. D., Zheng,W.,Wolschann, P., Buchbauer,
G., and Tropsha, A., Combinatorial QSAR of ambergris fragrance compounds. J. Chem.
Inf. Comput. Sci. 2004, 44, 582–595.
2. Kovatcheva, A., Golbraikh, A., Oloff, S., Feng, J., Zheng, W., and Tropsha, A., QSAR
modeling of datasets with enantioselective compounds using chirality sensitive molecular
descriptors. SAR QSAR Environ. Res. 2005, 16, 93–102.
230 Handbook of Chemoinformatics Algorithms
3. de Cerqueira, L. P., Golbraikh, A., Oloff, S., Xiao, Y., and Tropsha, A., combinatorial
QSAR modeling of P-glycoprotein substrates. J. Chem. Inf. Model. 2006, 46, 1245–1254.
4. Wang, X. S., Tang, H., Golbraikh, A., and Tropsha,A., Combinatorial QSAR modeling of
specificity and subtype selectivity of ligands binding to serotonin receptors 5HT1E and
5HT1F. J. Chem. Inf. Model. 2008, 48, 997–1013.
5. Golbraikh, A. and Tropsha, A., Beware of Q2! J. Mol. Graph. Model. 2002, 20, 269–276.
6. Saliner, A. G., Netzeva, T. I., and Worth, A.P., Prediction of estrogenicity: Validation of a
classification model. SAR QSAR. Environ. Res. 2006, 17, 195–223.
7. Hsieh, J. H., Wang, X. S., Teotico, D., Golbraikh, A., and Tropsha, A., Differentiation of
AmpC beta-lactamase binders vs. decoys using classification kNN QSAR modeling and
application of the QSAR classifier to virtual screening. J. Comput. Aided Mol. Des. 2008,
22, 593–609.
8. Li, Y., Pan, D., Liu, J., Kern, P. S., Gerberick, G. F., Hopfinger, A. J., and Tseng, Y. J.,
Categorical QSAR models for skin sensitization based upon local lymph node assay clas-
sification measures part 2: 4D-Fingerprint three-state and two-2-state logistic regression
models. Toxicol. Sci. 2007, 99, 532–544.
9. Efron, B., Bootstrap methods:Another look at the jackknife. The Annals of Statistics 1979,
7, 1–26.
10. Mooney, C. Z. and Duval, R. D., Bootstrapping. A Non-Parametric Approach to Statistical
Inference. Sage, Newbury Park, CA, 1993.
11. DiCiccio, T. J. and Efron, B., Bootstrap confidence intervals. Statist. Sci. 1996, 11, 189–
228.
12. Efron, B., Better bootstrap confidence intervals. J.Amer. Statist. Assoc. 1987, 82, 171–220.
13. Isaksson, A., Wallman, M., Göransson, H., and Gustafsson, M. G., Cross-validation and
bootstrapping are unreliable in small sample classification. Pattern Recogn. Lett. 2008,
29, 1960–1965.
14. Wickenberg-Bolin, U., Goransson, H., Fryknas, M., Gustafsson, M. G., and Isaksson, A.,
Improved variance estimation of classification performance via reduction of bias caused
by small sample size. BMC Bioinform. 2006, 7, 127.
15. Jaworska, J., Nikolova-Jeliazkova, N., and Aldenberg, T., QSAR applicabilty domain
estimation by projection of the training set descriptor space: A review. Altern. Lab Anim.
2005, 33, 445–459.
16. Jaworska,J. and Nikolova-Jeliazkova, N., Review of methods to assess a QSAR applicabil-
ity domain, http://ambit.acad.bg/nina/publications/2004/AppDomain_qsar04.ppt, 2008.
17. Nikolova-Jeliazkova, N. and Jaworska, J., An approach to determining applicability
domains for QSAR group contribution models: An analysis of SRC KOWWIN. Altern.
Lab Anim. 2005, 33, 461–470.
18. Netzeva, T. I., Gallegos, S. A., and Worth, A. P., Comparison of the applicability domain
of a quantitative structure–activity relationship for estrogenicity with a large chemical
inventory. Environ. Toxicol. Chem. 2006, 25, 1223–1230.
19. Fechner, N., Hinselmann, G., Schmiedl, C., and Zell, A., Estimating the applicability
domain of Kernel-based QSPR models using classical descriptor vectors. pdf. Chem.
Central J. 2008, 2(Suppl. 1), 2.
20. Afantitis, A., Melagraki, G., Sarimveis, H., Koutentis, P. A., Markopoulos, J., and Igglessi-
Markopoulou, O., A Novel QSAR model for predicting induction of apoptosis by 4-Aryl-
4H-chromenes. Bioorg. Med. Chem.
2006, 14, 6686–6694.
21.
Govindraju, V., Pattern Recognition Review (1)—Definitions, Bayesian Classifiers,
http://www.cedar.buffalo.edu/∼govind/CSE666/fall2007/Pattern_recognition_lecture_
slides_1.pdf, 2008.
Predictive Quantitative Structure–Activity Relationships Modeling 231
22. Guha, R., Dutta, D., Jurs, P. C., and Chen, T., R–NN curves:An intuitive approach to outlier
detection using a distance based method. J. Chem. Inf. Model. 2006, 46, 1713–1722.
23. Kuhne, R., Ebert, R. U., and Schuurmann, G., Model selection based on structural
similarity-method description and application to water solubility prediction. J. Chem.
Inf. Model. 2006, 46, 636–641.
24. Zhang, L., Zhu, H., Oprea, T. I., Golbraikh, A., and Tropsha, A., QSAR modeling of the
blood–brain barrier permeability for diverse organic compounds. Pharm. Res. 2008, 25,
1902–1914.
25. Tetko, I. V., Bruneau, P., Mewes, H. W., Rohrer, D. C., and Poda, G. I., Can we estimate
the accuracy of ADME-tox predictions? Drug Discov. Today 2006, 11, 700–707.
26. Netzeva, T. I., Worth, A., Aldenberg, T., Benigni, R., Cronin, M. T., Gramatica, P.,
Jaworska, J. S., et al., Current status of methods for defining the applicability domain
of (Quantitative) structure–activity relationships. The Report and Recommendations of
ECVAM Workshop 52. Altern. Lab Anim. 2005, 33, 155–173.
27. Manallack, D. T., Tehan, B. G., Gancia, E., Hudson, B. D., Ford, M. G., Livingstone, D. J.,
Whitley, D. C., and Pitt, W. R., A consensus neural network-based technique for dis-
criminating soluble and poorly soluble compounds. J. Chem. Inf. Comput. Sci. 2003, 43,
674–679.
28. Bruneau, P. and McElroy, N. R., LogD7.4 Modeling using Bayesian regularized neural
networks. Assessment and correction of the errors of prediction. J. Chem. Inf. Model.
2006, 46, 1379–1387.
29. Bruneau, P., Search for predictive generic model of aqueous solubility using Bayesian
neural nets. J. Chem. Inf. Comput. Sci. 2001, 41, 1605–1616.
30. Tetko, I.V., Sushko, I., Pandey,A. K., Zhu, H., Tropsha,A., Papa, E., Oberg,T., Todeschini,
R., Fourches, D., and Varnek, A., Critical assessment of QSAR models of environmental
toxicity against tetrahymena pyriformis: Focusing on applicability domain and overfitting
by variable selection 1. J. Chem. Inf. Model. 2008, 48, 1733–1746.
31. Sachs, L., Applied Statistics: A Handbook of Techniques. Springer, New York, 1984.
32. Shen, M., Beguin, C., Golbraikh,A., Stables, J. P., Kohn, H., and Tropsha,A., Application
of predictive QSAR models to database mining: Identification and experimental validation
of novel anticonvulsant compounds. J. Med. Chem. 2004, 47, 2356–2364.
33. Votano, J. R., Parham, M., Hall, L. H., Kier, L. B., Oloff, S., Tropsha, A., Xie, Q., and
Tong, W., Three new consensus QSAR models for the prediction of Ames genotoxicity.
Mutagenesis 2004, 19, 365–377.
34. Zhang, S., Wei, L., Bastow, K., Zheng, W., Brossi, A., Lee, K. H., and Tropsha, A., Antitu-
mor agents 252. Application of validated QSAR models to database mining: Discovery of
novel tylophorine derivatives as potential anticancer agents. J. Comput. Aided Mol. Des.
2007, 21, 97–112.
35. Zhu, H., Tropsha, A., Fourches, D., Varnek, A., Papa, E., Gramatica, P., Oberg, T., Dao, P.,
Cherkasov, A., and Tetko, I. V., Combinatorial QSAR modeling of chemical toxicants
tested against tetrahymena pyriformis. J. Chem. Inf. Model. 2008, 48, 766–784.
36. Vasanthanathan, P., Lakshmi, M., Arockia, B. M., Gupta, A. K., and Kaskhedikar, S. G.,
QSAR study of 3-phenyl-5-acyloxymethyl-2H,5H-furan-2-ones as antifungal agents: The
dominant role of electronic parameter. Chem. Pharm. Bull. (Tokyo) 2006, 54, 583–587.
37. Jorgensen, W. L. and Tirado-Rives, J., QSAR/QSPR and proprietary data. J. Chem. Inf.
Model. 2006, 46, 937.
38. Maggiora, G. M., On outliers and activity cliffs—why QSAR often disappoints. J
.
Chem.
Inf. Model. 2006, 46, 1535.
232 Handbook of Chemoinformatics Algorithms
39. Stouch, T. R., Kenyon, J. R., Johnson, S. R., Chen, X. Q., Doweyko, A., and Li, Y., In
silico ADME/Tox: Why models fail. J. Comput. Aided Mol. Des. 2003, 17, 83–92.
40. Oprea, T. and Tropsha,A., Target, chemical and bioactivity databases—integration is key.
Drug Discov. Today 2006, 3, 357–365.
41. Austin, C. P., Brady, L. S., Insel, T. R., and Collins, F. S., NIH molecular libraries initiative.
Science 2004, 306, 1138–1139.
42. PubChem, http://pubchem.ncbi.nlm.nih.gov/, 2009.

8
Structure Enumeration
and Sampling
Markus Meringer
CONTENTS
8.1 Isomer Counting................................................................234
8.1.1 Counting Permutational Isomers ......................................235
8.1.2 Counting Isomers of Acyclic Structures and Other Compound
Classes..................................................................239
8.2 Isomer Enumeration: Deterministic Structure Generation ...................241
8.2.1 Early Cyclic and Acyclic Structure Generators.......................241
8.2.1.1 Acyclic Structure Generators ...............................241
8.2.1.2 Cyclic Structure Generator ..................................242
8.2.2 Orderly Generation ....................................................246
8.2.2.1 Enumerating Labeled Graphs ...............................246
8.2.2.2 Enumerating Unlabeled Graphs.............................247
8.2.2.3 Introducing Constraints .....................................248
8.2.2.4 Variations and Refinements .................................249
8.2.2.5 From Simple Graphs to Molecular Graphs .................250
8.2.3 Beyond Orderly Generation ...........................................252
8.3 Isomer Sampling: Stochastic Structure Generation...........................253
8.3.1 Uniformly Distributed Random Sampling ............................253
8.3.2 Monte Carlo and Simulated Annealing ...............................254
8.3.3 Genetic Algorithms ....................................................257
8.4 Beyond Isomer Enumeration ..................................................259
8.4.1 Virtual Chemical Space................................................259
8.4.2 Combinatorial Libraries ...............................................261
8.4.2.1 Counting Combinatorial Libraries ..........................261
8.4.2.2 Generating Combinatorial Libraries ........................263
Acknowledgment .....................................................................264
References ............................................................................264
Chemical structure enumeration and sampling have been studied by mathematicians,
computer scientists, and chemists for quite a long time. Given a molecular formula
plus, optionally, a list of structural constraints, the typical questions are: (1) How
233
234 Handbook of Chemoinformatics Algorithms
many isomers exist? (2) Which are they? And, especially if (2) cannot be answered
completely: (3) How to get a sample?
In this chapter, we describe algorithms for solving these problems. The techniques
are based on the representation of chemical compounds as molecular graphs (see
Chapter 2), that is, they are mainly applied to constitutional isomers. The major
problem is that in silico molecular graphs have to be represented as labeled structures,
while in chemical compounds, the atoms are not labeled. The mathematical concept
for approaching this problem is to consider orbits of labeled molecular graphs under
the operation of the symmetric group. We have to solve the so-called isomorphism
problem.
According to our introductory questions, we distinguish several disciplines: count-
ing, enumerating, and sampling isomers. While counting only delivers the number of
isomers, the remaining disciplines refer to constructive methods. Enumeration typi-
cally encompasses exhaustive and non-redundant methods, while sampling typically
lacks these characteristics. However, sampling methods are sometimes better suited
to solve real-world problems.
There is a wide range of applications where counting, enumeration, and sampling
techniques are helpful or even essential. Some of these applications are closely linked
to other chapters of this book. Counting techniques deliverpure chemical information;
they can help to estimate or even determine sizes of chemical databases or compound
libraries obtained from combinatorial chemistry.
Constructive methods are essential to structure elucidation systems (see Chap-
ter 9). They are used to generate structures that fulfill structural restrictions obtained
from spectroscopy in a pregeneration step, while in a postgeneration step, virtual
spectra of the generated structures can be computed and compared with the measured
data in order to determine which of them achieves the best fit.
Other applications use structure enumeration algorithms in order to produce
candidate structures for virtual screening (see Chapter 5). Structure–activity and
structure–property relationships, as introduced in Chapter 6, can be used in com-
bination with structure enumeration or sampling as rudimentary approaches toward
inverse QSAR (see Chapter 10) and de novo design algorithms often have their roots
in conventional structure generation.
The nonquantitative aspects of reaction network generation (see Chapter 11) are
also based on methods similar to those used for isomer enumeration.
8.1 ISOMER COUNTING
Counting means that only the number of structures is calculated and the structures
themselves are not produced by the algorithm. The most powerful counting technique
available to chemists is Pólya’s theorem [1], see also Refs. [2,3]. There are, of course,
various predecessors, for example a paper by Lunn and Senior [4], who were the first
to note that group theory plays a role, and a paper by Redfield [5] that contained even
better results. However, Pólya’s paper gave rise to the development of a whole theory
that is nowadays called Pólya’s Theory of Counting. Typical applications are counting
of permutational isomers and acyclic compounds.
Structure Enumeration and Sampling 235
8.1.1 COUNTING PERMUTATIONAL ISOMERS
Pólya’s approach to the enumeration of molecules with a given molecular formula is to
subdividethe molecule in question into a skeleton and a set of univalent substituents.It
leads to the following challenge: Evaluate the set of essentially different distributions
of the substituents over the sites of the skeleton with respect to the given symmetry
group of the skeleton.
In mathematical terms, the symmetry group G acts on the set of mappings m
n
from
the n sites of the skeleton onto the m available substituents. The set of orbits under this
group operation, m
n
// G, is in a one-to-one relation with the different constitutions.
If the skeleton shows no symmetries, that is, if G is of order 1, then it is clear that
there are m
n
different substitutions. Note that m
n
has two different meanings, one for
denoting the set of mappings and another for its cardinality. If the order of G is larger
than 1, the situation is more interesting.
The resulting isomers are called permutational or substitutional isomers. For
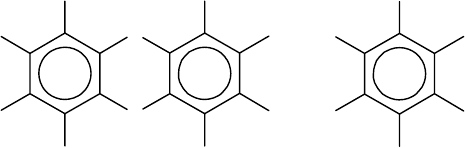
example the 22 permutational isomers of dioxin (tetrachlorodibenzo-p-dioxin) are
the essentially different distributions of four hydrogen and four chlorine atoms over
the eight sites of the skeleton depicted on the left:
O
O
81
2
3
45
6
7
6
5
1
2
3
4
Counting these isomers is described in detail in Kerber’s comprehensive book [6]
on finite group actions. In this section we will discuss the example of permutational
isomers of dichlorobenzene, which are based on the benzene skeleton sketched on
the right.
First we will try to describe Pólya’s approach in general. The symmetry group G
of the skeleton with respect to the n binding sites is required as input. The procedure
works with the topological symmetry group as well as with the geometrical symmetry
group. Of course, the results might differ. For ways to compute a molecule’s symmetry
group, see Chapter 2. A suitable data structure for representing groups is described
by Sims [7].
The reader should be familiar with the cycle notation of permutations, which is
briefly described in Example 8.1.1. At this point, it is useful to know that every
permutation has a unique decomposition into disjoint cycles. For more details of
permutations and cycles, the reader is referred to Ref. [6].
The cycle index of a permutation g ∈ G is a monomial in variablesz
k
. It is definedas
Z(g) =
n
'
k=1
z
k
c
k
(g)
, (8.1)

236 Handbook of Chemoinformatics Algorithms
where c
k
(g) is the number of cycles of g having length k. The cycle index Z(G) of G
is the averaged sum of cycle indices of group elements:
Z(G) =
1
|G|
g∈G
n
'
k=1
z
k
c
k
(g)
. (8.2)
In order to obtain a counting series from the cycle index, a so-called generating
function has to be inserted. Having m > 1 different chemical elements to choose from,
a suitable generating function is y
1
+···+y
m
. Inserting the generation function into
the cycle index means that every occurrence of z
k
in Z(G) is replaced by y
1
k
+···+
y
m
k
.Ifm = 2 the easier generation function 1 +x can be used instead, and during
insertion, z
k
is replaced by 1 +x
k
.
The coefficient of the monomial
2
m
i=1
y
j
i
i
of the counting series equals the number
of isomers with j
i
substituents of type i. In the case where we have just two different
elements to substitute, say H and Cl, the coefficient of x
j
equals the number of isomers
with j hydrogen atoms. To summarize, we can formulate the following.
ALGORITHM 8.1.1 COUNTING PERMUTATIONAL ISOMERS BY
PÓLYA’S THEOREM
1. Calculate the cycle index of the skeleton with respect to the binding sites
2. Insert the generation function into the cycle index
3. Evaluate the coefficients of the counting series
This formal description of how to solve the counting problem in general needs to be
illustrated by an example. Counting C
6
H
4
Cl
2
constitutions with a benzene skeleton
will be explained step by step.
Example 8.1.1: Counting Isomers of Dichlorobenzene
Below we see the benzene skeleton together with its symmetry axes (1), ..., (6).
1
2
34
5
6
(1)
(2)
(3)
(4)
(6) (5)
Besides six axis reflections, the benzene skeleton allows some more symmetry
operations, namely five proper rotations.Table 8.1 lists all the symmetry operations
and the according permutations of benzene’s symmetry group D
6h
. E denotes the
identity, C
+/−
i
represents a rotation by 360/i degrees, where the sign describes
the direction of the rotation, and σ
( j)
v
represents a reflection at axis ( j).

Structure Enumeration and Sampling 237
TABLE 8.1
Permutations of the Automorphism Group D
6h
of Benzene
List Cycle Cycle
Operation Representation Representation Index
E [123456] (1)(2)(3)(4)(5)(6) z
1
6
C
+
6
[234561] (123456) z
6
1
C
−
6
[612345] (654321) z
6
1
C
+
3
[345612] (135)(2 4 6) z
3
2
C
−
3
[561234] (531)(6 4 2) z
3
2
C
2
[456123] (14)(2 5)(3 6) z
2
3
σ
(1)
v
[165432] (1)(4)(2 6)(3 5) z
1
2
z
2
2
σ
(2)
v
[543216] (3)(6)(1 5)(2 4) z
1
2
z
2
2
σ
(3)
v
[321654] (2)(5)(1 3)(4 6) z
1
2
z
2
2
σ
(4)
v
[654321] (16)(2 5)(3 4) z
2
3
σ
(5)
v
[432165] (14)(2 3)(5 6) z
2
3
σ
(6)
v
[216543] (12)(3 6)(4 5) z
2
3
Permutations are given in two notations. The list representation might appear
more straightforward to the reader, because for a permutation π the ith component
in the list simply defines the image of i, that is the list representation of π is
[π(1), ..., π(n)].
The cycle notation is a little more difficult to understand, but gives direct access
to the cycle index, which is needed to compute counting series. The cycle repre-
sentation consists of 1 to n cycles, which are enclosed by round brackets. Each
cycle itself consists of 1 to n elements. Cycles of only one element (i) show that i
is fixed under the permutation, that is, π(i) = i. Sometimes such cycles are even
suppressed in cycle notations. Cycles (i
1
, ..., i
l
) with more than one element indi-
cate that i
1
is mapped onto i
2
, i
2
is mapped onto i
3
, and so on. The length l of
the cycle is determined by the minimum number of applications of π that map i
again onto i, that is, l = min{h : π
h
(i) = i}. In particular, this means that the last
element of the cycle, i
l
, is mapped onto i
1
, and generally the cycle of i
1
can be
written as [i
1
, π(i
1
), π
2
(i
1
), ..., π
l−1
(i
1
)].
Finally, the cycle indices of the elements of the automorphism group can be
derived directly from the cycle notations using Equation 8.1. This results in the
cycle index
Z(D
6h
) =
1
12
(z
1
6
+4z
2
3
+2z
3
2
+2z
6
1
+3z
1
2
z
2
2
)
for benzene’s automorphism group D
6h
. Inserting the generating function 1 +x
leads to the counting series
C(D
6h
) =
1
12
((1 + x)
6
+4(1 +x
2
)
3
+2(1 +x
3
)
2
+ 2(1 + x
6
) + 3(1 +x)
2
(1 + x
2
)
2
)
= 1 +x + 3x
2
+3x
3
+3x
4
+x
5
+x
6
.
