Ellis A.M., Feher M., Wright T. Electronic and Photoelectron Spectroscopy: Fundamentals and Case Studies
Подождите немного. Документ загружается.


20
Rotationally resolved laser
excitation spectrum of propynal
Concepts illustrated: near-symmetric rotor approximation; asymmetric rotors and
asymmetry splitting; parallel and perpendicular bands.
Vibrationally resolved LIF excitation spectra of propynal were met in the previous Case
Study. In the present Case Study the focus is on the rotationally resolved laser excitation
spectrum of propynal. This molecule is nominally an asymmetric rotor, since the only
symmetry it possesses is a reflection plane (C
s
point group). However, as we will see, it is
a near-prolate symmetric rotor and therefore its rotationally resolved electronic spectrum
can be largely understood in terms of the properties of a prolate symmetric top.
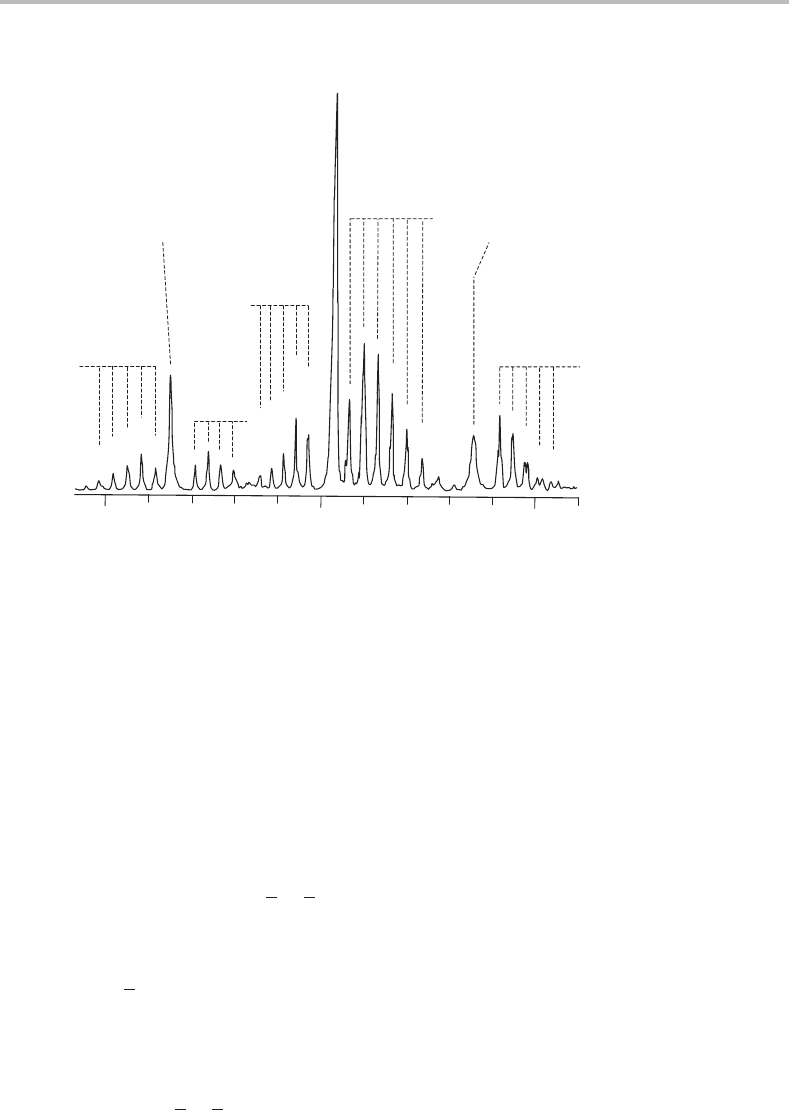
Figure 20.1 shows the excitation spectrum for the 6
1
0
band, where mode ν
6
is dominated
by C
C stretching character. This was taken from original work by Stafast and co-workers
[1]inwhich propynal was seeded into a pulsed supersonic jet. The origin (0
0
0
) band has
very similar rotational structure.
20.1 Assigning the rotational structure
The rotational structure is relatively simple to assign, although it might look quite com-
plicated at first sight. We will attempt to interpret this spectrum by treating propynal as a
prolate symmetric top, and will subsequently consider what happens when this constraint
is removed.
P, Q, and R branches are readily identified in the central portion of the spectrum in
Figure 20.1. The intense Q branches are the most obvious features, and once identified
then it is relatively straightforward to see that each is flanked by fully resolved P and R
branches. On both sides of the central, and most intense, P/Q/R system there are additional,
weaker P/Q/R systems. With some experience, this structure suggests to the spectroscopist
that transitions out of different K levels are being observed. For a prolate symmetric top
the rotational energy levels are described by equation (6.15) and the transition selection
rules are
Parallel transitions K = 0,J = 0, ±1
Perpendicular transitions K =±1,J = 0, ±1
165
166 Case Studies
Laser wavenumber/cm
−1
2712027115 27125
)(
0
JR
r
420
)(
0
JQ
r
)(
1
JQ
r
)(
1
JR
r
13 5
)(
0
JP
r
246
135
)(
1
JP
p
)(
1
JQ
p
)(
1
JR
p
13
Figure 20.1 Rotationally resolved laser excitation spectrum of the
˜
A
1
A
−
˜
X
1
A
0
0
0
band of jet-cooled
propynal. (Reproduced with permission from H. Stafast, H. Bitto, and J. R. Huber, J. Chem. Phys. 79
(1983) 3660, American Institute of Physics.)
A parallel transition is one in which the transition dipole moment is oriented along the
a inertial axis. If the A rotational constants are similar in the upper and lower electronic
states, then the second term on the right-hand side of (6.15)isirrelevant and we find that
the rotational structure should consist of single P, Q, and R branches. This is not what is
observed in Figure 20.1,sothe transition must be dominated by perpendicular character.
Using equation (6.15) transitions are expected at
ν = ν
0
+ [(A
− B
)K
2
+ B
J
(J
+ 1)]
−[(A
− B
)K
2
+ B
J
(J
+ 1)] (20.1)
where
v
0
is the transition wavenumber for the pure 6
1
0
transition, i.e. in the absence of rota-
tional structure. For simplification, assume that A
=A
and B
=B
so that the superscripts
can be dropped. The above equation then simplifies to
ν = ν
0
+ (A − B)(K
2
− K
2
) + B[J
(J
+ 1) − J
(J
+ 1)] (20.2)
For Q branch transitions J
= J
and therefore the second term on the right of (20.2)
disappears. Consequently, for a perpendicular transition, Q branches are expected at the

20 Rotationally resolved spectrum of propynal
167
following positions:
K
= 1 ← K
= 2 ν = ν
0
− 3(A − B)
K
= 0 ← K
= 1 ν = ν
0
− (A − B)
K
= 1 ← K
= 0 ν = ν
0
+ (A − B)
K
= 2 ← K
= 1 ν = ν
0
+ 3(A − B)
K
= 3 ← K
= 2 ν = ν
0
+ 5(A − B)
Thus a series of Q branches are expected separated by 2(A − B). The most intense Q branch
in a spectrum is expected to be that corresponding to K
=1 ←K
=0, since K
=0 will be
the most populated K level in the ground electronic state. Transitions from other K
levels
are possible but will be progressively weaker as K
increases.
The features in Figure 20.1 can now be readily explained. The strong Q branch in
the centre of the spectrum must be due to K
= 1 ← K
= 0 transitions, with the high
intensity deriving from unresolved contributions from various Q(J) transitions. The weaker
Q branches to higher and lower wavenumbers are, respectively, due to K
= 2 ← K
= 1
and K
= 0 ← K
= 1 transitions. We shall refer to these transitions with different values
of K
and K
as K sub-bands.
A specific labelling system is used for rotational transitions in electronic spectra of
symmetric tops, which is an extension of that employed for linear molecules. P, Q and R
branch transitions are labelled in the usual manner by specifying the rotational quantum
number J in the lower state, i.e. P(J)orR(J). However, superscripts and subscripts are added
to specify a particular sub-band. For example, in the
r
P
0
(J) transition the pre-superscript
‘r’reveals that K =+1, while the ‘0’ subscript refers to the value of K in the lower state.
In this way we can uniquely identify the upper and lower state rotational quantum numbers
and this compact notation has been employed in Figure 20.1.
The P and R branches in each K sub-band are simple to interpret. From the second term on
the right-hand side of equation (20.2), we expect P and R branch structure exactly analogous
to that for linear molecules, i.e. a spacing of approximately 2B between adjacent members in
a specific branch. However, certain members of a specific branch may be missing. For exam-
ple, the first members of the R branches in both the K
= 0 ←K
= 1 and K
= 2 ←K
= 1
sub-bands are absent whereas that in the K
= 1 ← K
= 0 sub-band is clearly seen.
This is due to the fact that for K
= 0any value of J
is possible but for K
= 1 the
lowest allowed value of J
is 1, since K is the projection of J and therefore J ≥ K .
Although less obvious from the spectrum because of overlap with the stronger K
=
1 ← K
= 0 sub-band, the first member of the P branch in the K
= 2 ← K
= 1
sub-band is
r
P
1
(3) since J
≥ 2 for K
= 2. Missing lines such as these make it possi-
bletoconfirm the K quantum numbers in the upper and lower states, and this type of
argument is commonly used to assign quantum numbers in rotationally resolved spectra.
20.2 Perpendicular versus parallel character
Why is the electronic transition perpendicular rather than parallel? In Case Study 19 it was
suggested that the electronic transition involved is a π * ← n transition on the carbonyl
168 Case Studies
group. In other words, an electron is moved from a non-bonding orbital on the C Ogroup
to a π antibonding molecular orbital. In the non-bonding orbital the electron density is
oriented in the plane of the molecule, whereas in the π * orbital it is perpendicular to
the plane. In electronic transitions the transition dipole moment reveals the direction in
which the instantaneous shift in charge takes place. Since the charge shifts from an in-
plane to out-of-plane orientation, the transition dipole moment must be perpendicular to the
molecular plane. In other words, it is approximately perpendicular to the a inertial axis (see
below), and explains why perpendicular character dominates in the rotationally resolved
spectrum.
H
H
O
a
b
CCC
20.3 Rotational constants
In the prolate rotor limit the rotational constants can easily be determined from the spectrum
in Figure 20.1.Wewill no longer assume that A
= A
but we will continue to assume that
B
= B
because the Q(J) transitions in a specific sub-band are unresolved (which is only
possible if B
is very similar to B
). The separation between the observed Q branches can
then be expressed as follows:
r
Q
1
(J ) −
r
Q
0
(J ) = 3A
− A
− 2B = 3.27 cm
−1
(20.3)
r
Q
0
(J ) −
p
Q
1
(J ) = A
+ A
− 2B = 3.78 cm
−1
(20.4)
The wavenumbers are estimates taken from the spectrum. Similarly, 2B can be estimated
from the average spacing between members of a particular branch, giving B ≈ 0.15 cm
−1
.
Simultaneous equations (20.3) and (20.4) can then be solved to yield A
= 1.91 and
A
= 2.17 cm
−1
.
Clearly these are only estimates of the rotational constants. In reality propynal is an
asymmetric top so there is little point in pushing the analysis in terms of a symmetric rotor
too far. Instead, it is better to consider the effect that asymmetry has on the rotational energy
levels.
20.4 Effects of asymmetry
The impact of asymmetry is difficult to discern in Figure 20.1. This is partly because
propynal is a good approximation to a prolate symmetric rotor, but also because of the
modest resolution in the spectrum. Nevertheless, the keen-eyed reader may have noticed

20 Rotationally resolved spectrum of propynal
169
−1.0 −0.8 −0.6
0
00
1
01
1
11
1
10
2
12
2
11
2
20
2
21
3
22
3
21
60
50
40
30
20
10
0
Energy
Asymmetry
K
a
= 0
K
a
= 1
K
a
= 2
Figure 20.2 Variation of rotational energies of an asymmetric top as a function of the degree of
asymmetry. This diagram was calculated for A = 10 and C = 1, the energy units being arbitrary.
The asymmetry is expressed as an asymmetry parameter, κ, such that κ =−1 corresponds to a
prolate symmetric top (for further details see, for example, Reference [4]). Propynal is a very good
approximation to a prolate symmetric top – in its ground electronic state it has κ =−0.99.
that some of the lines in the
r
R
1
branch are resolved into doublets. This splitting is caused
by the breakdown of symmetric rotor behaviour.
In an asymmetric top the three rotational constants A, B, and C are all different. As a
result K is no longer a good quantum number. In order to specify a particular rotational
level a new labelling system must be introduced. The accepted notation is J
K
a
K
b
,where J
has its usual definition. The quantities K
a
and K
b
are integers referring to the value of K
with which a particular rotational level correlates in the prolate and oblate symmetric rotor
limits, respectively.
Figure 20.2 shows how the energies of rotational levels change in moving from a sym-
metric rotor to an increasingly asymmetric top. At the extreme left the energies are those of a

170 Case Studies
prolate symmetric top. The rotational energy is unaffected by the sense of rotation about the
a axis, which contributes a two-fold degeneracy to each level with K =0.
1
In an asymmetric
top this degeneracy is removed, and the extent of the splitting increases as the molecule
becomes more asymmetric. It is noticeable that the splitting, often referred to as asymmetry
doubling, is largest for levels correlating with K
a
= 1 (compare the splitting of the 2
12
/2
11
levels with the 2
20
/2
21
pair). This can be attributed to the higher speed of rotation about
the a axis as K
a
increases, which increases the prolate symmetric rotor character. Also, for
agivenK
a
, the asymmetry doubling has an approximately quadratic dependence on J.
The effect of asymmetry should be most noticeable in transitions involving K
a
= 1in
the upper or lower electronic state. In fact, for reasons beyond the scope of this book (see
Reference [3] for more details), selection rules prevent the direct observation of asymmetry
doubling in the K
= 1 ←K
= 0 and K
= 0 ←K
= 1 sub-bands. It is, however, visible in
the R branch of K
= 2 ← K
= 1. Although asymmetry doubling in both upper and lower
rotational levels contribute, the splitting will be dominated by the much larger asymmetry
splitting in K
= 1. A detailed analysis of this asymmetry structure is possible and has
yielded the following rotational constants (in cm
−1
) for propynal [2]:
Ground electronic state
A = 2.2694
B = 0.1610
C = 0.1501
Excited electronic state
A = 1.8893
B = 0.1630
C = 0.1498
These can be compared with the estimates for A and B shown earlier from the symmetric
rotor model and the agreement is seen to be quite reasonable given the approximation
involved.
References
1. H. Stafast, H. Bitto, and J. R. Huber, J. Chem. Phys. 79 (1983) 3660.
2. J. C. D. Brand, W. H. Chan, D. S. Liu, J. H. Callomon, and J. K. G. Watson, J. Mol. Spectrosc.
50 (1974) 304.
3. Molecular Spectra and Molecular Structure. III. Electronic Spectra and Electronic Structure
of Polyatomic Molecules,G.Herzberg, Malabar, Florida, Krieger Publishing, 1991.
4. Angular Momentum,R.N.Zare, New York, Wiley, 1988.
1
There is also 2 J + 1degeneracy for each rotational level so the overall degeneracy is 2(2 J + 1) for K = 0levels.

21
ZEKE spectroscopy of Al(H
2
O)
and Al(D
2
O)
Concepts illustrated: atom–molecule complexes; ZEKE–PFI spectroscopy; vibrational
structure and the Franck–Condon principle; dissociation energies; rotational structure of
an asymmetric top; nuclear spin statistics.
The study of molecular complexes in the gas phase provides important information on
intermolecular forces and spectroscopy has played a vital role in this field. As an illustra-
tion, the complex formed between an aluminium atom and a water molecule is described
here.
To obtain Al(H
2
O), it is necessary to bring together aluminium atoms and water
molecules. Getting water into the gas phase is easy, but aluminium poses more of a problem
since at ordinary temperatures the solid has a very low vapour pressure. An obvious solu-
tion is to heat the aluminium in an oven. However, the high temperature has a concomitant
downside; if water is passed through (or near) the oven the high temperature will almost
certainly prevent the formation of a weakly bound complex such as Al(H
2
O). Instead, the
heat may allow the activation barriers to be exceeded for other reactions, leading to products
such as the insertion species HAlOH.
A solution to this apparent quandary is to make Al(H
2
O) by the laser ablation–supersonic
jet method, which was mentioned briefly in Chapter 8 (see Section 8.2.3). Any involatile
solid, including metals, can be vaporized by focussing a high intensity pulsed laser beam
onto the surface of the solid. The resulting plume of gaseous material above the surface,
which includes metal atoms, can then be rapidly cooled by mixing with an excess of inert
carrier gas, such as helium or argon. If a small amount of water vapour is seeded into the
flowing carrier gas, formation of Al(H
2
O) complexes can occur. These are then rapidly
cooled further by expanding the gas mixture into vacuum to form a supersonic jet (see
Section 8.2.2).
In a recent study, Agreiter et al. formed Al(H
2
O) and Al(D
2
O) by the above procedure
and obtained spectra of this complex for the first time using ZEKE spectroscopy [1]. This
provided new information on both the neutral complexes and the corresponding cations, as
described below.
171

172 Case Studies
21.1 Experimental details
Al(H
2
O) is unlikely to be the sole product when laser ablating solid aluminium in the
presence of H
2
Ovapour. Despite the effort to cool the gas mixture, other reactions are almost
certainly unavoidable. Furthermore, a variety of clusters and complexes might be formed
involving multiple metal atoms and/or multiple water molecules. Experimental conditions
can be optimized to favour production of Al(H
2
O), e.g. by adjusting the partial pressure
of water vapour, but there will always be other species in the supersonic jet. Consequently,
some means of selectively detecting the spectrum of Al(H
2
O) is beneficial.
Agreiter and co-workers recorded spectra using the PFI version of ZEKE, in which the
laser wavelength is tuned to just below the ionization threshold and the complex is then
ionized by application of a delayed pulsed electric field (see Section 12.6 for more details).
The apparatus employed by Agreiter et al. was also equipped with a time-of-flight mass
spectrometer, and so it proved possible to estimate the ionization energies of Al(H
2
O)
n
complexes with different n by tuning the laser wavelength and looking for the onset of
photoionization in a given mass channel. In this way, Agreiter et al. were able to confirm
earlier work by Misaizu and co-workers in which the ionization energies of Al(H
2
O)
n
complexes were found to decrease rapidly as a function of n [2]. Since pulsed field ionization
is only observed close to the threshold for ionization, this information provides the means
of distinguishing between the spectra of the various possible Al(H
2
O)
n
complexes. The
ionization energy of Al(H
2
O) is approximately 5.1 eV, much lower than that expected for
chemical products such as HAlOH. It is therefore possible to be confident that the ZEKE
spectra recorded in the region close to 5.1 eV (
243 nm) originate from Al(H
2
O).
21.2 Assignment of the vibrationally resolved spectrum
ZEKE spectra of Al(H
2
O) and Al(D
2
O) are shown in Figure 21.1. Both spectra show an
obvious vibrational progression. In addition, there is finer structure, which is particularly
noticeable in the case of Al(D
2
O). This additional structure will be discussed later.
It is worth briefly reviewing what ZEKE spectroscopy reveals. In essence, resonant
transitions between energy levels of the neutral molecule and the ion are recorded. Conse-
quently, assuming most of the Al(H
2
O) and Al(D
2
O) complexes are initially in the zero-
point vibrational level, as is reasonable given that they are entrained in a supersonic jet,
then the observed vibrational structure is representative of the corresponding cations.
The fact that a single vibrational progression dominates the spectrum makes the assign-
ment relatively easy. The active mode has a frequency of approximately 328 cm
−1
for
Al
+
(H
2
O), as deduced from the spacing between adjacent peaks in the progression. The
only challenge is to identify the specific vibrational mode responsible. Al(H
2
O) and its
cation each have six degrees of vibrational freedom, which are illustrated in Figure 21.2.
1
1
The form of the six vibrations can be readily deduced. H
2
O will contribute the same three vibrational modes as the
free H
2
O molecule, although the mode frequencies will differ from those of the free molecule. The formation of
an Al
O bond will then add three further vibrations, an intermolecular (Al O) stretching mode and two bending
modes, one an in-plane and the other an out-of-plane (umbrella-like) deformation.
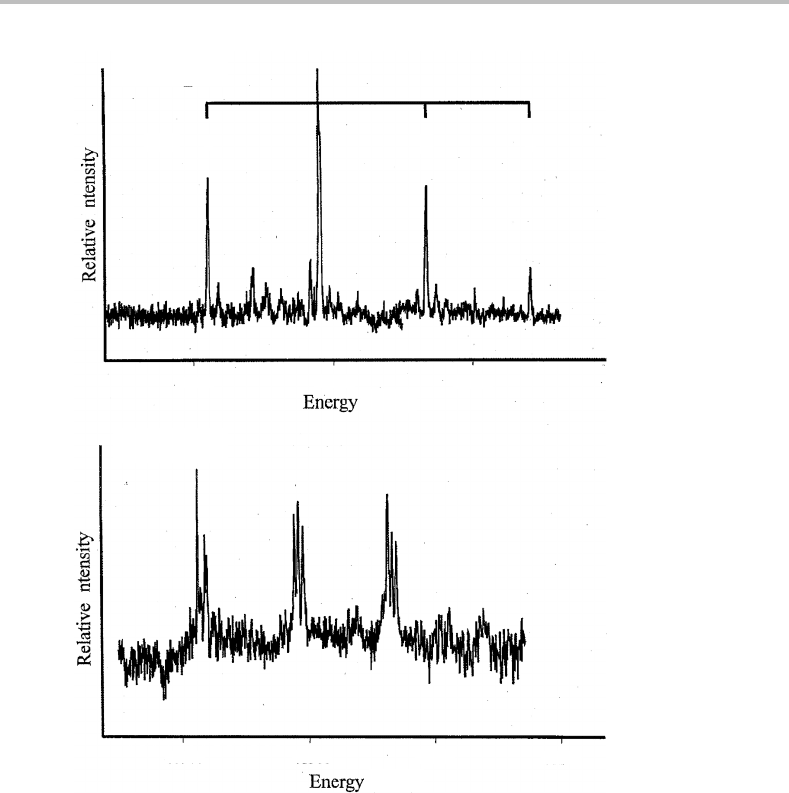
21 ZEKE spectroscopy of Al(H
2
O) and Al(D
2
O)
173
A1
+
—H
2
O
v
+
=
0 2 3
i
41000 41400 41800
(cm
−1
)
A1
+
—D
2
O
i
(cm
−1
)
40 900 41300 41700 42100
Figure 21.1 ZEKE–PFI spectra of Al(H
2
O) and Al(D
2
O). Single vibrational progressions dominate
both spectra and the vibrational quantum number in the ion formed is shown above the Al(H
2
O)
spectrum. (Reproduced from J. K. Agreiter, A. M. Knight, and M. A. Duncan, Chem. Phys. Lett. 313
(1999) 162, with permission from Elsevier.)
It will be assumed for the moment that the molecule has the C
2v
structure shown in
Figure 21.2, with the Al coordinated to the O atom. However, it is worth emphasizing
that as yet we have presented no evidence to support this assumption.
The six vibrational modes can be divided into two groups, vibrations localized primarily
on the water molecule and vibrations that are intermolecular in character. The former are
essentially the same vibrations as found in a free water molecule, although with somewhat
different frequencies because of the binding to an aluminium atom. We can be sure that
these vibrations are not responsible for the progression in Figure 21.1 since all three water
vibrations will possess far higher frequencies than 328 cm
−1
.
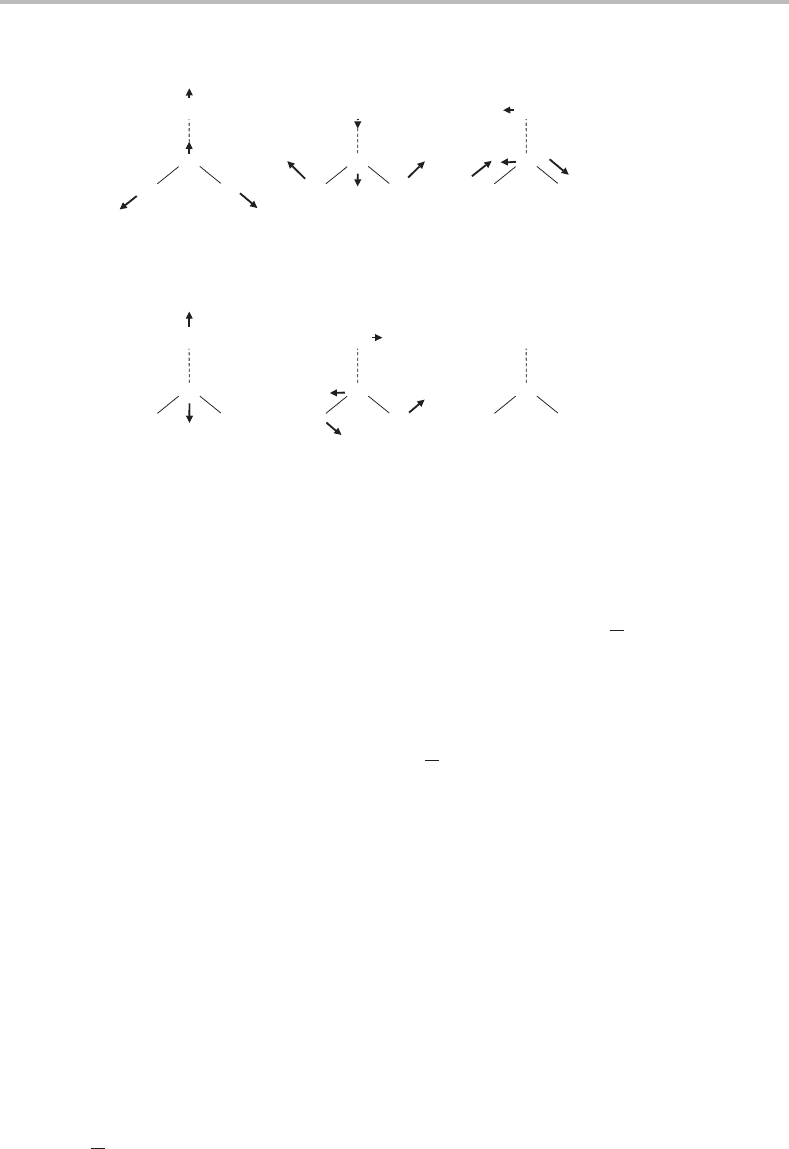
174 Case Studies
O
H
H
Al
Water vibrations
Intermolecular vibrations
Symmetric (a
1
)
O–H stretch
O
H
H
Al
O
H
H
Al
Bend (a
1
) Antisymmetric (b
2
)
O–H stretch
O
H
H
Al
Al–O stretch (a
1
)
O
H
H
Al
O
H
H
Al
In-plane bend (b
2
)Out-of-plane (b
1
)
deformation
+
+
-
+
Figure 21.2 Schematic illustration of the six vibrational modes of Al(H
2
O). C
2v
point group symmetry
has been assumed for the complex.
The formation of a bond between Al and H
2
O introduces three additional vibrational
modes, the intermolecular modes. One of these vibrations is the Al
O stretch, which is a
totally symmetric motion (a
1
symmetry in the C
2v
point group). The other two intermolec-
ular vibrations are bending modes, one involving in-plane twisting of the water molecule
relative to the Al atom, while the other is an out-of-plane deformation. These two bending
modes will be non-totally symmetric in C
2v
symmetry. If the complex has C
2v
symmetry
in both neutral and ionic states, then the Al
O stretch is the obvious assignment for the
observed vibrational progression.
However, it is possible that a lower symmetry complex may be formed in either the
ion or the neutral system, and in this case one or both of the bending modes may become
Franck–Condon active. For example, if the complex is non-planar but the Al atom remains
equidistant from the two H atoms, then the molecule will have a single plane of symmetry
and will belong to the C
s
point group. In this case the out-of-plane deformation would be
totally symmetric and significant vibrational structure might result if there is a change in
the equilibrium deformation angle on photoionization.
A comparison of the spectra for the deuterated and non-deuterated complexes establishes
the assignment. The vibrational motion in the deformation mode is dominated by motion
of the two hydrogen atoms. A large change in vibrational frequency would therefore be
expected in switching from Al(H
2
O) to Al(D
2
O). The separations between adjacent peaks
in the vibrational progressions show no such change, the decline in frequency being only
12 cm
−1
. The main vibrational structure in Figure 21.1 can therefore be assigned to the
Al
O stretching vibration.
