Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.


CHAPTER 4
SEMICONDUCTORS
Our technologically advanced way of life would not be possible without the
semiconductor industry. The first semiconductor device known as a transistor was
discovered at Bell Labs in the late 1940s, and was widely used shortly thereafter for
radio electronics. Today, transistors are still pervasive in every microelectronic
component such as CD/DVD players, cellular phones, modes of transportation
(e.g., planes, automobiles, etc.), and computers. In fact, modern computer chips
now contain over 2 billion individual transistors – all on a surface that is smaller than
a postage stamp!
This chapter will investigate the various types of semiconducting materials,
focusing on the influence of their structure on overall properties. We will also detail
the many applications for semiconductors, especially within the framework of
micro-electronic circuitry. It should be noted that nanostructural materials represent
a more recent realm of semiconduct ing materials, of use for next-generation solar
devices, sensors, batteries, etc. However, these materials will not be considered
in this chapter, but will instead be detailed in Chapter 6 that focuses solely on
nanotechnology.
4.1. PROPERTIES AND TYPES OF SEMICONDUCTORS
As their name implies, semiconductors possess electrical conductivity intermediate
between conductors such as metals and insulators such as ceramics. In Chapter 2,we
discussed the band structure for the molecular orbitals of infinite lattices. The lack of an
energy gap between the filled (valence) and empty (conduction) bands indicated that no
thermal energy was required to facilitate electrical conductivity. That is, metals are able
to transport electrons through their lattices even at absolute zero. By contrast, semi-
conductors possess a bandgap between valence and conduction bands (Figure 4.1).
An appropriate amount of energy must be supplied to the material in order to promote
an electron from the valence band to the conduction band, wherein electron transport
may occur. For semiconductors, any temperature greater than 0 K is sufficient for such
electron promotion; if the bandgap is too large, the material is unable to conduct
electricity, and is known as an insulator. The bandgap for semiconductors are typically
in the range 150–290 kJ mol
1
(i.e., 1–4 eV). Since the bandgap of Si is 1.12 eV, only
239
radiation with wavelengths ca. 1,100 nm (near IR, visible, UV, X-ray, etc.)will
be absorbed by silicon semiconductors, as the absorption of less-energetic radiation
will place the electron in the bandgap, where there are no available energy states.
The absorption of visible light by silicon results in its opacity, relative to transparent
glasses that do not absorb radiation within this wavelength regime.
Group 14 provides an interesting case-study for the most dramatic change in
electrical conductivity among its congeners. In fact, all types of conductors are
present in this group, from insulating carbon (diamond) to metallic tin and lead.
Since the electronic configurations o f these atoms are all {ns
2
np
2
}, the change in
electrical conductivities is related to the bond strength for the atoms comprising the
crystal lattice. Recall that the indivi dual atoms in diamond, Si, Ge, Sn, and Pb are
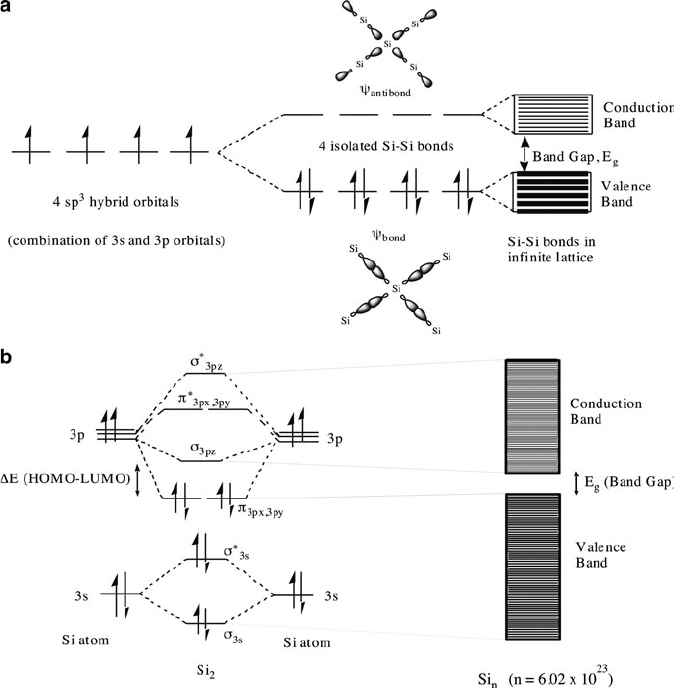
Figure 4.1. Electronic band diagrams for silicon. Shown is (a) bands resulting from overlap of sp
3
hybrid
orbitals and (b) bands resulting from overlap of molecular orbitals.
240 4 Semiconductors
tetrahedrally linked through the crystal lattice due to sp
3
hybridization. As the
individual s and p orbitals that comprise the hybrid orbital become more diffuse
(e.g., Sn, Pb), the bonding electrons are less tightly bound to individual atoms, and
become more polarizable. This results in delocalized metallic bonding in Sn and Pb
relative to very strong localized bonding in diamond. For intermediate Si and Ge, the
bonding between individual atoms is weaker than C, allowing the possibility for
thermal motion to break bonds in the solid-state lattice, by promoting bonding
electrons into the conduction band and propagating electrical conductivity.
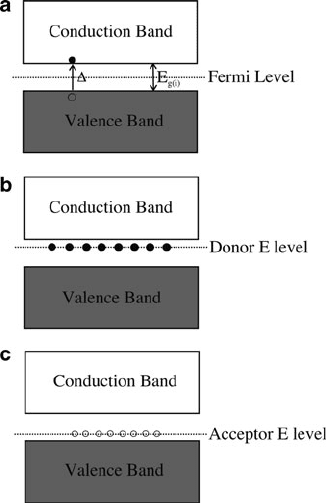
There are two types of semiconductors: intrinsic and extrinsic (Figure 4.2).
Intrinsic semiconductors contain the same numbers of free bonding electrons (e
)
and holes (h
+
), created from the migration of electrons from the valence to conduc-
tion bands. The temperature-dependent concentration of e
/h
+
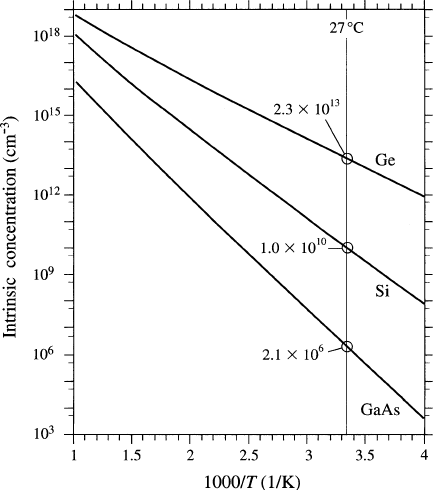
carriers is known as
the intrinsic concentration ,n
i
(Figure 4.3). As we discusse d in Chapter 2, the
number of electrons that are able to traverse the bandgap is governed by the Fermi
function, which gives the probability of an electron occupying an available energy
state. The density of states (DOS), or numb er of available energy levels, is also
paramount for the promotion of electrons from valence-conduction bands. The DOS
begin at the bottom of the valence band and continue to the Fermi level – the highest
Figure 4.2. Band diagrams for intrinsic and extrinsic semiconductors. Shown are (a) an intrinsic
semiconductor, with an equivalent number of free electrons and holes, (b) an n-type extrinsic semiconductor,
with a greater number of electrons, and (c) a p-type extrinsic semiconductor, with an excess of holes.
4.1. Properties and Types of Semiconductors 241
occupied state at absolute zero (i.e., 273.15
C). For metals that readily exhibit
electrical conductivity, the Fermi level lies within the conduction band due to the
lack of a bandgap. However, unlike metals, the DOS for conduction electrons in a
semiconductor do not coincide with the Fermi level, but rather begin at the top of the
bandgap. Hence, the placement of the Fermi level represents the relative ease at
which an electron is promoted from the valance band to the conduction band in a
bulk semiconductor. Quantitatively, the density of occupied states per unit volume is
the product of the DOS and the Fermi function, f(E); the density of holes is the
product of the DOS and [1 – f(E)].
For extrinsic semiconductors, the Fermi level corr esponds to a level slightly above
or below conduc tion or valence bands, depending on the dopants introduced into the
lattice – either an excess of electrons or holes (Figure 4.2b, c). Since our frame of
reference for semiconducting ability is the Group 14 element of Si, the terms
electron-deficient and electron-rich dopants refer to atoms possessing <4 valence
electrons (e.g., B, Al) and >4 electrons (e.g., N, P), respectively. By convention, if
additional electrons are introduced into the lattice, the sem iconductor is designated as
n-type, whereas the doping of additional holes yields a p-type semiconductor desig-
nation. From the convenient “n” and “p” notation, one may immediately recognize
whether there are excess negative or positive carriers in the lattice.
Figure 4.3. The intrinsic carrier concentration vs. temperature for Ge, Si, and GaAs. Reproduced with
permission from Kasap, S. O. Principles of Electronic Materials and Devices, 3rd ed., McGraw-Hill:
New York, 2007. Copyright 2006 The McGraw-Hill Companies.
242 4 Semiconductors
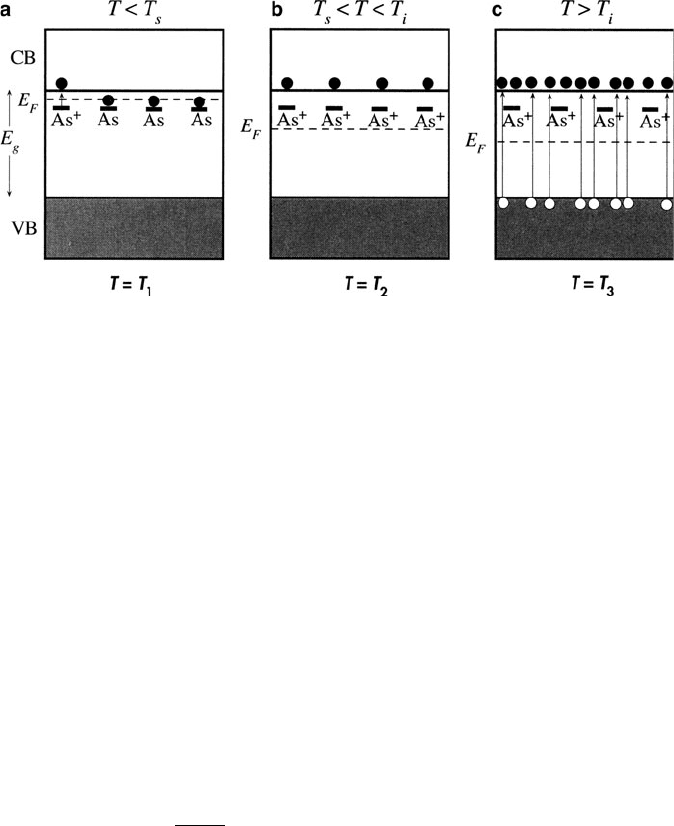
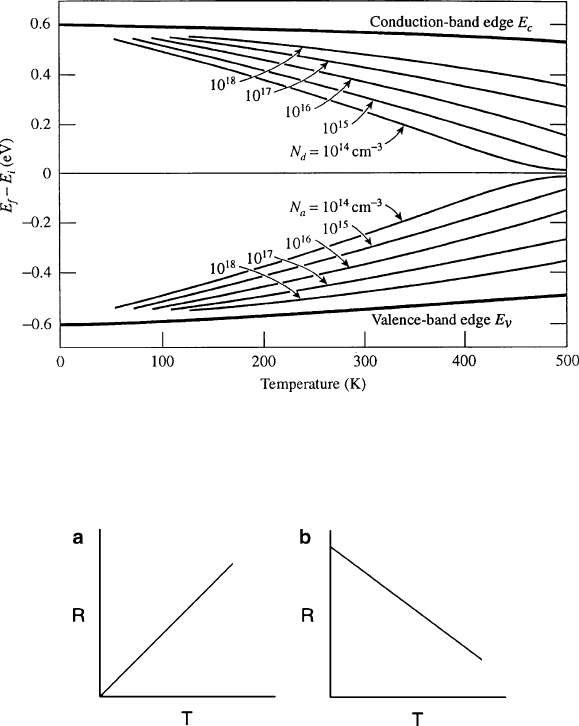
Figure 4.4 illustrates the effect of temperature on the carrier concentration of a
semiconductor. At low temperatures, the e
/h
+
concentration of extrinsic semicon-
ductors is governed by the ionization of the dopants (e.g.,B
/h
+
,Al
/h
+
for p-type;
P
+
/e
,As
+
/e
for n-type – Figure 4.4a). At the saturation temperature,T
s
, all donors
or acceptors have been ionized resulting in no further increase in the carrie r
concentration (Figure 4.4b). As the temperat ure is increased further, the intrinsic
temperature,T
i
, is reached when the thermal promotion of electrons across the
bandgap exceeds the concentration of acceptors/donors. Hence, at temperatures
above T
i
, an extrinsic semiconductor will exhibit an intrinsic carrier concentration,
with [e
] ¼ [h
+
] (Figure 4.4c). For a given carrier concentration, the lattice constant
will increase concomitantly with temperature. Accordingly, this will decrease the
energy required to break bonds thereby lowering the bandgap (Eq. 1 – the Varshni
equation, Figure 4.5).
E
g
¼ E
g
0
AT
2
B+T
ð1Þ
where E
g
0
is the bandgap at T ¼ 0 K (GaAs ¼ 1.519 eV, Si ¼ 1.7 eV); A and B are
material-specific constants (e.g., Si: A ¼ 4.73 10
4
eV K
1
,B¼ 636 K; GaAs:
A ¼ 5.405 10
4
eV K
1
,B¼ 204 K), and T ¼ temperature (in K).
The temperature dependence is quite different for metals and semiconductors. At
a temperature of 0 K, a semiconductor will behave as a perfect insulator. However,
metals will exhibit electrical conductivity at absolute zero due to the delocalized
electron density and lattices described in Chapter 3. However, as the temperature is
increased, the resp ective conductivities of these materials will be reversed, with
Figure 4.4. Position of the Fermi level (E
F
) and relative ionization of n-type Si with increasing temperature.
Below the saturation temprature (T
s
), only a few As dopants are ionized; however, at increasing temperatures,
more As atoms become ionized until the intrinsic temperature is reached (T
i
). At that temperature, all dopants
have been ionized, and the electrical conductivity results from promotion of electrons across the bandgap.
Reproduced with permission from Kasap, S. O. Principles of Electronic Materials and Devices,3rded.,
McGraw-Hill: New York, 2007. Copyright 2006 The McGraw-Hill Companies.
4.1. Properties and Types of Semiconductors 243
metals showing a decrease and semiconductors an increase in conductivity
(Figure 4.6). The thermal motion of metal atoms causes less efficient electron
mobility through the lattice, whereas a temperature increase causes the bandgap to
narrow for semiconductors, resulting in more effective electrical conductivity.
As the temperature continues to increase for semiconductors, the linear relationship
does not continue to hold, and the resistance begins to increase analogous to metals.
Instead, the atomic vibrations caused by the elevated temperature begin to outweigh
the thermally-induced decrease of the bandgap.
As Figure 4.7 illustrates, when thermal energy promotes a bonding electron from
the valence band to the conduction band, the released electrons are free to migrate
Figure 4.5. The effect of increasing temperature on the position of the donor/acceptor energy levels
within the bandgap for various doping levels, N
D
or N
A
. Reproduced with permission from Kasap, S. O.
Principles of Electronic Materials and Devices, 3rd ed., McGraw-Hill: New York, 2007. Copyright 2006
The McGraw-Hill Companies.
Figure 4.6. Resistivity–temperature relationships for (a) metals and (b) semiconductors.
244 4 Semiconductors
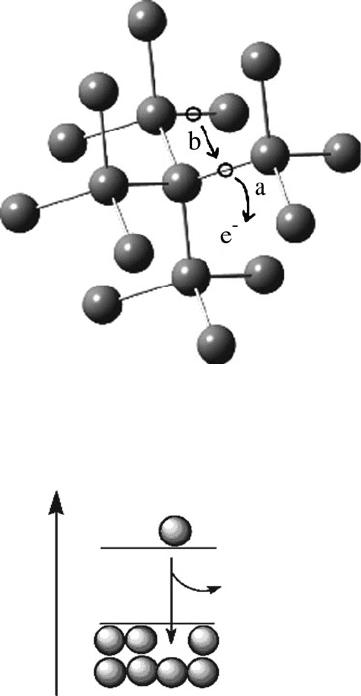
throughout the lattice. However, the vacancies (i.e., holes) left behind are also free to
move – in the opposite direction as electrons. One may consider these holes as
positively charged species formed from loss of an electron. Thus, electrons and holes
represent the two types of carriers that correspond to electrical conductivity in
semiconductors.
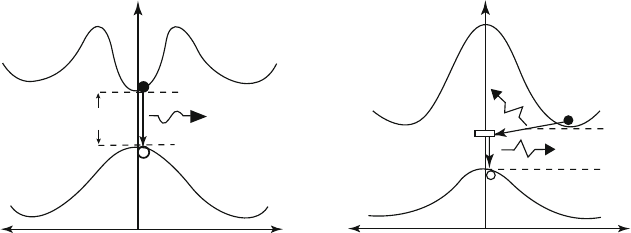
Since an electron that has been promoted to the conduction band will have a
greater energy than those left in the valence band, there is a possibility for the
electron to lose this excess energy. The spontaneous return of electrons in the
conduction band to the valance band is known as recombination, and is usually
accompanied by light emission and heat (Figure 4.8). This phenomenon happens all
the time for excited-state molecules. For instance, consider what happens when one
Figure 4.7. Illustration of creation/migration of electrons and holes created through Si–Si bond
thermolysis. Shown are (a) the release of an electron and concomitant formation of a hole and (b) the
migration of an electron from a nearby bond to fill the vacancy.
Conduction Band
Valence Band
Energy
hn or D
Figure 4.8. Schematic of recombination of electron-hole pairs generating either a photon of energy or heat.
4.1. Properties and Types of Semiconductors 245
supplies sodium atoms with sufficient ener gy to promote an electron from the 3s
energy level into an empty 3p, or 4s orbital. The ele ctron does not stay in the higher
energy level very long (typically ca.10
6
s) before it is spontaneously dropped to its
original ground state, releasing heat and/or light. There are three types of recombi-
nation
[1]
:
(i) Band-to-band: an electron moves from the conduction band into an empty state
in the valence band associated with the hole. This yields a radiative emission,
whose wavelength is inversely proportional to the bandgap.
(ii) Trap-assisted: an electron falls into an energy level within the bandgap that is
associated with a dopant atom or structural defect.
(iii) Auger recombination: when a conduction-band electron and valence-band hole
recombine, rather than emitting light the energy may kick off an outer-shell
electron known as an Auger electron.
To understand the band structure and recombination process in more detail, we
must revisit some concepts of quantum physics discussed in Chapter 2. Recall that
matter and light exhibit both wave-and particle-like behavior. This duality may be
expressed by the de Broglie equation, which equates the wavelength and momen-
tum, p, of a particle (Eq. 2). The potential energy of an electron in a crystal lattice
depends on its location, and will be periodic due to the regular array of lattice atoms.
The periodic wave functions that result from solving the Schr
€
odinger equation are
referred to as Bloch wave functions. Each wave function represents the energy of an
electron at a specific location in the lattice, referred to as k-space. Hence, an E-k
diagram may be constructed, with the potential energy of the electron on the y-axis,
and the wave vector, k, on the x-axis. The wave vector represents lattice directions of
the semiconductor crystal; changing values of k represents a change in momentum
of the electron. A comparison of the simpl ified E-k diagrams for Si or Ge and a
compound semiconductor such as GaAs is shown in Figure 4.9. For GaAs, the
k
k
-k
-k
E
E
E
c
E
c
E
v
E
v
E
r
E
g
CB
CB
VB
VB
k
vb
k
cb
Photon
Phonon
Direct bandgap
a
b
Figure 4.9. Comparison of (a) direct bandgap (e.g., GaAs) and (b) indirect bandgap (e.g., Si, Ge)
materials. Reproduced with permission from Kasap, S. O. Principles of Electronic Materials and
Devices, 2nd ed., McGraw-Hill: New York, 2002.
246 4 Semiconductors
minimum of the conduction band (CB) is directly above the maximum of the
valence band (VB). Accordingly, we refer to such solids as direct bandgap materi-
als. In contrast, for Si and Ge, the CB minimum and VB maximum are offset,
resulting in an indirect bandgap.
l ¼
h
p
ð2Þ
For Si, in order for an electron at the bottom of the CB to recombine with a hole from
the top of the VB, the momentum of the electron must shift from k
cb
to k
vb
(Figure 4.9b). However, this is not allowed by the Law of Conservation of Momen-
tum. Instead, an indirect recombination mechanism must take place, wherein the
electron is captured by an interstitial defect with energy E
r
, which facilitates its
relaxation to the top of the VB. This process is accompanied by the emission of
phonons, or lattice vibrations rather than light emission. In contrast, efficient
electron-hole recombination may occur without any change in momentum for direct
bandgap materials, resulting in the emission of photons. We will describe some
important applications for dir ect bandgap semiconductors later in this chapter.
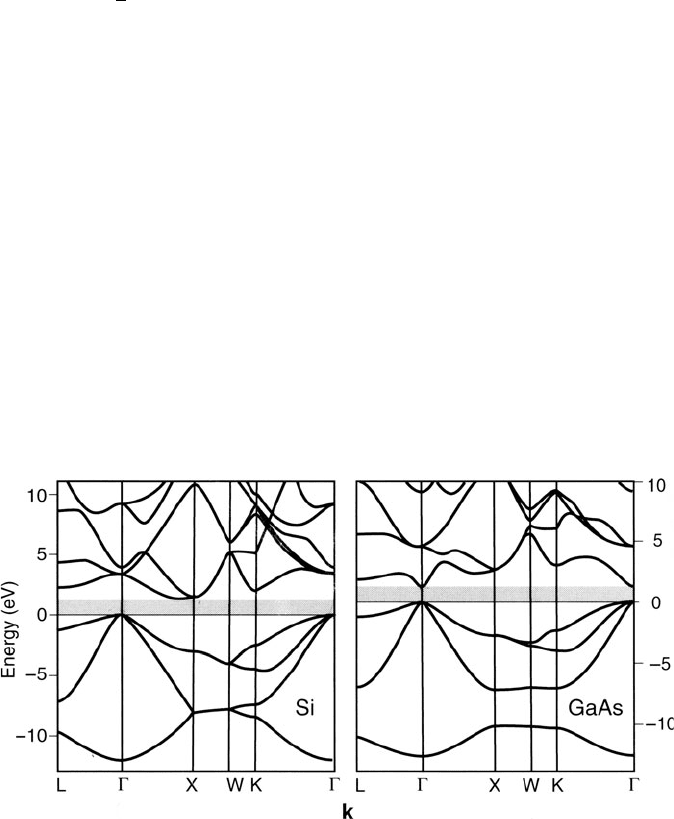
The band diagram for real solids is not as straight forward as what we have
illustrated thus far. Figure 4.10 presents the real band diagram s for indire ct and
direct bandgap semiconductors Si and GaAs, respectively, showing a great deal of
complexity associated with valence and conduction bands.
[2]
As noted in Chapter 2,
the Brillouin zones (BZ; primitive unit cell of the reciprocal lattice) for 3-D crystal
lattices are complex polyhedra (Figure 2.76). Hence, the band energy varies in 3-D
k-space and the resultant lines are slices through the BZ polyhedron in specific
Figure 4.10. Electronic energy bands for Si and GaAs. The bands below the gray zone (bandgap) are
completely filled (valence band – VB), and those above are completely empty (conduction band – CB) at
0 K. Reproduced with permission from Hofmann, P. Solid State Physics: An Introduction, Wiley:
New York, 2008. Copyright 2008 Wiley-VCH Verlag GmbH & Co.
4.1. Properties and Types of Semiconductors 247
